Preparation method of ulipristal acetate and key intermediate thereof
一种醋酸乌利司他、酸性条件的技术,应用在医药领域,能够解决成本高、收率低、不适合工业化生产等问题,达到成本低、收率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、3
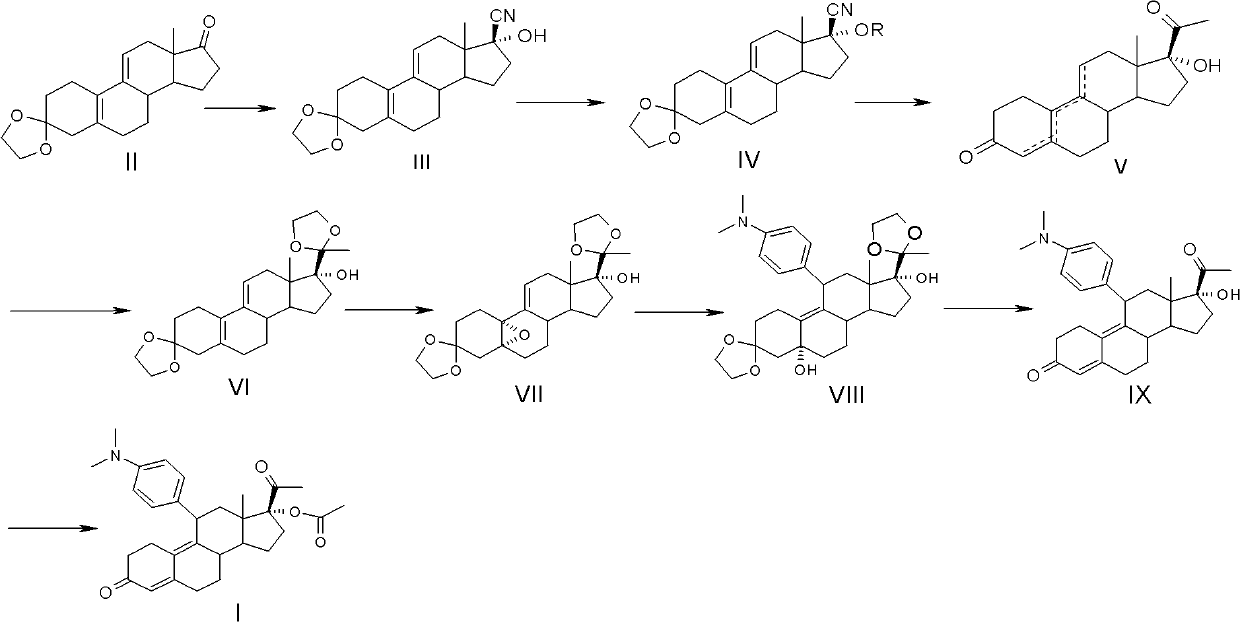
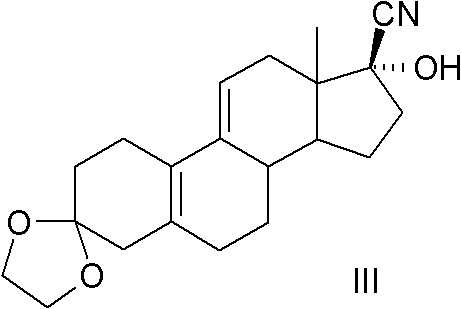
[0062] Example 1, Preparation of 3,3-ethylenedioxy-17β-cyano-17α-hydroxyl-19-norpregna-5(10), 9(11)-diene (compound III):
[0063] Add 3-ketal (2.0kg, 6.37mol), methanol (12L), sodium cyanide (343g, 7.0mol) and glacial acetic acid (440ml) into the reaction flask, stir the reaction overnight at room temperature, then pour into 24L ice Stir in water for 30 minutes to filter, wash the filter cake three times with water, and dry to obtain 2.06kg of white powder, mp: 176-178°C (decomposition), yield 95%, HPLC purity above 98%.
[0064] MS: 342(M+1)
[0065] Single crystal tests showed the absolute configuration as follows:
[0066]
Embodiment 2、17
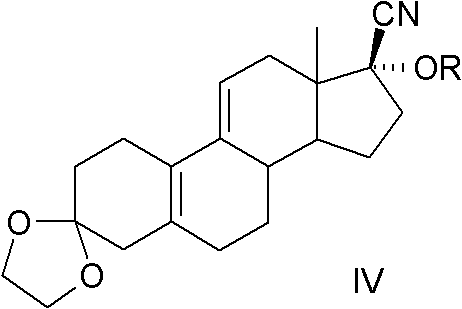
[0067] Example 2, 17α-[(±)1-(1-ethoxy)ethyl]oxy-17β-cyano-3,3-ethylenedioxy-19-norpregna-5(10 ), the preparation of 9(11)-diene:
[0068] Compound III (2.0kg, 5.87mol) obtained in Example 1, THF (14L) and p-toluenesulfonic acid (12.0g, 70mmol) were added to the reaction flask, under ice-water cooling, vinyl ether (668ml, 7.04mol), kept stirring for 4h, added triethylamine (15ml), stirred for 10 minutes, the aqueous phase was extracted with dichloromethane, washed with water, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to obtain light yellow or colorless oil 2.43 kg, the yield is quantitative.
[0069] TLC (developer ethyl acetate:petroleum ether=1:5) of the above oil showed two compounds, solids were precipitated after cryogenic freezing, and most of them were obtained by crystallization with 10 times the amount of ethyl acetate petroleum ether (1:2). A highly reactive product, HPLC > 90%. A small amount of the mixture was separated by column...
Embodiment 3、17
[0072] Example 3, 17α-[(±)1-(1-ethoxy)ethyl]oxy-17β-cyano-3,3-ethylenedioxy-19-norpregna-5(10 ), the preparation of 9(11)-diene:
[0073]Add compound III (50.0g, 0.147mol), dichloromethane (500ml) and p-toluenesulfonic acid (0.3g, 1.74mmol) into the reaction flask, under ice water cooling, add vinyl ether (17ml, 0.18mol ), insulated and stirred for 4h, after the reaction was completed, the same treatment was performed to obtain light yellow oil 17α-[(±)1-(1-ethoxy)ethyl]-17β-cyano-3,3-ethylenedioxy- 19-norpregna-5(10), 9(11)-diene 60.7g, quantitative yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com