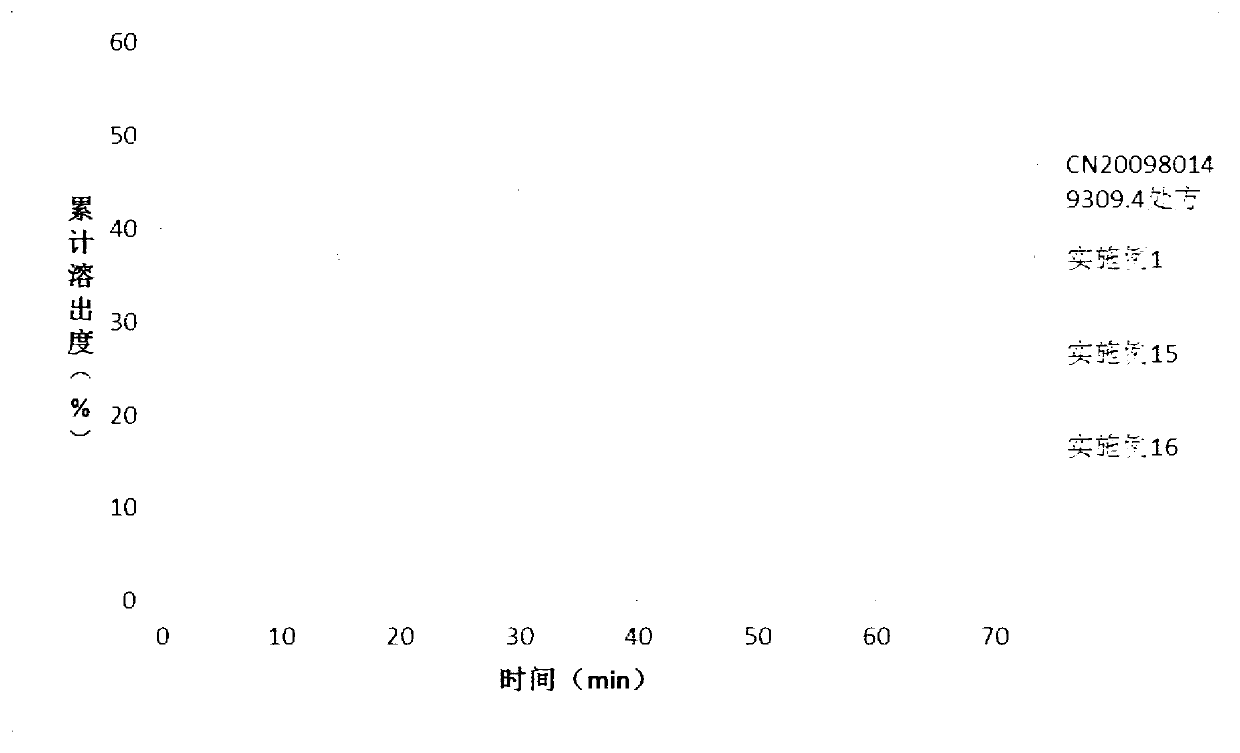
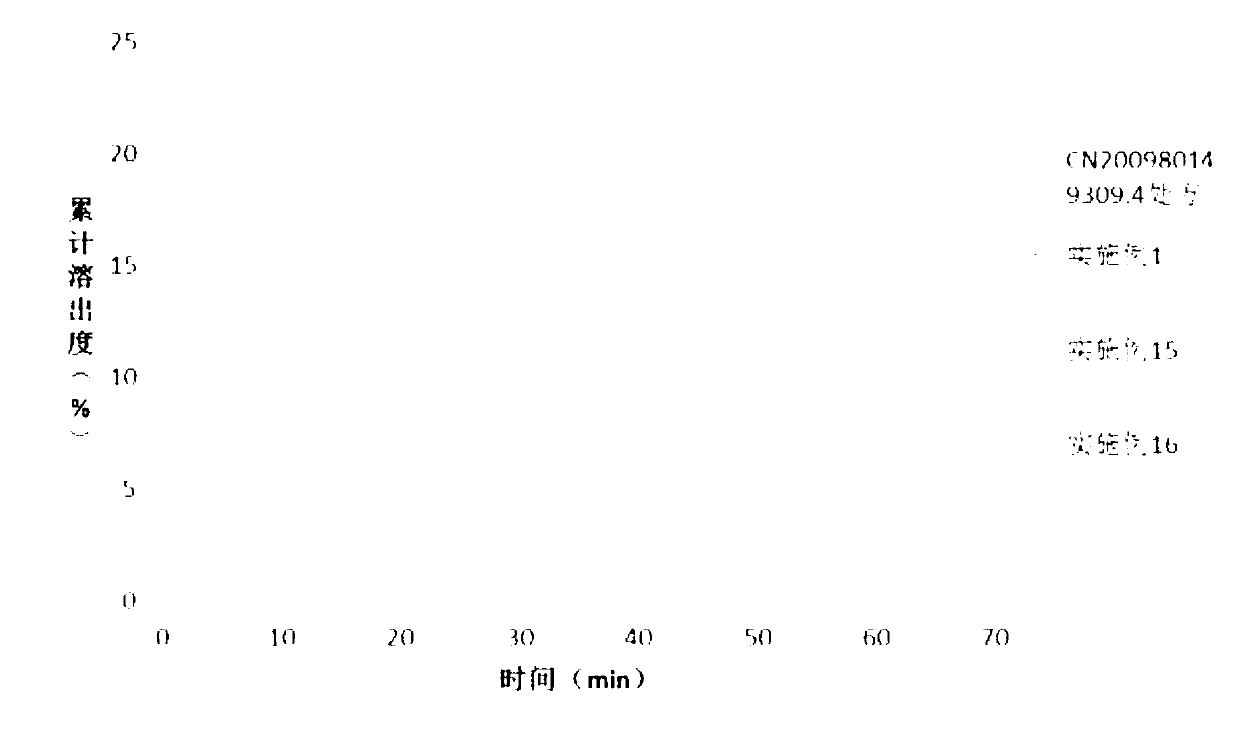
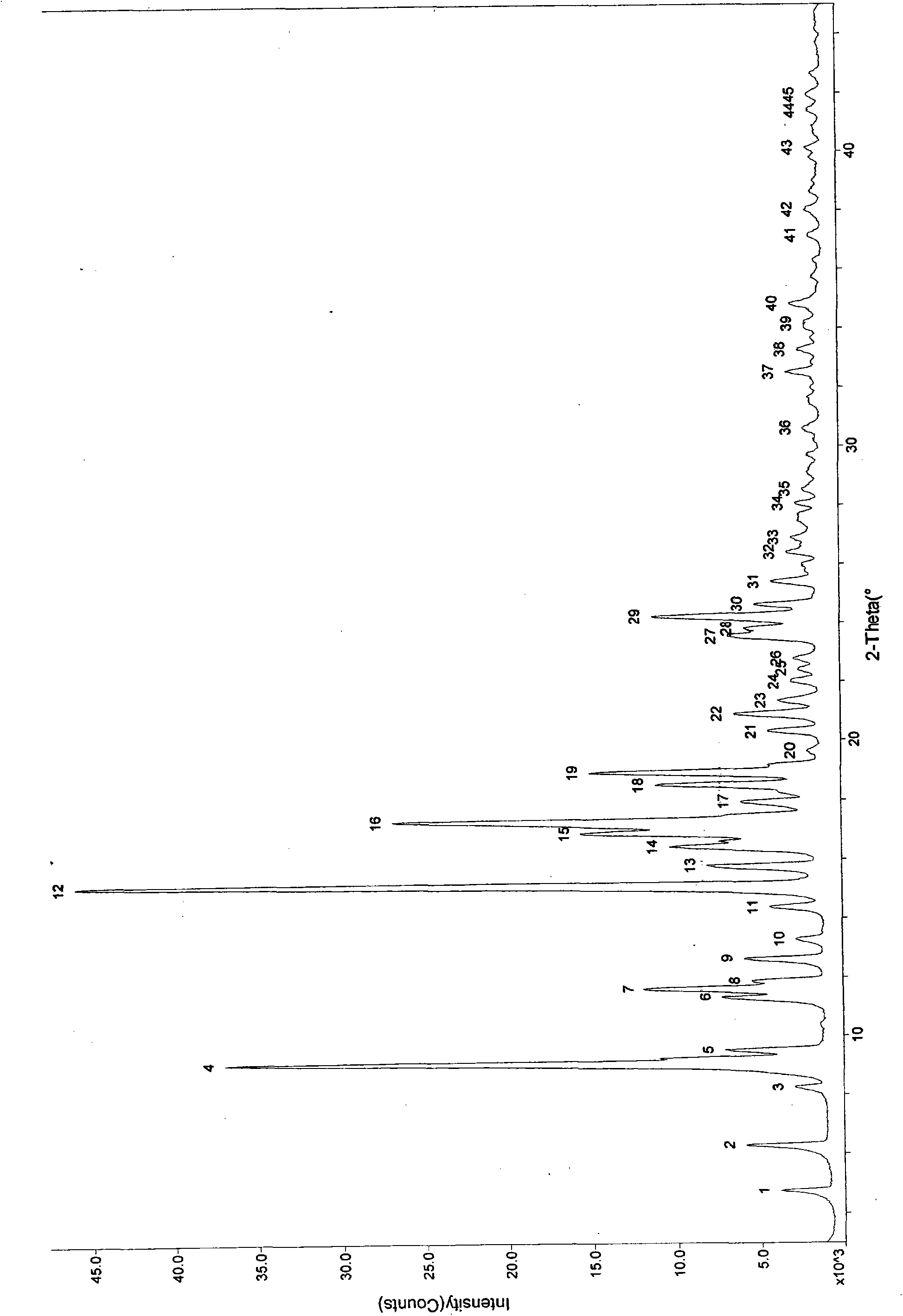
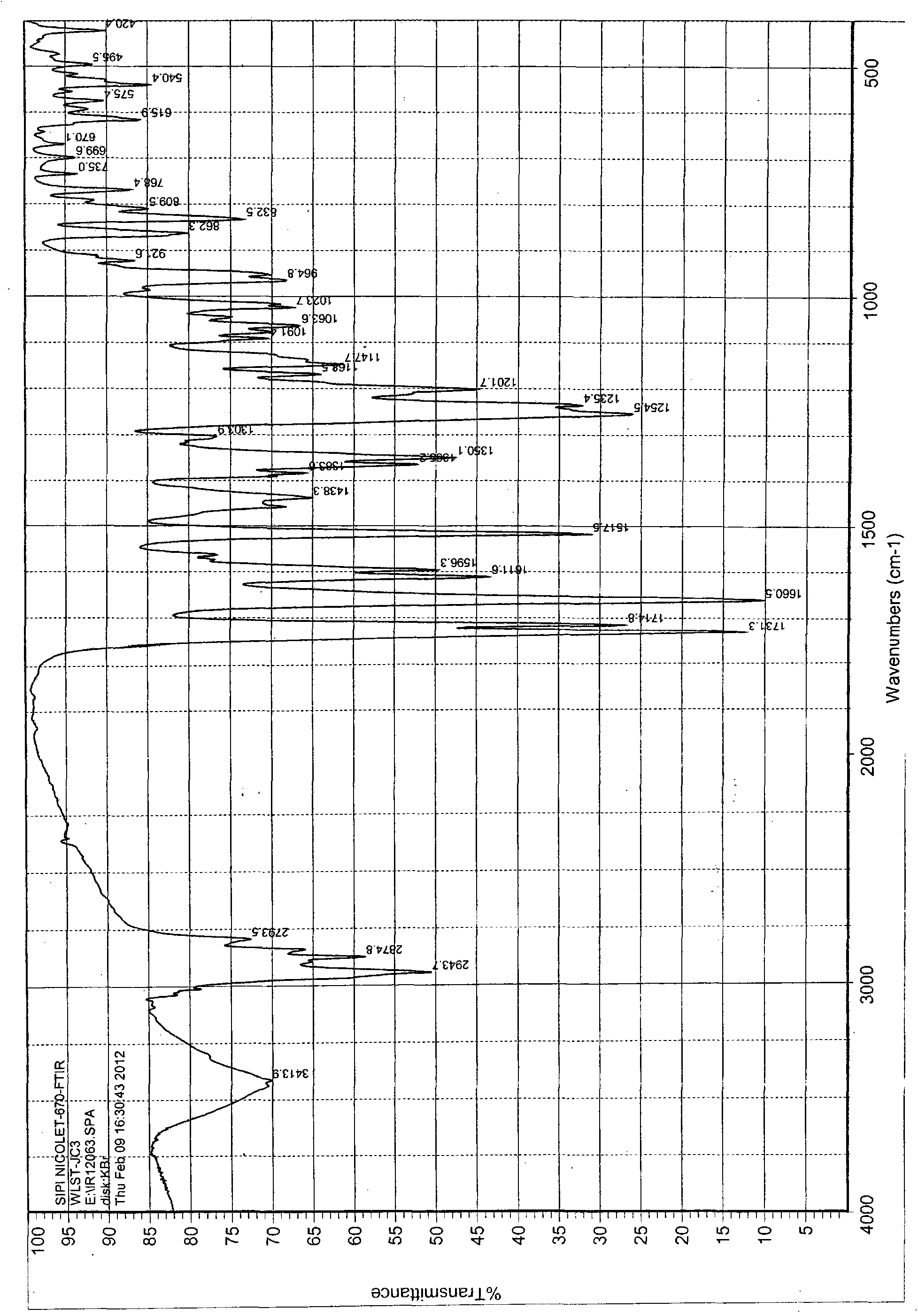
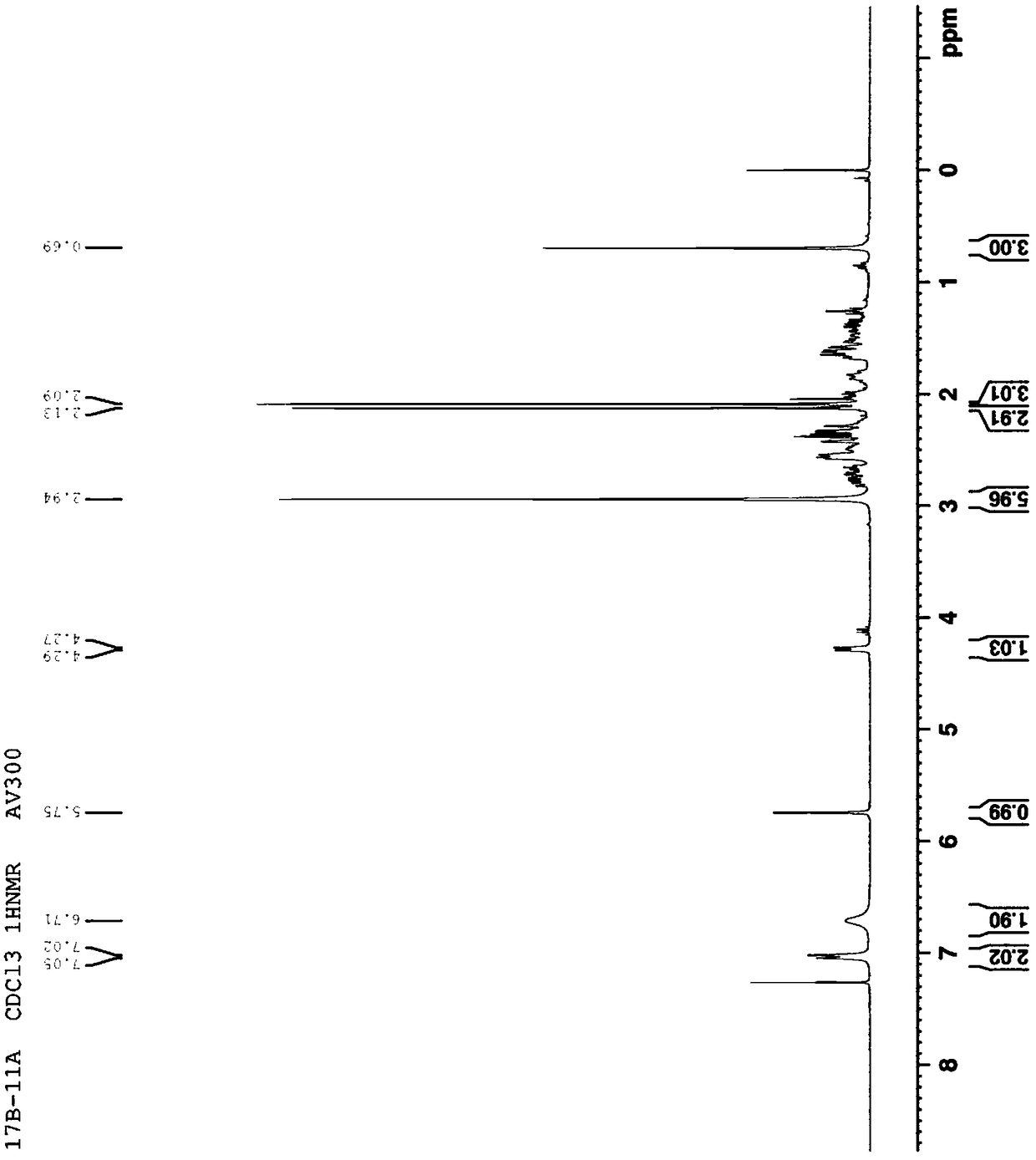
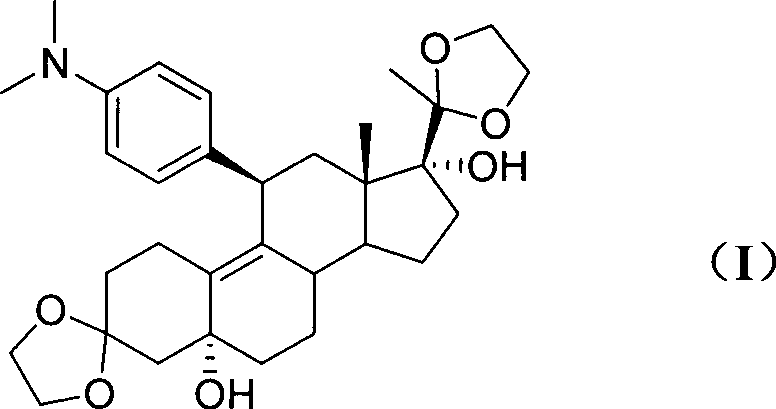
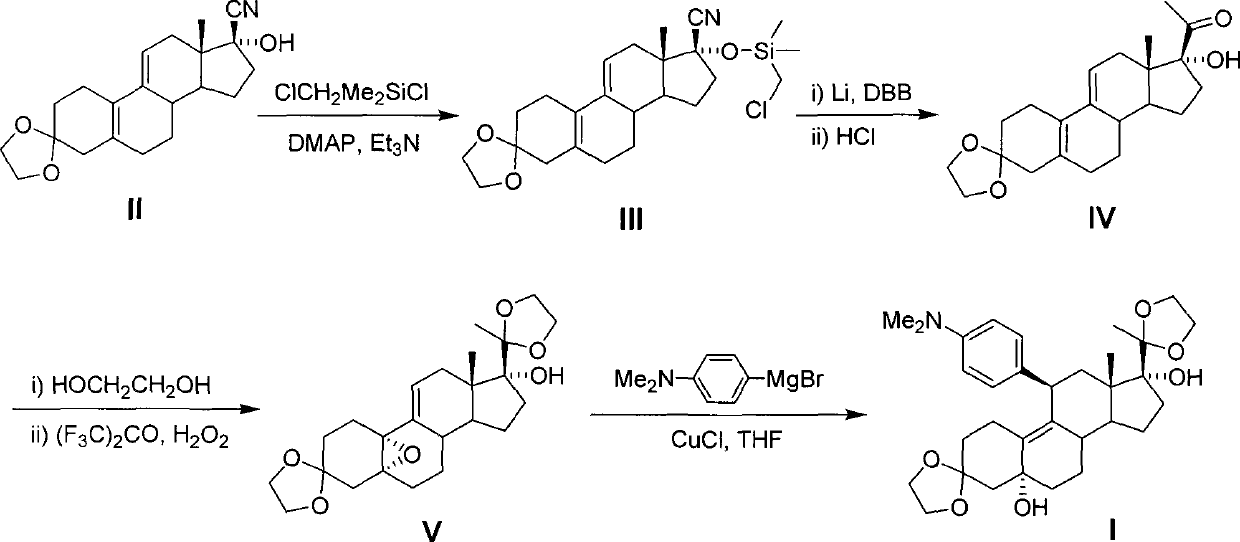
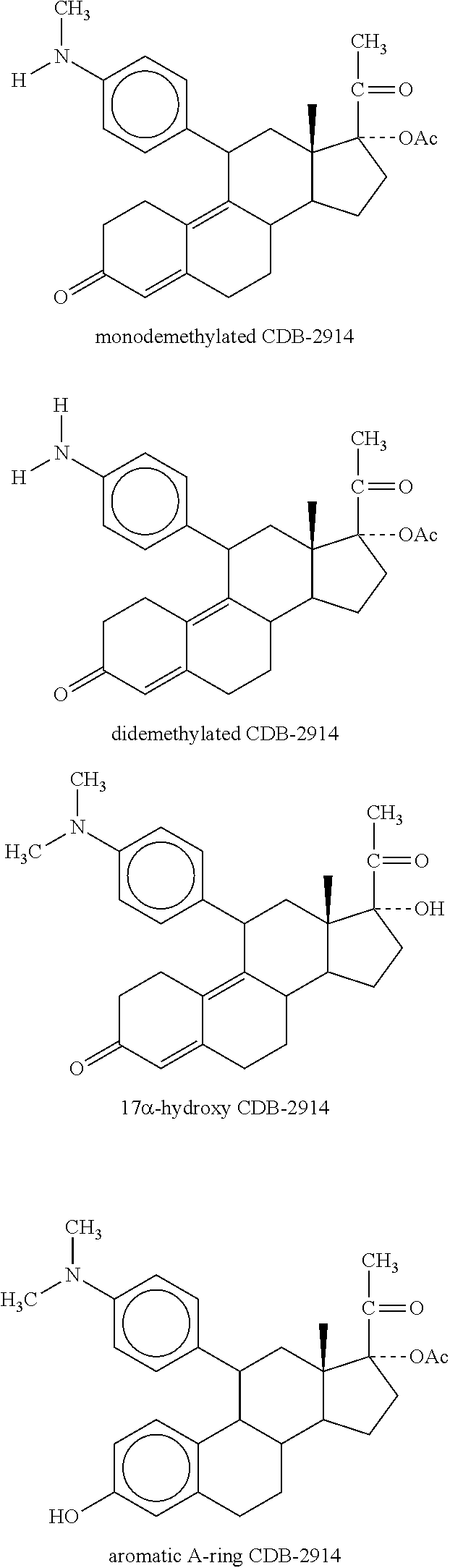
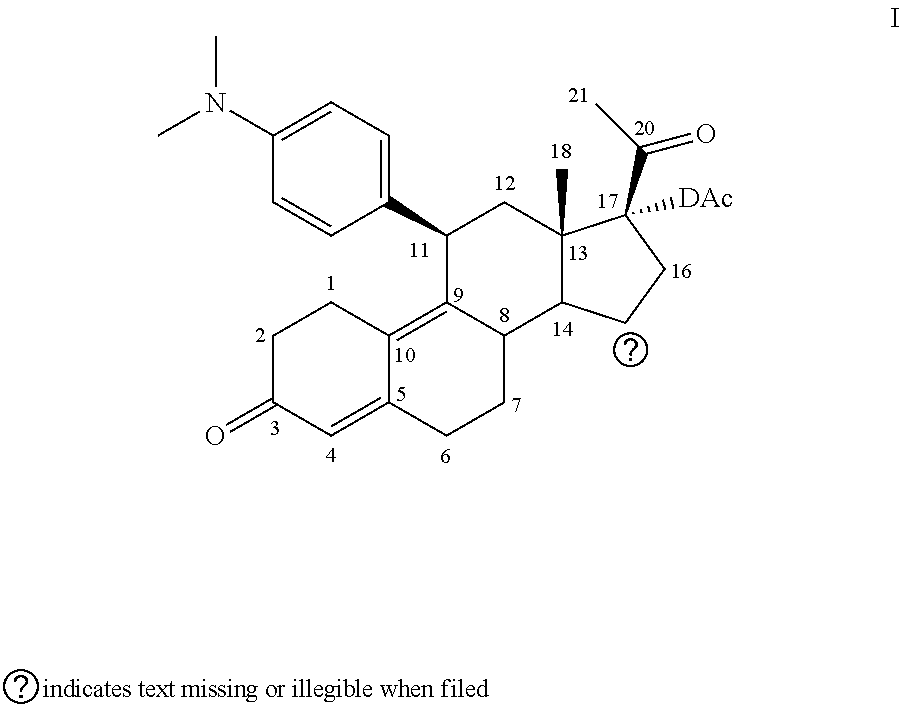
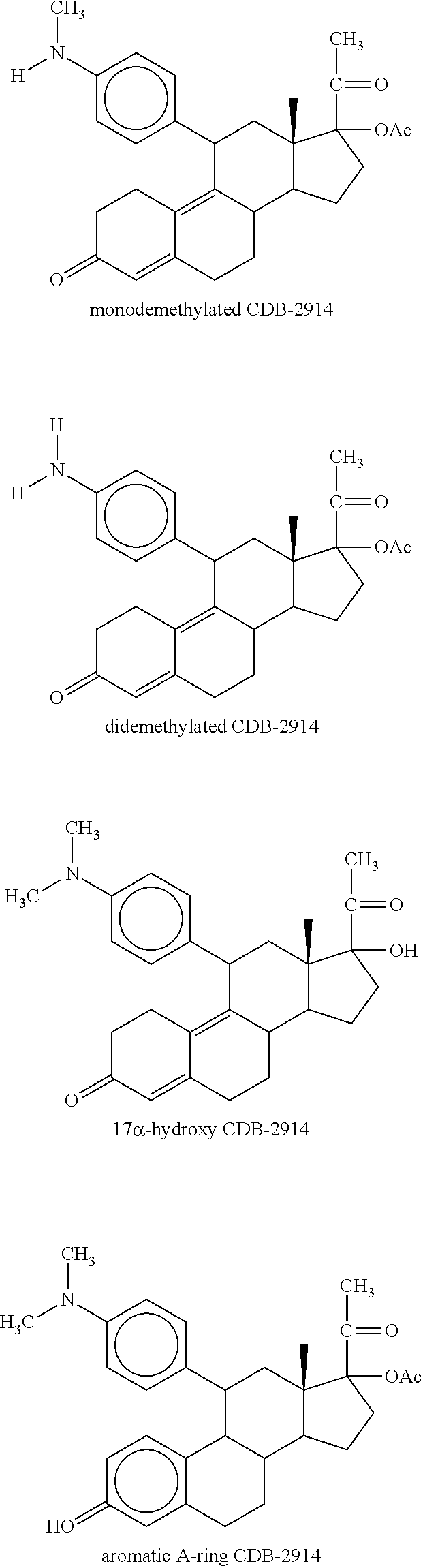
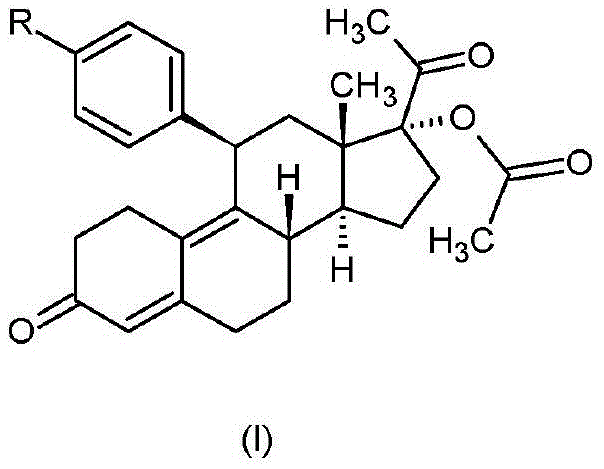
Patents
Literature
72 results about "Ulipristal acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ulipristal is used by women to prevent pregnancy after birth control failure (such as a broken condom) or unprotected sex. This medication is an emergency contraceptive and should not be used as a regular form of birth control.
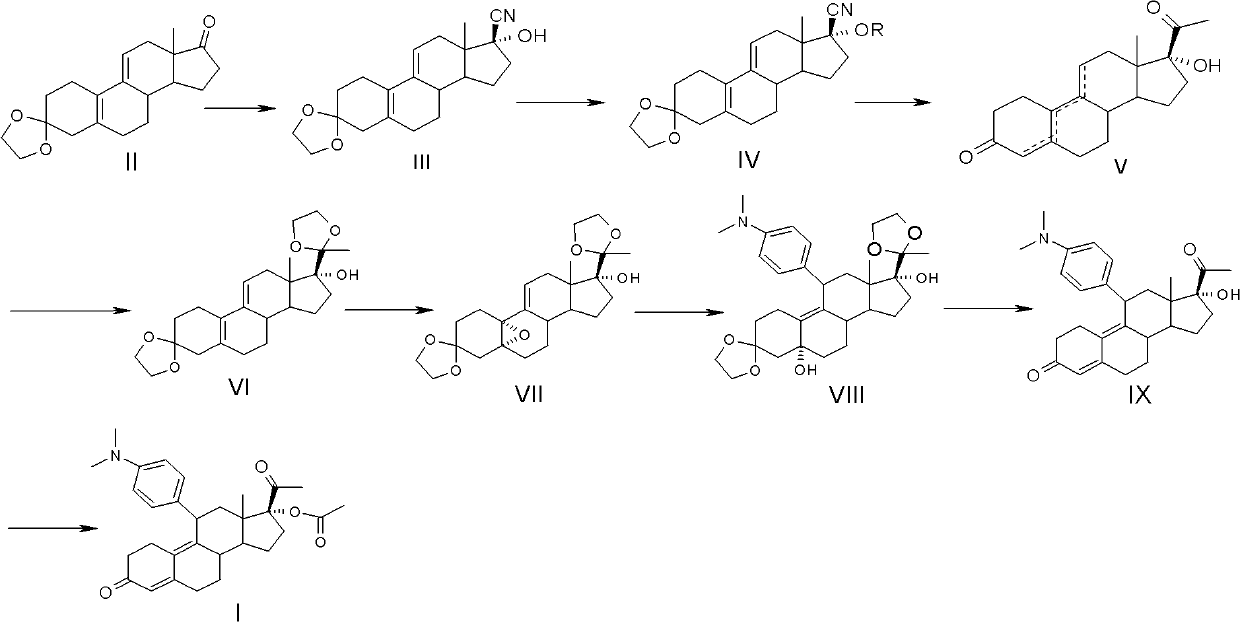
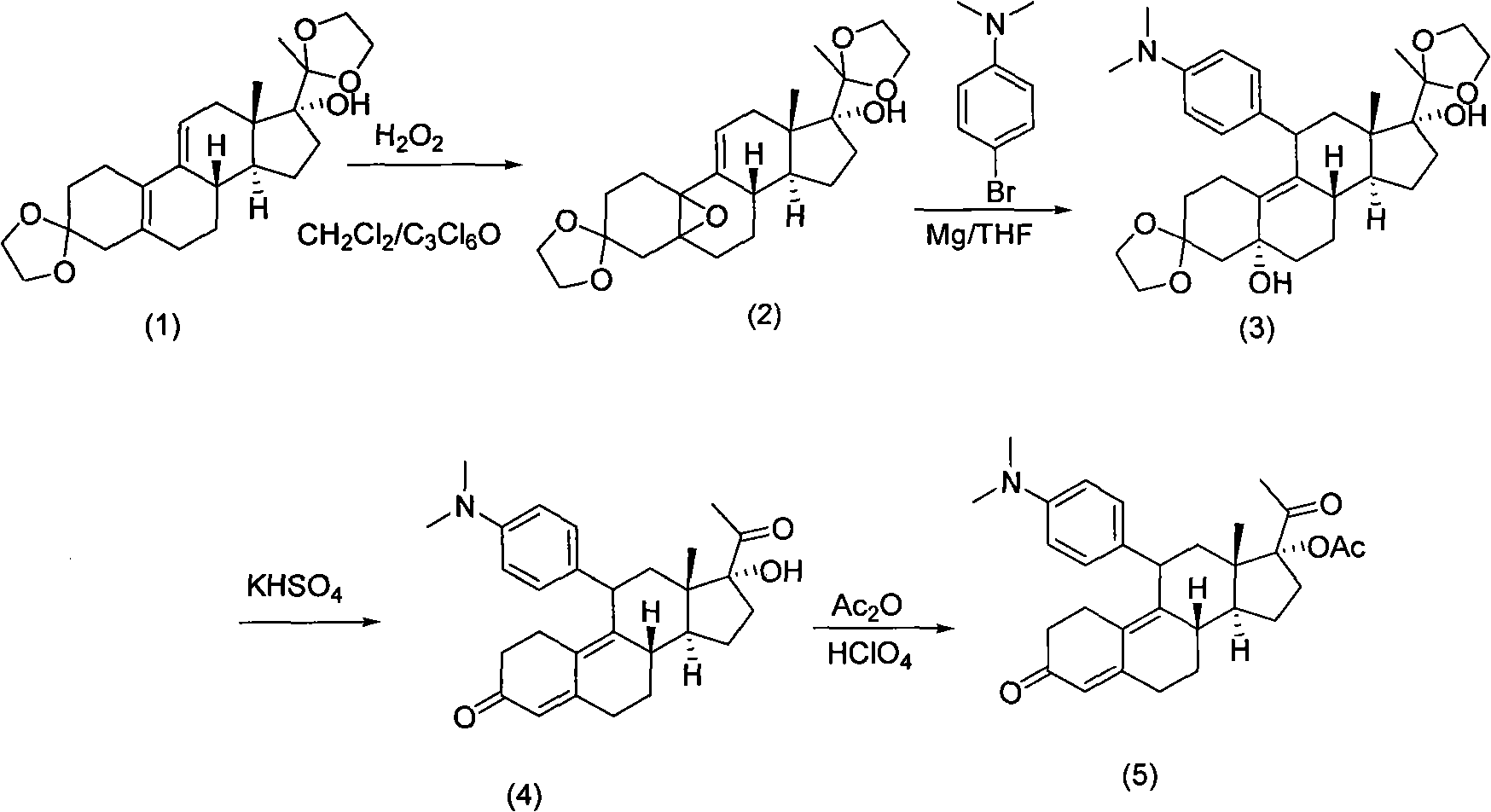
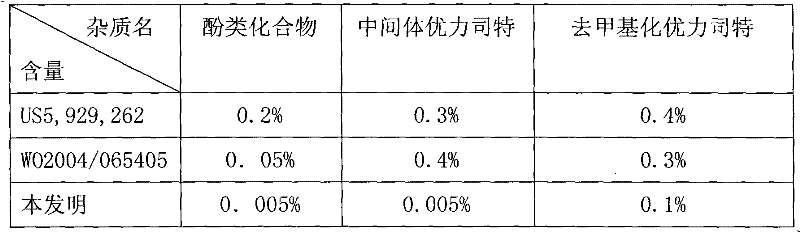
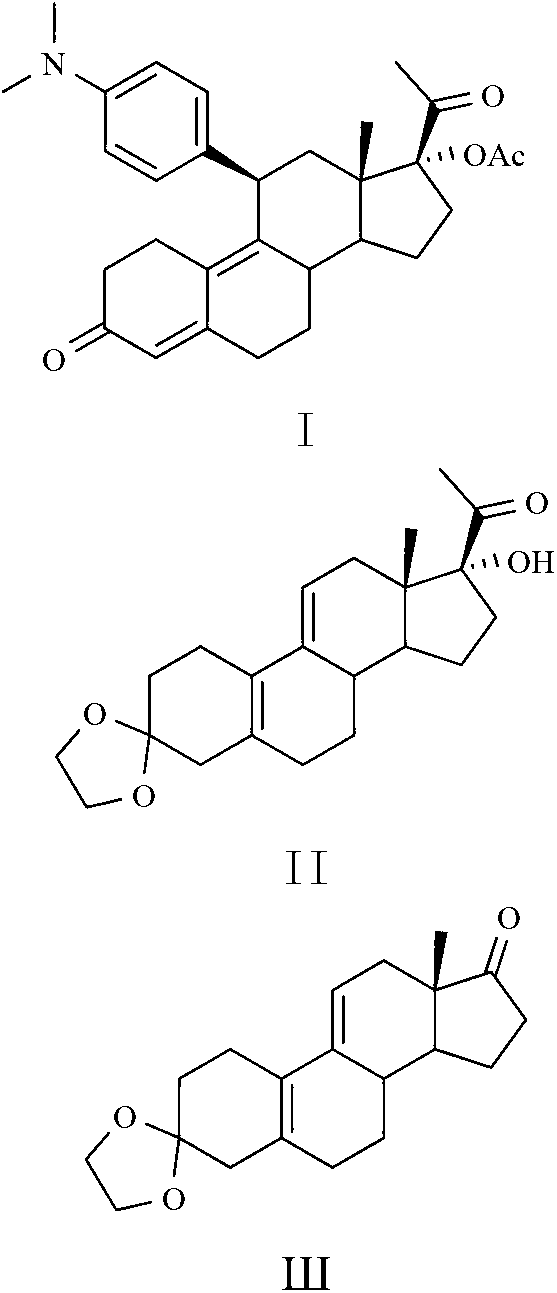
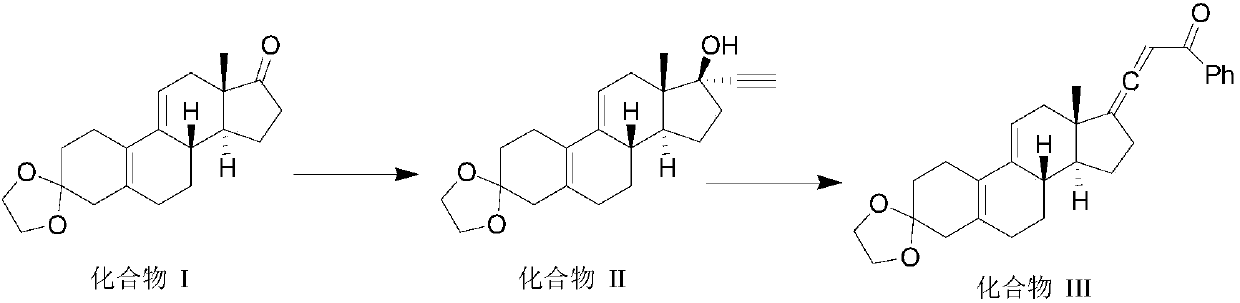
Preparation method of ulipristal acetate and key intermediate thereof
ActiveCN102516345AHigh puritySimple methodKetal steroidsSteroids preparationEthylenedioxyGrignard reagent
Owner:UTOPHARM SHANGHAI +1
Synthesis method of progesterone receptor regulating agent ulipristal
The invention provides a new synthesis method of a progesterone receptor regulating agent ulipristal acetate. The method has simple and short steps and mild conditions and is easy to operate, the obtained product has low cost, high yield and high purity, and the method is easy to amplify and is suitable for industrial production.
Owner:杭州容立医药科技有限公司
Method for purifying ulipristal serving as synthetic progesterone receptor regulator
ActiveCN102241722ASuitable for industrialized mass productionSteroidsPR - Progesterone receptorImpurity
The invention provides a method for purifying ulipristal serving as a synthetic progesterone receptor regulator. By adopting the method, high-purity ulipristal acetate can be obtained by quickly removing various impurities. The method is easy and convenient to operate, high in yield and suitable for industrial production.
Owner:杭州容立医药科技有限公司
Synthetic method of ulipristal acetate
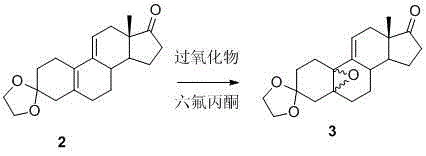
The invention relates to a synthetic method of ulipristal acetate. According to the method, 3, 3-(ethylenedioxy group) steroidal estrogen-5(10), 9(11)-diene17-ketone is used as a raw material and reacted with tosylmethyl isocyanide under the condition of alkalinity to obtain 3, 3-(ethylenedioxy group) steroidal estrogen-5(10), 9(11)-diene17-cyanogen; the 3, 3-(ethylenedioxy group) steroidal estrogen-5(10), 9(11)-diene17-cyanogen and a methyl grignard reagent are reacted to obtain methyl ketone; the methyl ketone and trialkyl ester phosphate are reacted in the oxidation environment under alkalinity conditions to obtain 3, 3-ethylenedioxy group-17 alpha-hydroxyl-19-norpregna-5(10), 9(11)-diene-20-ketone; the 3, 3-ethylenedioxy group-17 alpha-hydroxyl-19-norpregna-5(10), 9(11)-diene-20-ketone uses ethylene glycol to protect 20-carbonyl under the acid catalysis to obtain a key intermediate; and the intermediate is synthesized into a target product through four steps of reaction by means of known methods. The synthetic method is concise in paths, easy to operate, convenient to post-process, low in cost, high in total yield and easy to amplify, and raw materials are easily obtained.
Owner:SUZHOU KANGRUN PHARMA +1
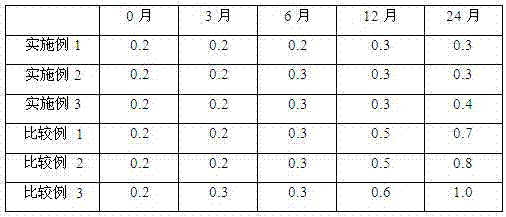
Polycrystal forms of ulipristal acetate and preparation method thereof
ActiveCN102675395AAvoid problems such as new impuritiesShorten the timeOrganic active ingredientsSteroidsInfraredX-ray
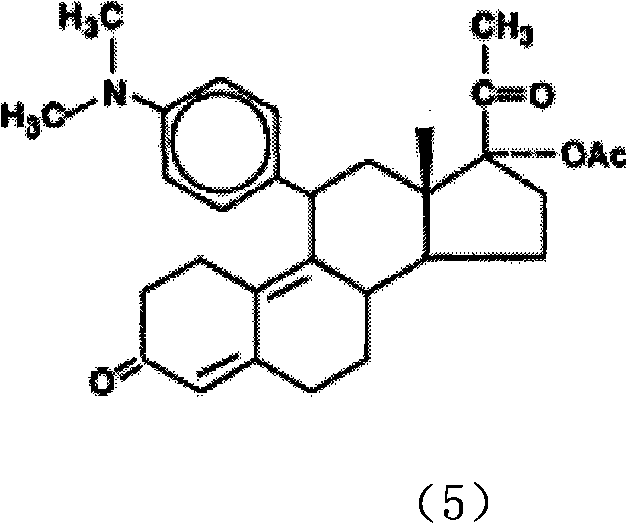
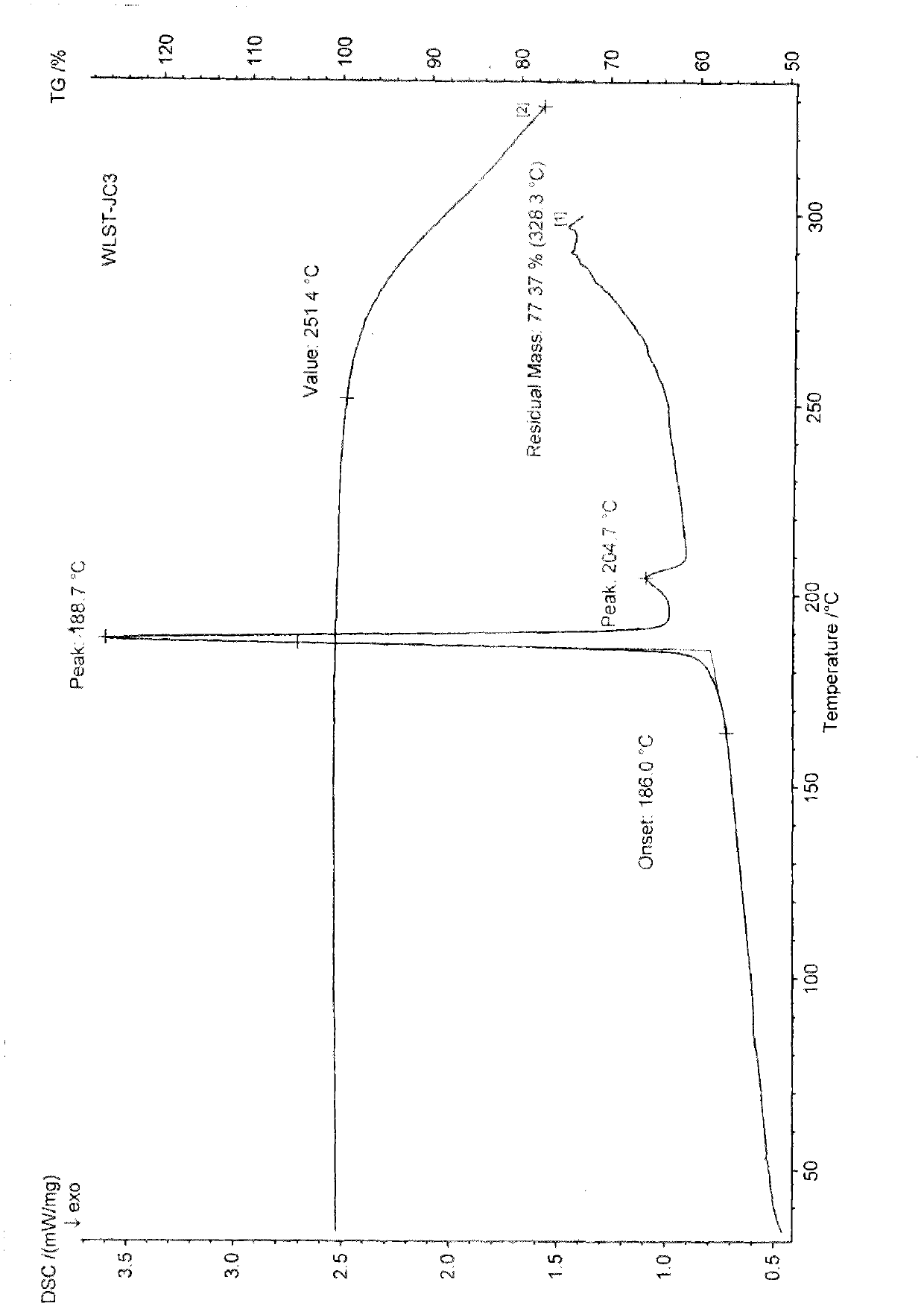
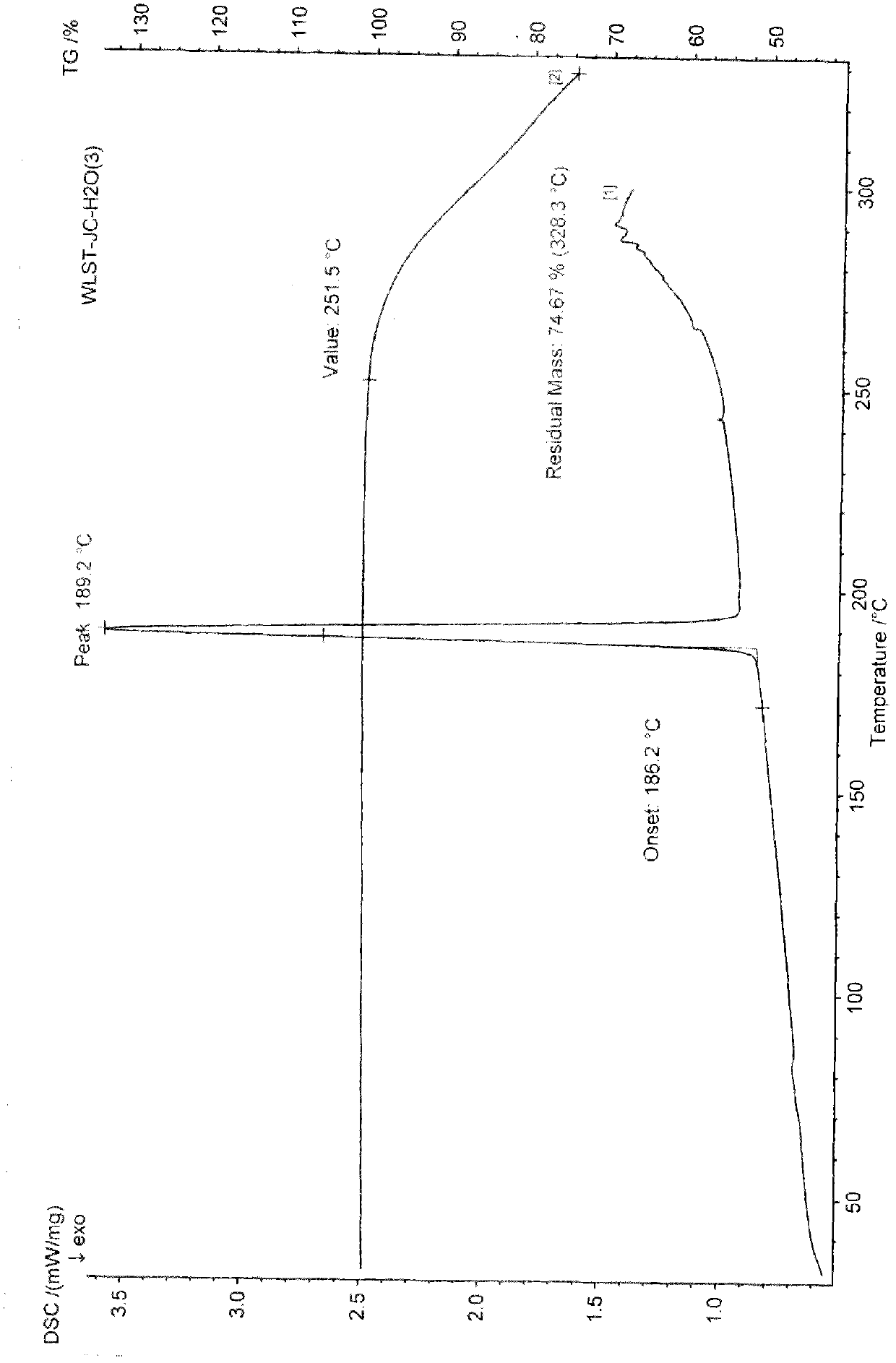
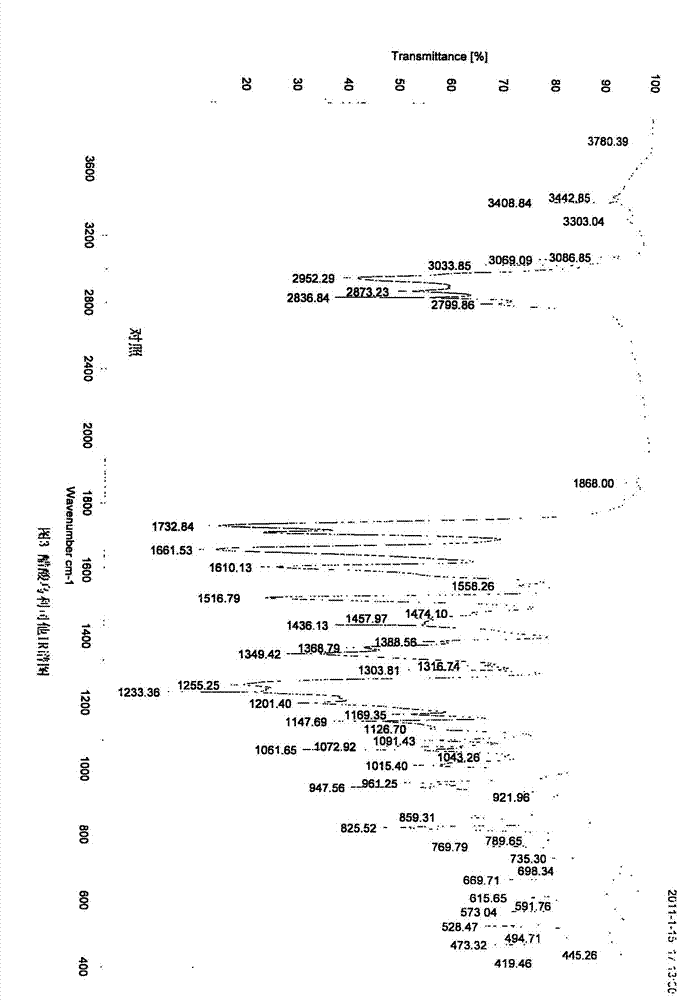
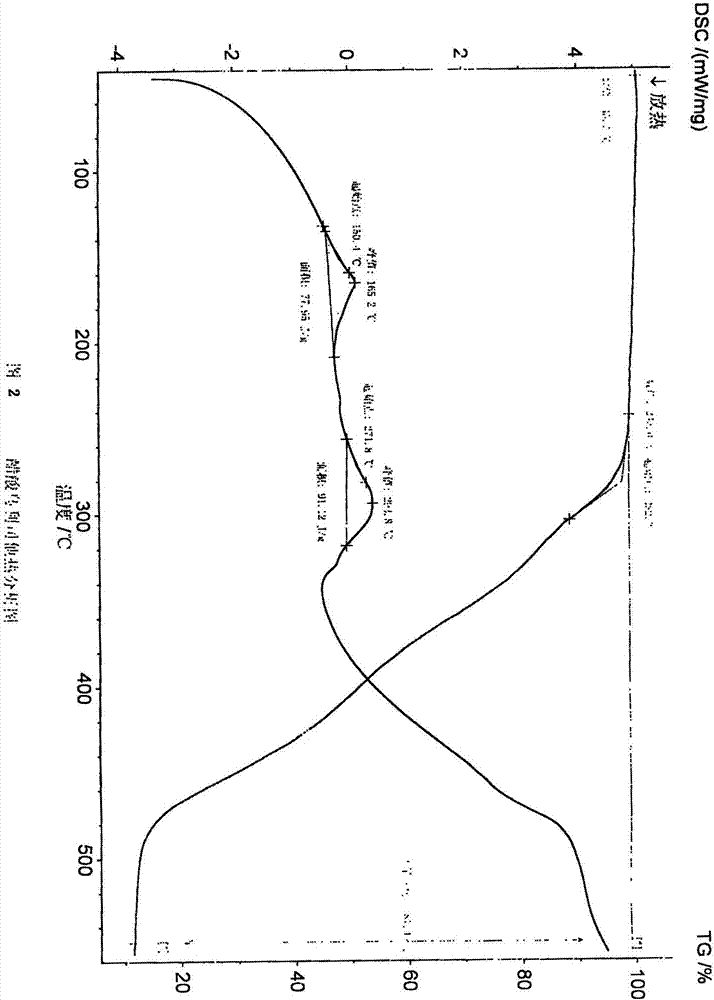
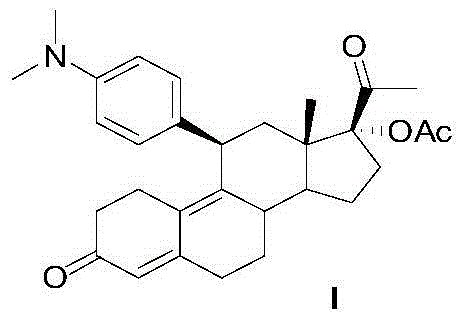
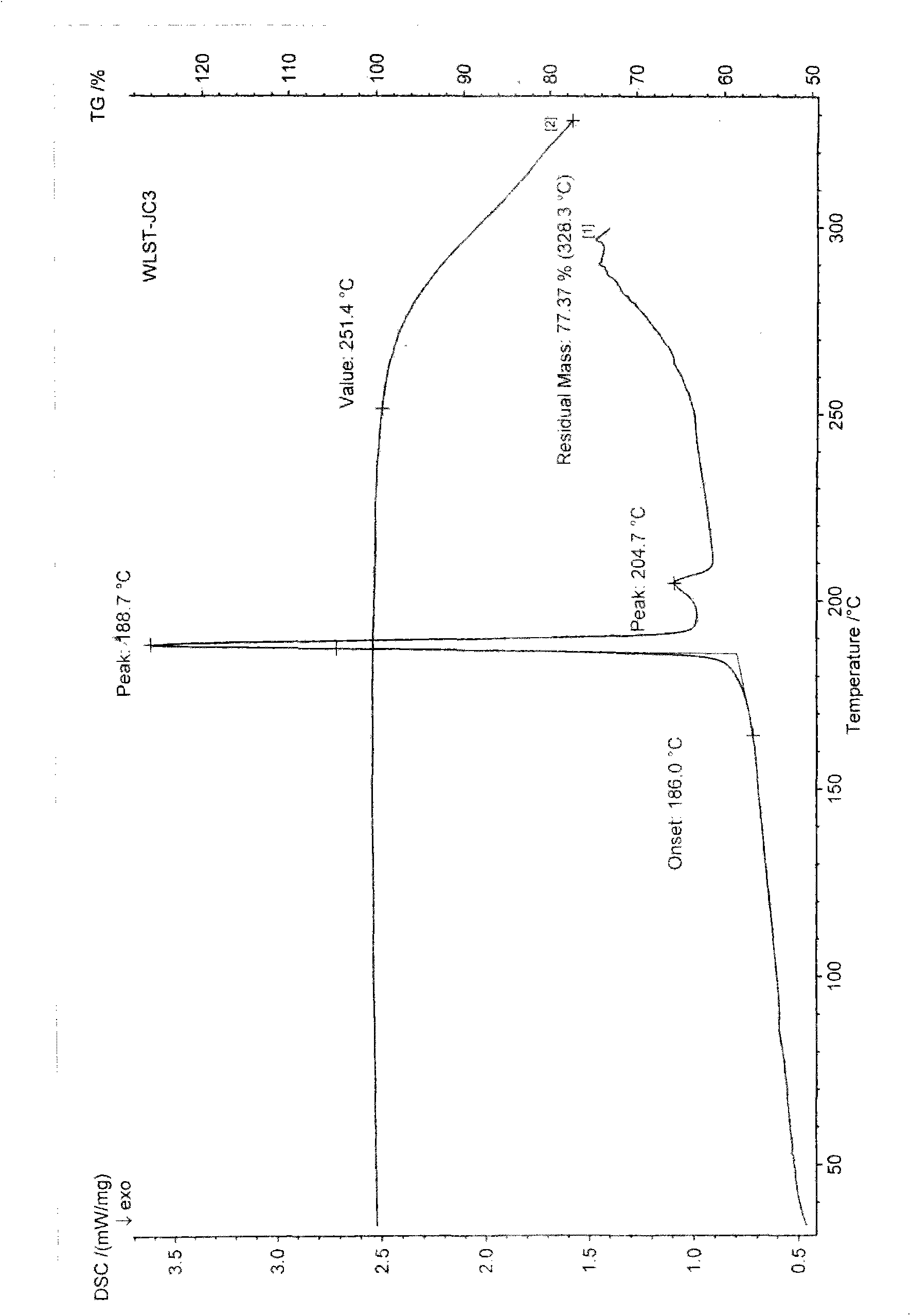
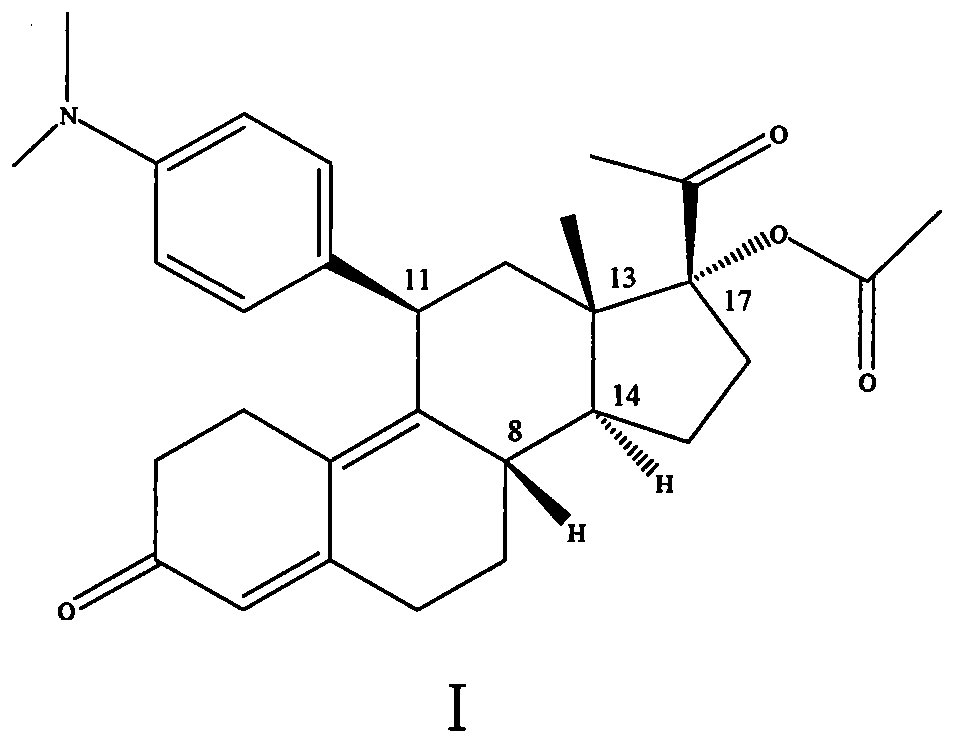
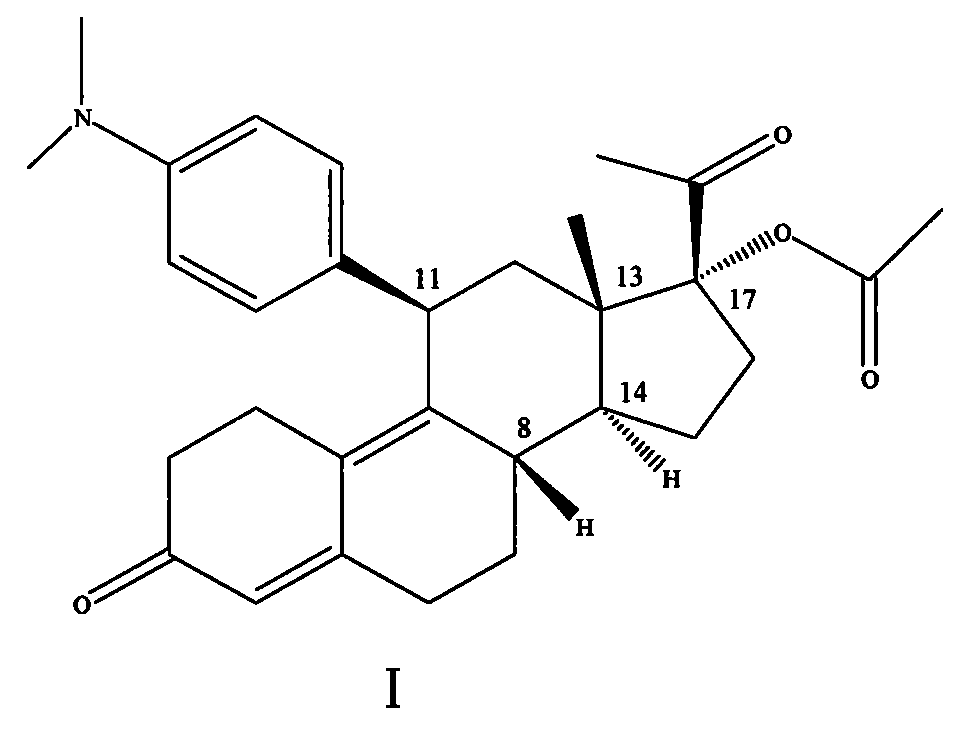
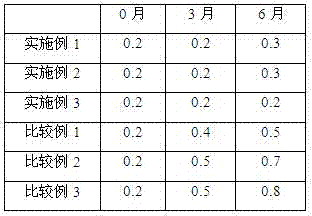
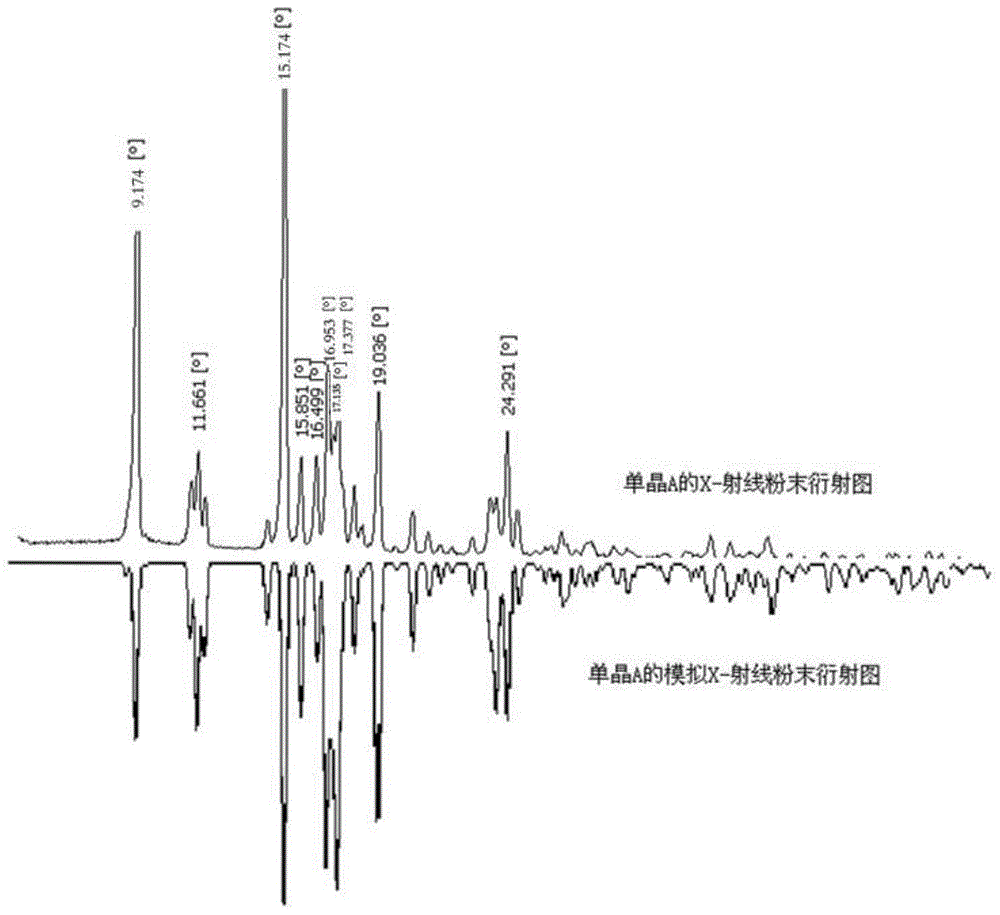
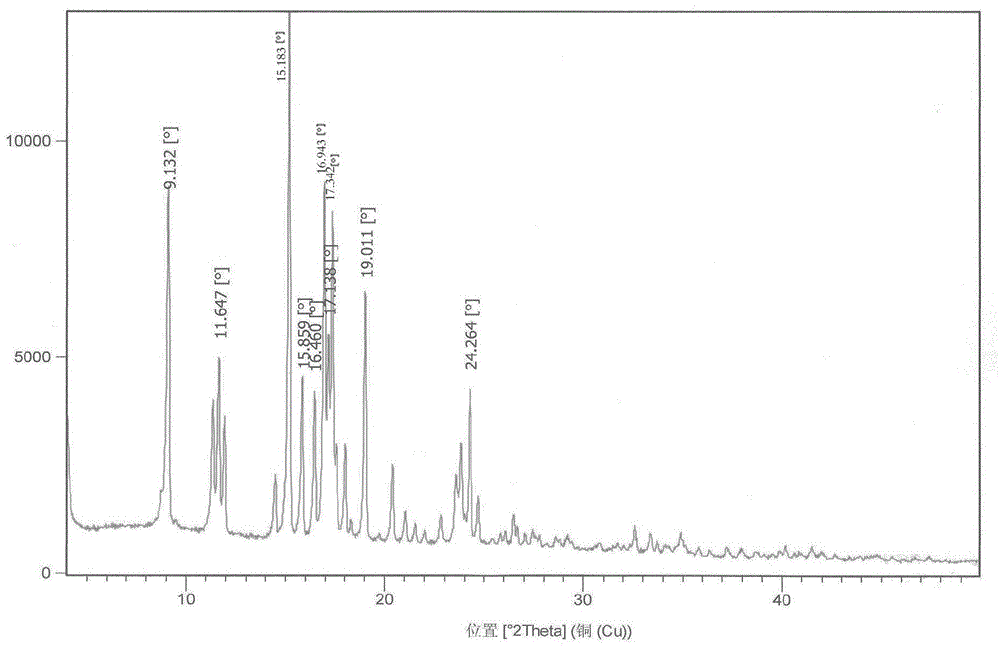
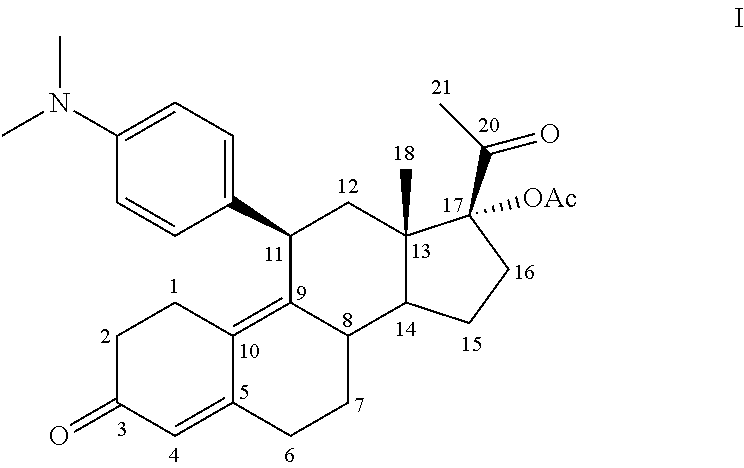
The invention discloses a polycrystal form A1 and a polycrystal form A2 of ulipristal acetate (formula I) free of solvate and a preparation method, a pharmaceutical composition and medical application of the polycrystal forms. By verification of melting point, X-ray diffraction powder spectrum (XRD), infrared spectrum (IR), differential thermal analysis spectrum (DSC) and thermogravimetric spectrum (TG), the polycrystal form A1 and polycrystal form A2 of ulipristal acetate prepared by the method disclosed by the invention are extremely stable to temperature, illumination and humidity, and favorable to long-term storage; the used crystallizing solvent is safe and easy to remove; the obtained polycrystal form A1 and the polycrystal form A2 can be directly used for preparation processing; and the preparation method is simple in operation and suitable for industrial production. The formula I is shown in the description.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Ulipristal acetate dispersible tablet and preparation method thereof
InactiveCN102871977AHigh dissolution rateRapid dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsMedicineMicrometer
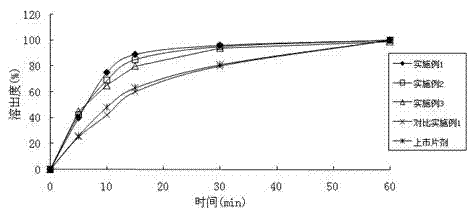
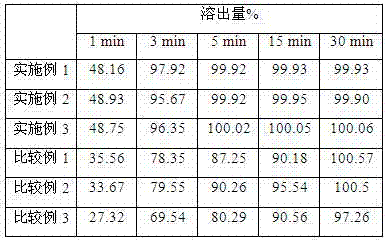
The invention relates to a ulipristal acetate preparation and a preparation method thereof, and particularly relates to a ulipristal acetate dispersible tablet with improved dissolution rate and a preparation method thereof. In order to solve the problem that the ulipristal acetate tablet in the prior art is low in dissolution rate and bioavailability and affects emergency contraception effects, the invention provides the ulipristal acetate dispersible tablet. The ulipristal acetate dispersible tablet is prepared from a premix compound of ulipristal acetate and a disintegrating agent and a excipient, wherein the content of ulipristal acetate is 1-30%(w / w) and accounts for 50-80% of the total weight of the premix compound, the particle sizes of 70-100% ulipristal acetate and the disintegrating agent are less than or equal to 75 micrometers. The ulipristal acetate dispersible tablet disclosed by the invention can take effect quickly in vivo, can realize more effective emergency contraception effect, and is wider in application range.
Owner:CHINA RESOURCES ZIZHU PHARMA
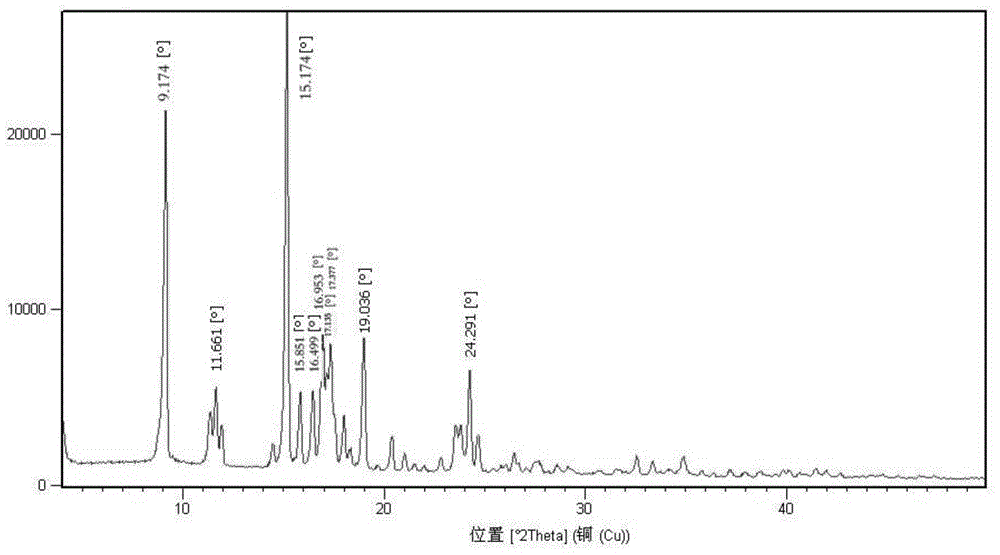
Ulipristal acetate crystals and preparation method thereof
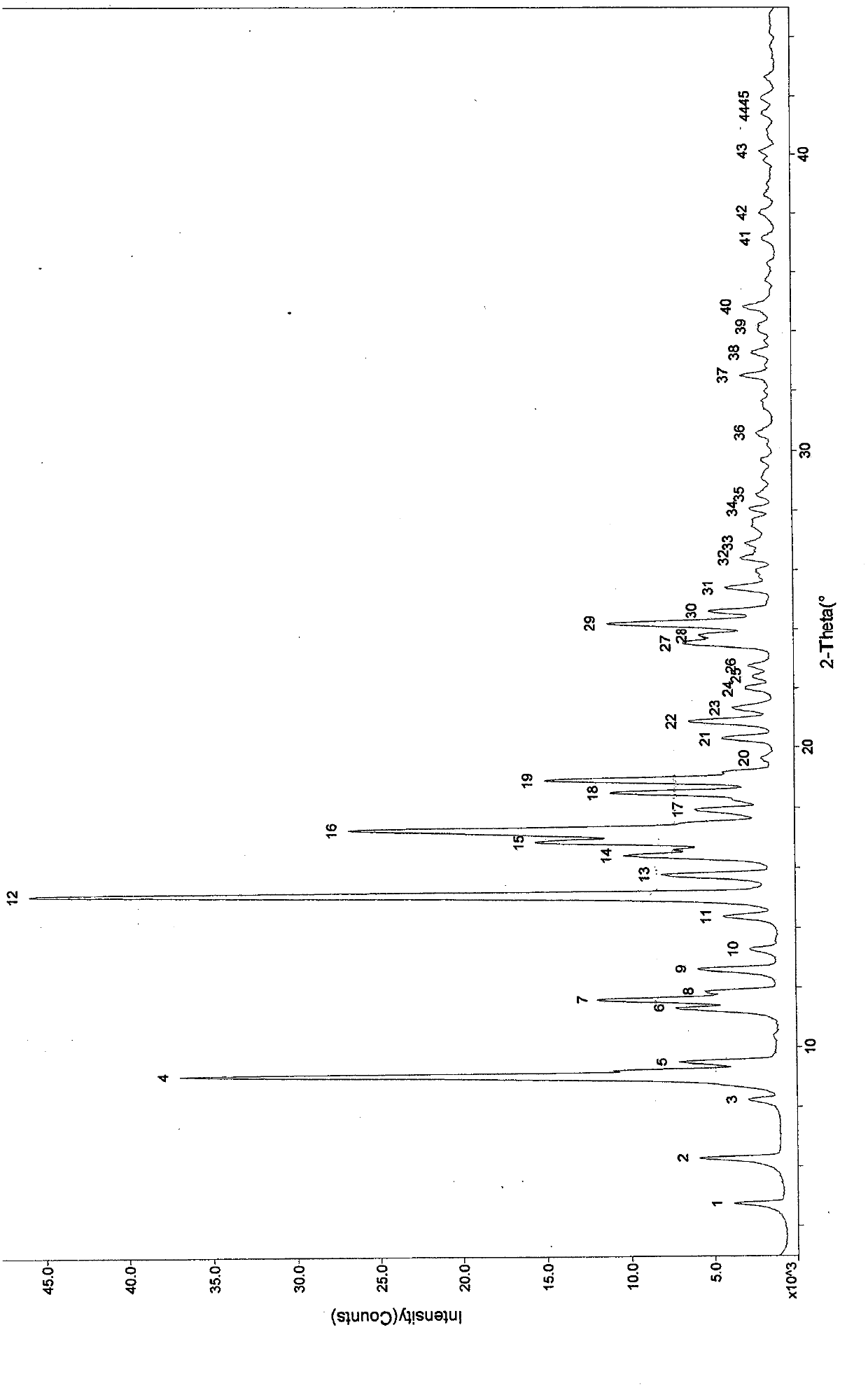
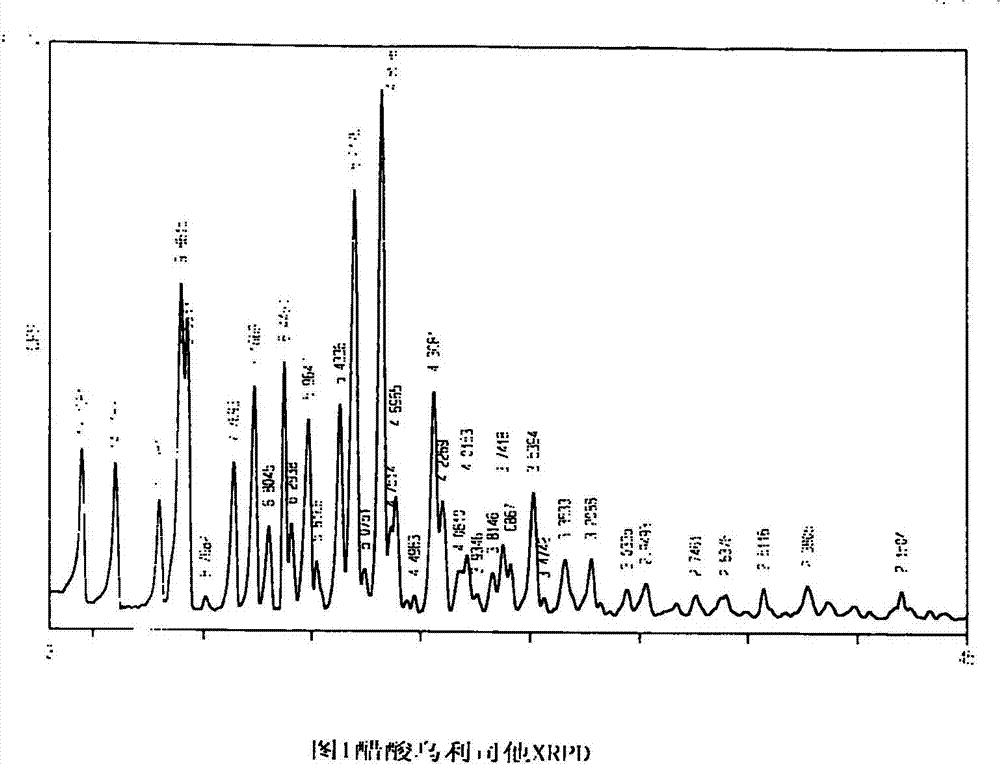
The invention provides ulipristal acetate crystals. In the powder X ray of diffraction pattern of the crystals radiated by copper Ka rays with wavelength lambda being 1.54 angstroms, obvious main peaks appear at distances from the surface of the crystal surface (d) equals to 19.70+ / -0.05, 14.72+ / -0.05, 9.86+ / -0.05, 7.77+ / -0.05, 7.16+ / -0.05, 6.45+ / -0.05, 5.96+ / -0.05, 5.43+ / -0.05, 5.21+ / -0.05, 4.86+ / -0.05, and 4.31+ / -0.05. Compared with the conventional crystals, the ulipristal acetate crystals are more pure and stable. The invention also provides a method for preparing the crystals. The method comprises the following steps of: recrystallizing ulipristal acetate in a soluble solvent; washing the crystals in a solvent; and drying the crystals in vacuum to obtain finished products. The preparation method is characterized by simple operation and high product purity.
Owner:SHANDONG CHENGCHUANG BLUE OCEAN PHARM TECH CO LTD
Ulipristal acetate medicine composition
InactiveCN103083326AExcellent pharmacological technical characteristicsOrganic active ingredientsSenses disorderEthylic acidPharmaceutical drug
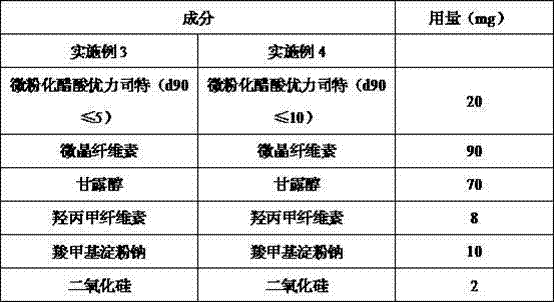
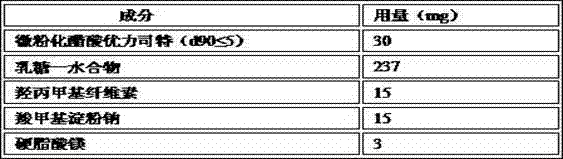
The invention relates to an ulipristal acetate medicine composition comprising, by weight, 3-10% of ulipristal acetate micro-particles, 70-90% of a diluting agent, 3-6% of a bionding agent, 4-12% of a disintegrant, and 0-2% of a lubricating agent. The particle size D90 of the micro-particles is no higher than 10mum. The diluting agent is selected from lactose, microcrystalline cellulose, mannitol, and mixtures thereof. The bonding agent is hydroxypropyl methylcellulose. The disintegrant is sodium carboxymethyl starch. The lubricating agent is selected from magnesium stearate, silicon dioxide, talc powder, and mixtures thereof. The composition has excellent dissolution profile, good friability, and good homogeneity.
Owner:四川尚锐生物医药有限公司
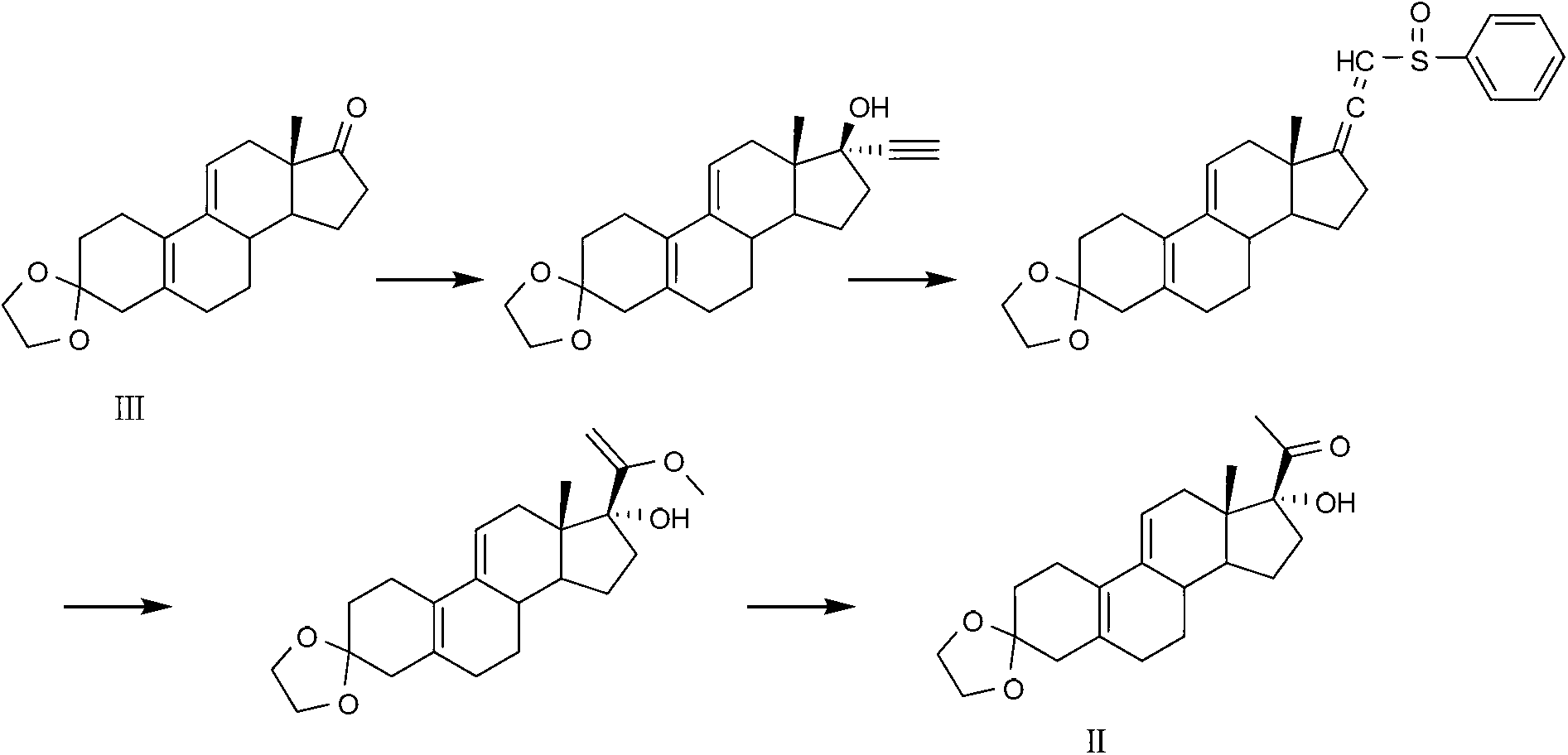
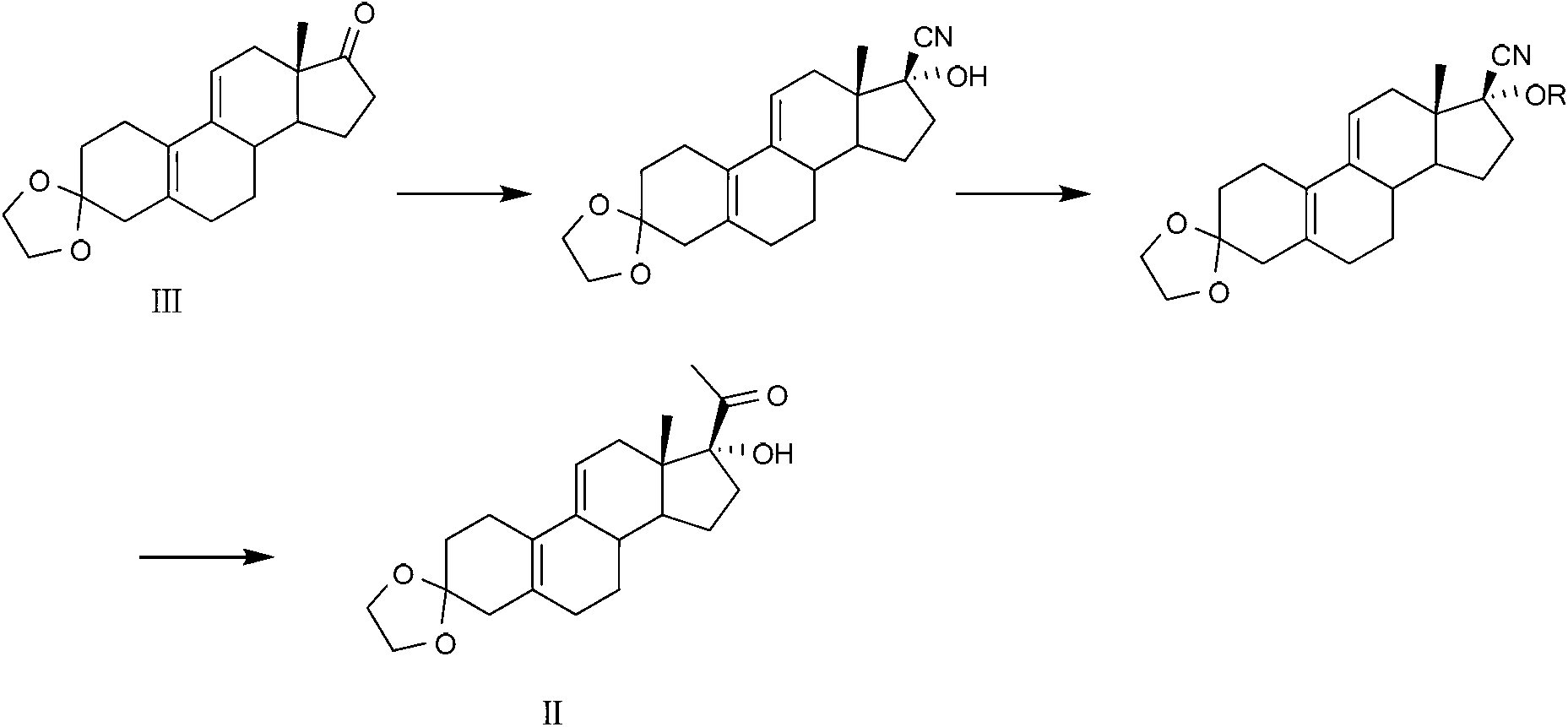
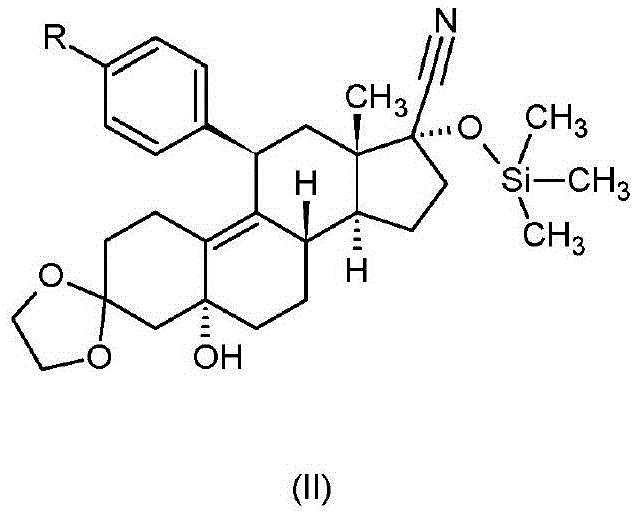
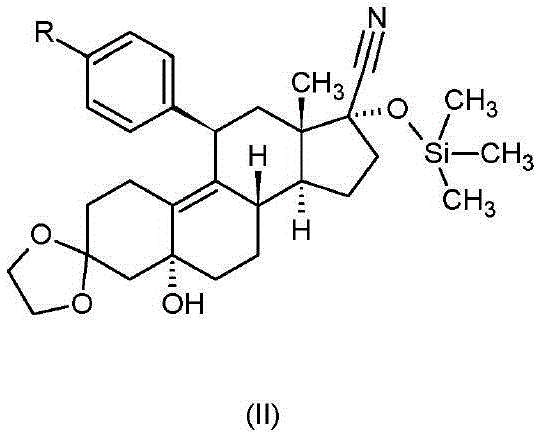
Ulipristal acetate intermediate product and preparation method thereof
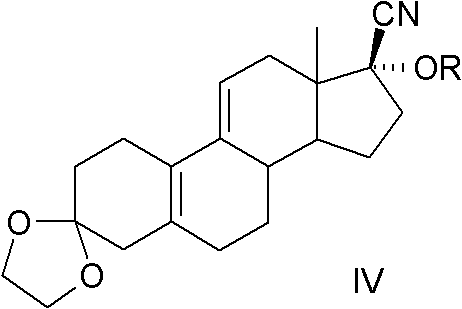
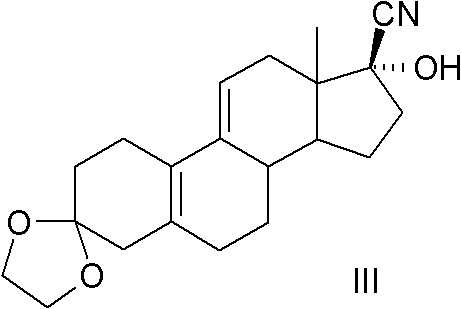
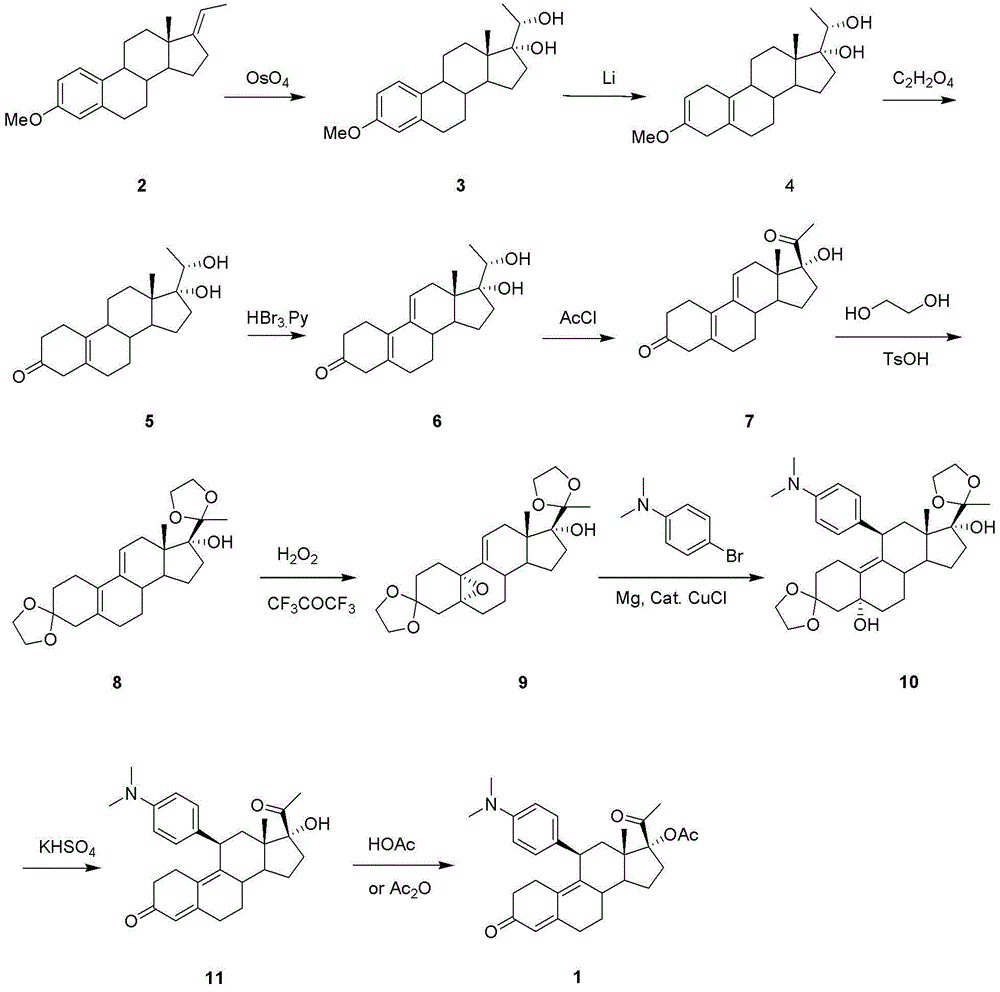
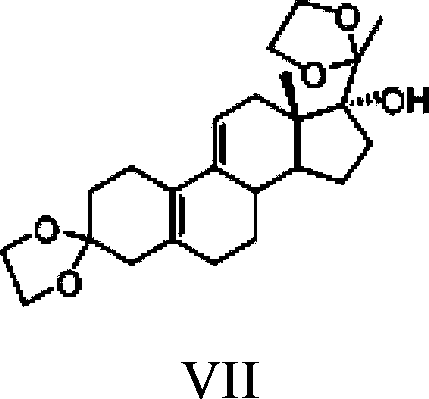
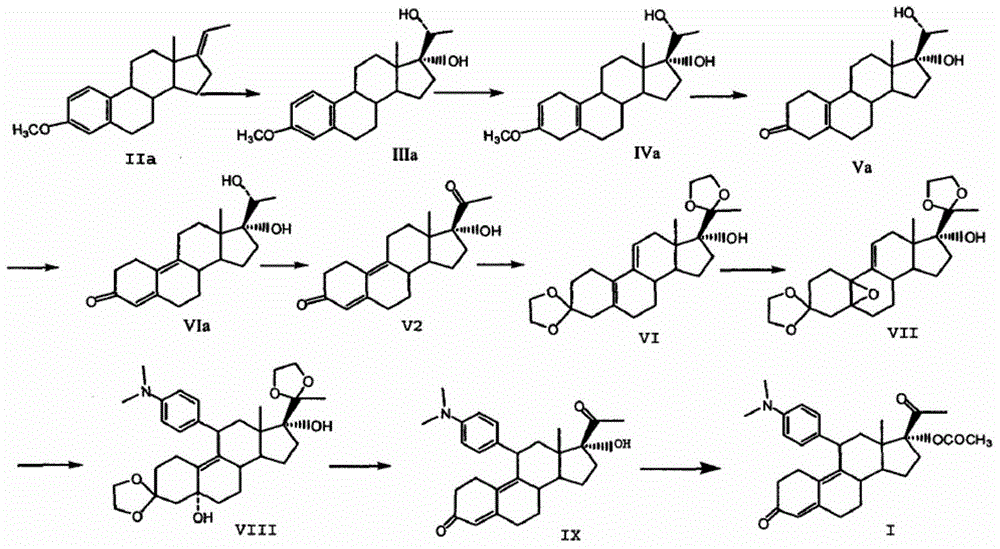
The invention relates to an ulipristal acetate intermediate product and a preparation method thereof. In particular, the invention discloses multiple intermediate products for preparing 3,3,20,20-bis (ethylene dioxy)-17 alpha-hydroxy-19-norpregna-5(10),9(11)-diene (namely, a compound represented as formula VII), and a preparation method thereof, wherein structures of the intermediate products are represented as formula I, formula II, formula III, formula IV or formula V. Furthermore, the invention discloses a method for preparing the compound represented as formula VII. The method disclosed by the invention is easily available in raw material, mild in reaction condition, high in yield, relatively low in cost, low in byproduct content in reaction process, high in target product purity and applicable to industrial production.
Owner:JIANGSU PUXIN PHARMA CO LTD
Preparation method of ulipristal acetate and intermediate thereof
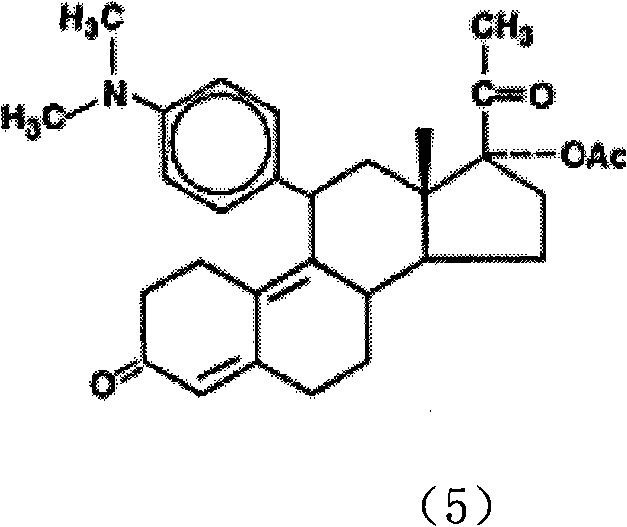
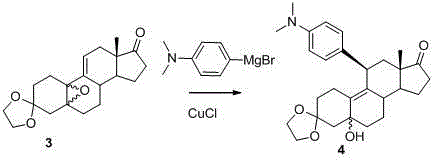
The invention discloses a preparation method of ulipristal acetate and an intermediate thereof, and belongs to the field of pharmaceutical synthesis. The preparation method of the ulipristal acetate comprises the following steps: by taking 3,3-(ethylenedioxy)-19-methylestra-5(10),9(11)-diene-3,17-diketone as raw material, enabling the raw material to react with sodium acetylide or potassium acetylide to obtain a compound III, carrying out high-selectivity epoxidation by oxide to obtain a compound IV, subsequently enabling the compound IV to react with 4-(N,N-dimethyl amino) phenyl magnesium bromide Grignard reagent to obtain a compound V, then enabling the compound V to react with phenyl sulfonic acid chloride to obtain a compound VI, enabling the compound VI to respectively react with sodium methoxide and trimethyl phosphate to obtain a compound VII, hydrolyzing and removing a protection group to obtain a compound VIII, finally carrying out acetylation reaction to obtain the ulipristal acetate, wherein the reaction formulae are as shown in the description. The method is short in synthetic route, mild in reaction conditions, high in yield and purity of products, low in cost, stable and controllable in quality, and is suitable for industrial production.
Owner:CHENGDU ORGANOCHEM CO LTD
Solid dispersion and solid preparation of ulipristal acetate
ActiveCN103006603AHigh dissolution rateImprove bioavailabilityOrganic active ingredientsPill deliveryMANNITOL/SORBITOLDissolution
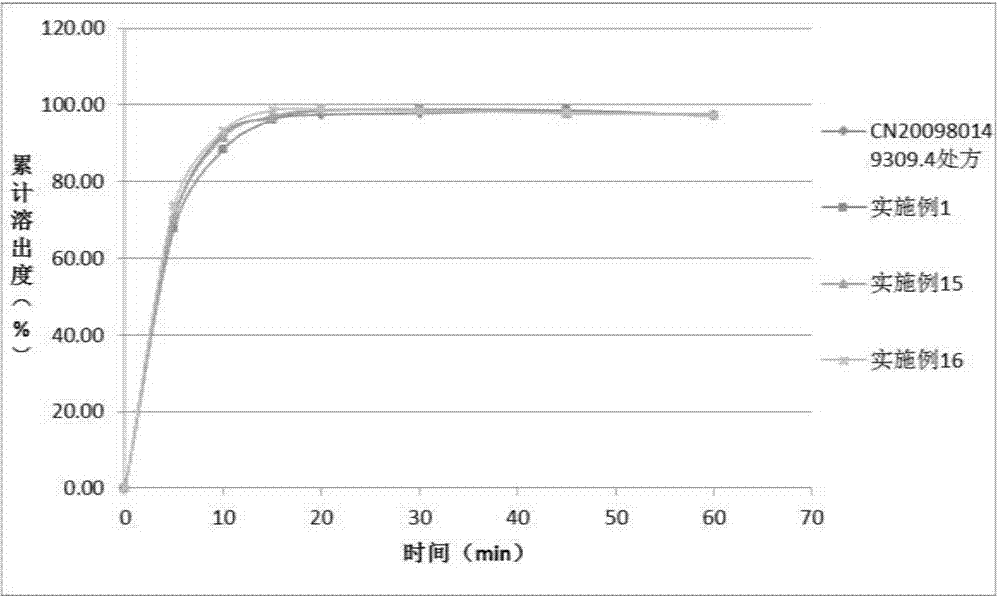
The invention discloses solid dispersion of ulipristal acetate which is prepared from 1 part by weight of ulipristal acetate and 3-20 parts by weight of auxiliary material, wherein the auxiliary material is one or combination of more than two selected from lactose, mannitol, sorbitol and povidone. The invention also discloses a solid preparation of the ulipristal acetate which is prepared from the solid dispersion of the ulipristal acetate and the conventional preparation auxiliary material, wherein the conventional preparation auxiliary material comprises filler, a disintegrating agent, a binding agent, a lubricating agent or flow aid. The solid preparation of the ulipristal acetate disclosed by the invention has higher dissolution rate compared with the ulipristal acetate tablet disclosed by the Chinese patent with the publication number of CN102245173A, the dissolution rate of the ullipristal acetate in a slightly soluble medium can be greatly increased, further bioavailability of the ulipristal acetate is improved and a pharmaceutical effect is guaranteed.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Polycrystalline type for ulipristal acetate and preparation method thereof
ActiveCN103755765AAvoid problems such as new impuritiesShorten the timeOrganic active ingredientsSteroidsX-raySolvent
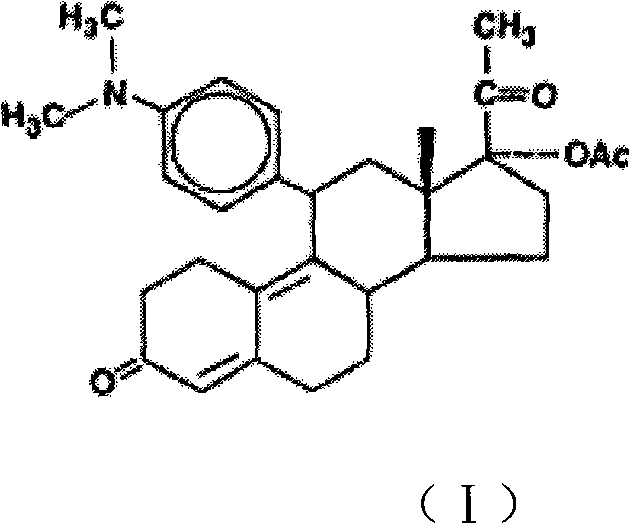
The invention discloses a polycrystalline type A1 and a polycrystalline type A2 of ulipristal acetate (shown as a formula I) not containing any solvate, and preparation methods, a medicinal composition and medicinal application thereof. As proved by smelting point, X-ray powder diffraction (XRD), infrared spectrogram (IR), differential thermal analysis spectrogram (DSC) and thermal weight loss spectrogram (TG), the polycrystalline type A1 and the polycrystalline type A2 of ulipristal acetate prepared by using the methods are extremely stable under the conditions of temperature, illumination and moisture, and contribute to long-time storage. A used crystallization solvent is safe and is easy to remove, the prepared polycrystalline type A1 and polycrystalline type A2 can be directly applied to preparation processing, and the preparation methods are easy to operate, and suitable for industrial production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
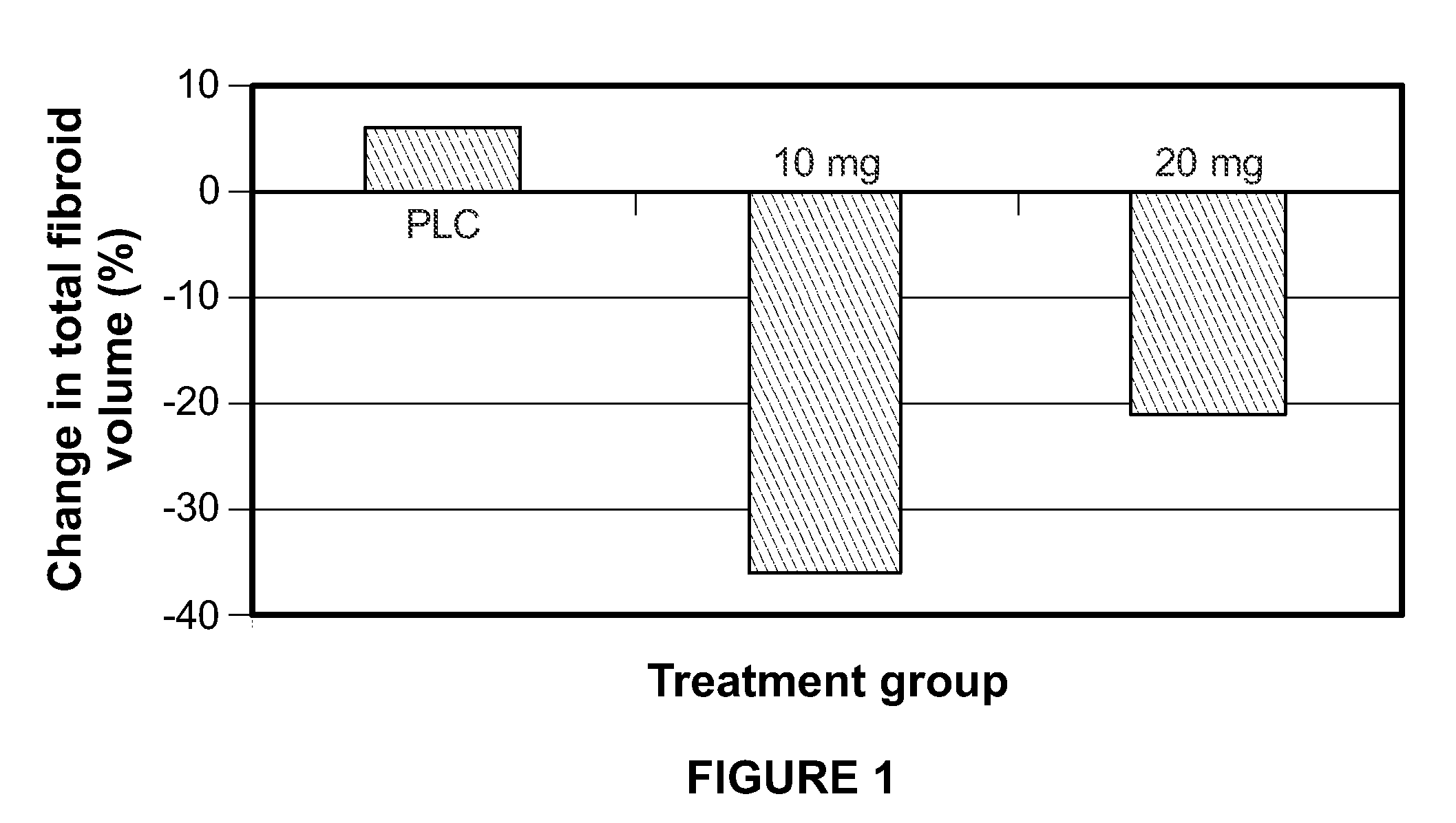
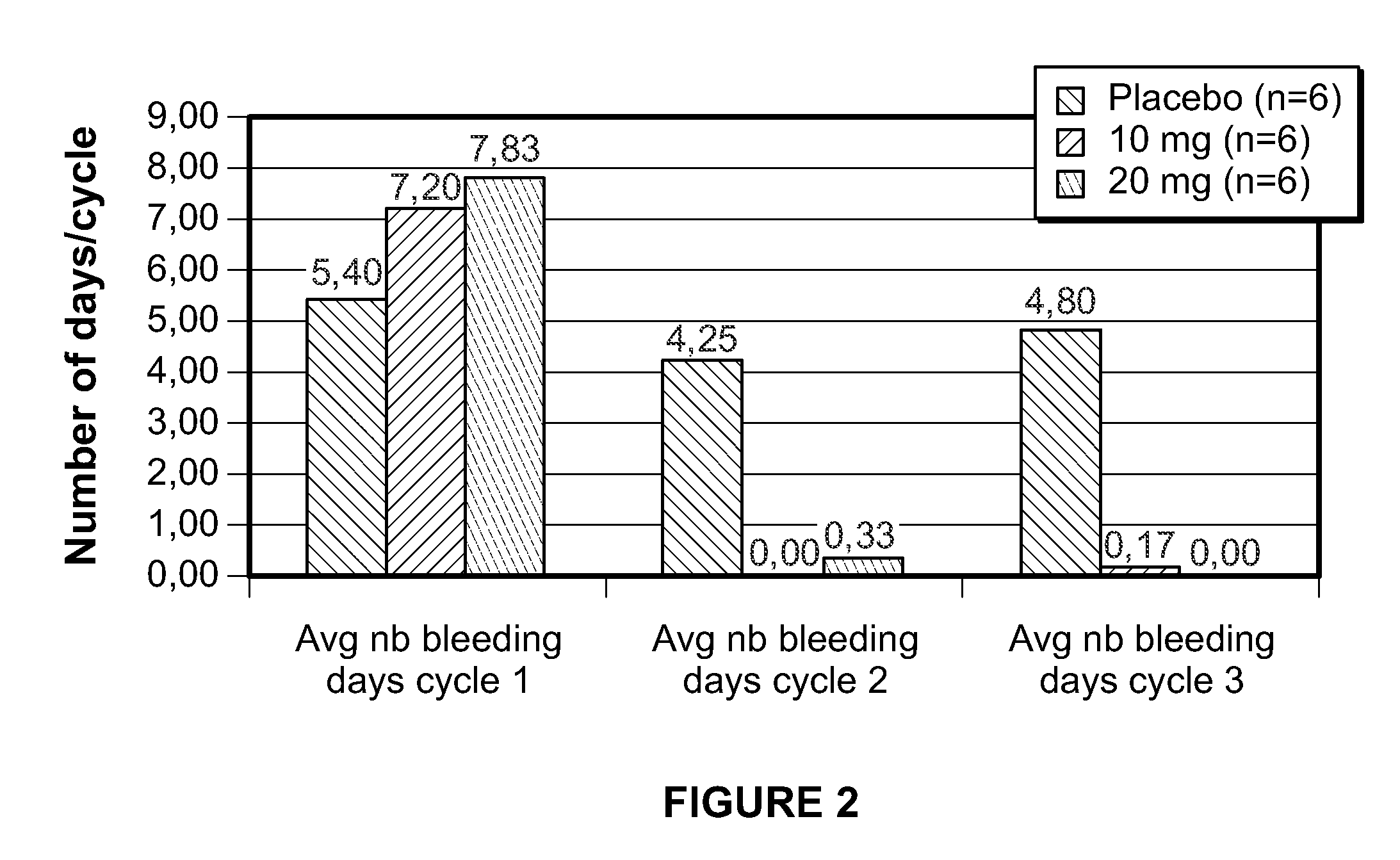
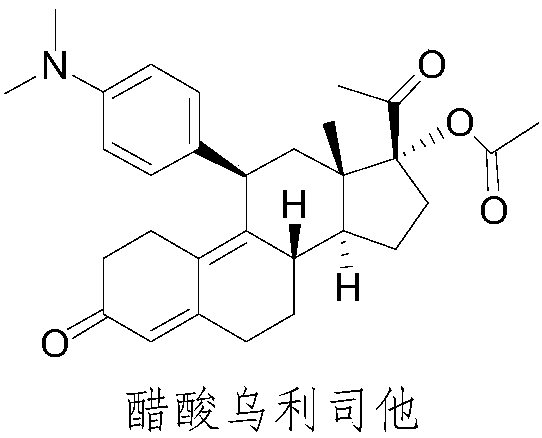
Method for treating uterine fibroids
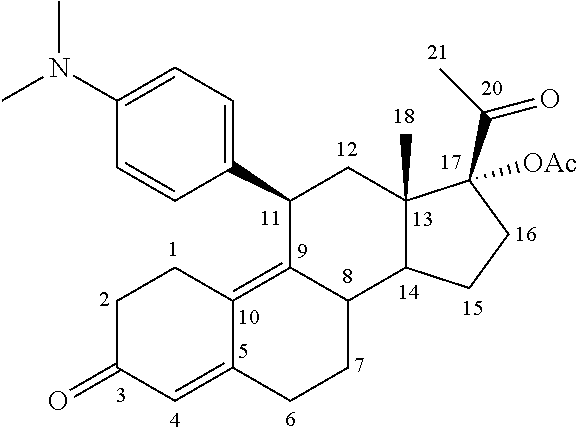
ActiveUS8299050B2Reduce and stop bleedingSmall sizeOrganic active ingredientsPill deliveryMetaboliteUlipristal acetate
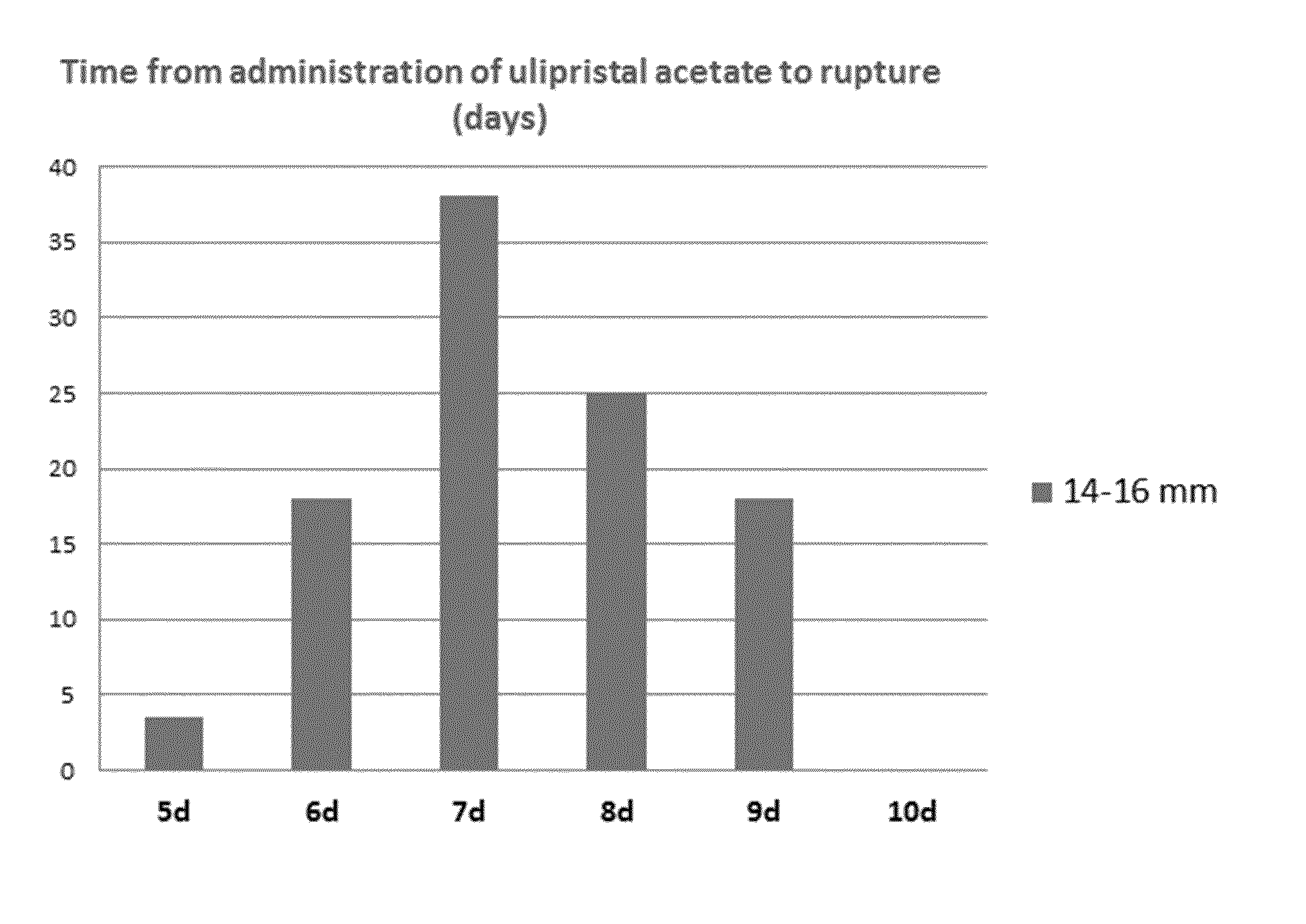
The invention relates to a method for treating uterine fibroids, which method comprises administering to a patient in need thereof, an effective amount of 17α-acetoxy-11β-[4-N,N-dimethylamino-phenyl)-19-norpregna-4,9-diene-3,20-dione (ulipristal acetate) or any metabolite thereof. More particularly, the method is useful for reducing or stopping bleeding in a patient afflicted with uterine fibroids, and / or for reducing the size of uterine fibroids.
Owner:UNITED STATES OF AMERICA +1
Novel method for synthesizing ulipristal acetate
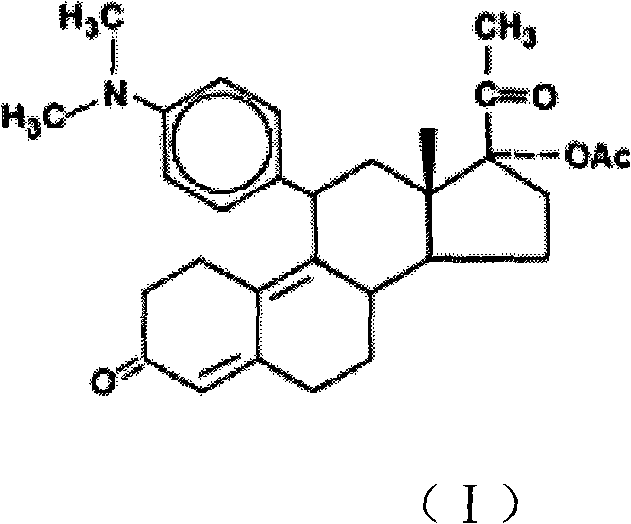
InactiveCN102942612AIngenious ideaHigh yieldSteroidsBulk chemical productionBiotechnologyPR - Progesterone receptor
The invention provides a novel method for synthesizing a progesterone receptor regulator, ulipristal acetate of formula I. According to the invention, the method has the advantages of ingenious conception, high yield and high purity, the cost is greatly reduced, and the method is suitable for industrial production.
Owner:SICHUAN UNIV
Ulipristal acetate dispersible tablet and preparation method thereof
InactiveCN107982230ADisintegrates quicklyHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionSURFACTANT BLEND
The invention discloses a ulipristal acetate dispersible tablet, which consists of the following components in percentage by weight: 3-15% of ulipristal acetate, 60-90% of a filler, 5-30% of povidone,2-7.5% of a surfactant, 2-5% of a disintegrant and 0.05-2% of a lubricant. The invention also discloses a preparation method of the ulipristal acetate dispersible tablet, wherein the preparation method comprises the following steps: dissolving the povidone in an ethanol solution, and then sequentially adding the surfactant and the ulipristal acetate, so that a prepared material is obtained; and uniformly mixing the filler and the disintegrant, adding the prepared material, implementing granulating and drying, straightening particles, adding the lubricant, uniformly mixing the materials and conducting tabletting, so that the dispersible tablet is obtained. The dispersible tablet prepared by the invention is high in disintegration rate, high in dissolution rate, good in dispersion uniformity and high in ulipristal acetate content; and the dispersible tablet is convenient to take and convenient to carry, and the compliance of clinical administration is greatly improved.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
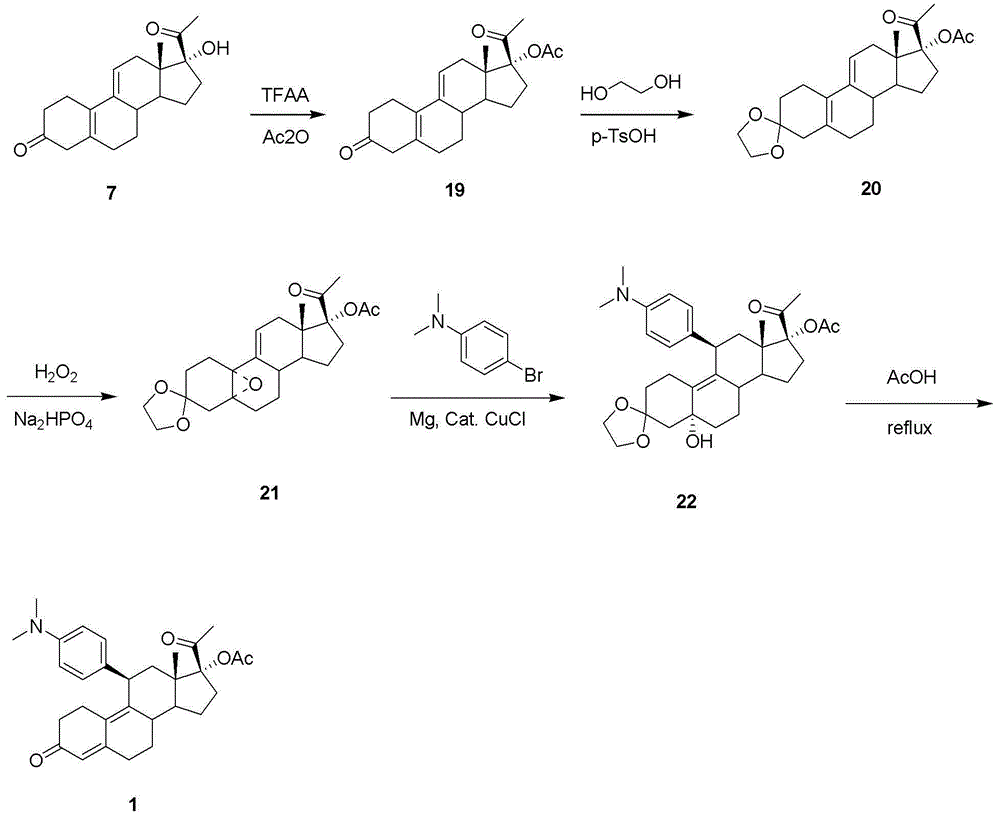
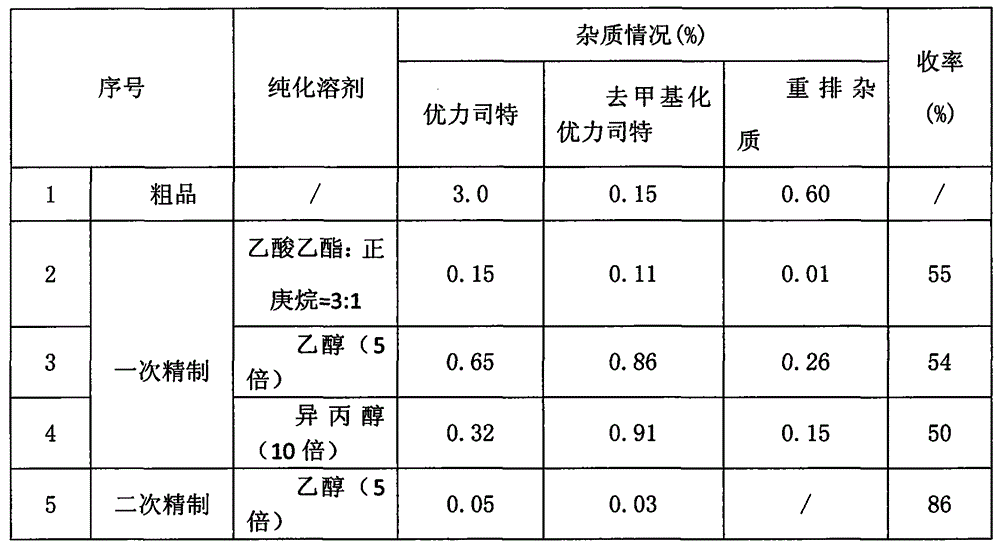
Preparation method of ulipristal acetate bulk drug impurities
The invention belongs to the technical field of steroid hormone drug preparation, and particularly relates to an ulipristal acetate bulk drug impurity preparation method, which comprises: dissolving ulipristal acetate in a solvent, carrying out a reaction under acetic anhydride and acetyl bromide conditions, and treating to obtain the ulipristal acetate bulk drug impurity, wherein the chemical name of the ulipristal acetate bulk drug impurity is 17 alpha-acetoxy-3-acetoxy-11beta-[4-(N,N-dimethylamino)phenyl]estra-1,3,5(10)-trien-20-one, the reaction process is defined in the specification, thesolvent is dichloromethane, and the mole number of acetic anhydride is 0.5-3 times that of ulipristal acetate. The product provided by the invention is high in purity and high in yield, and is used as a reference substance in an ulipristal acetate production process for controlling the quality of ulipristal acetate.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
High-purity ulipristal acetate
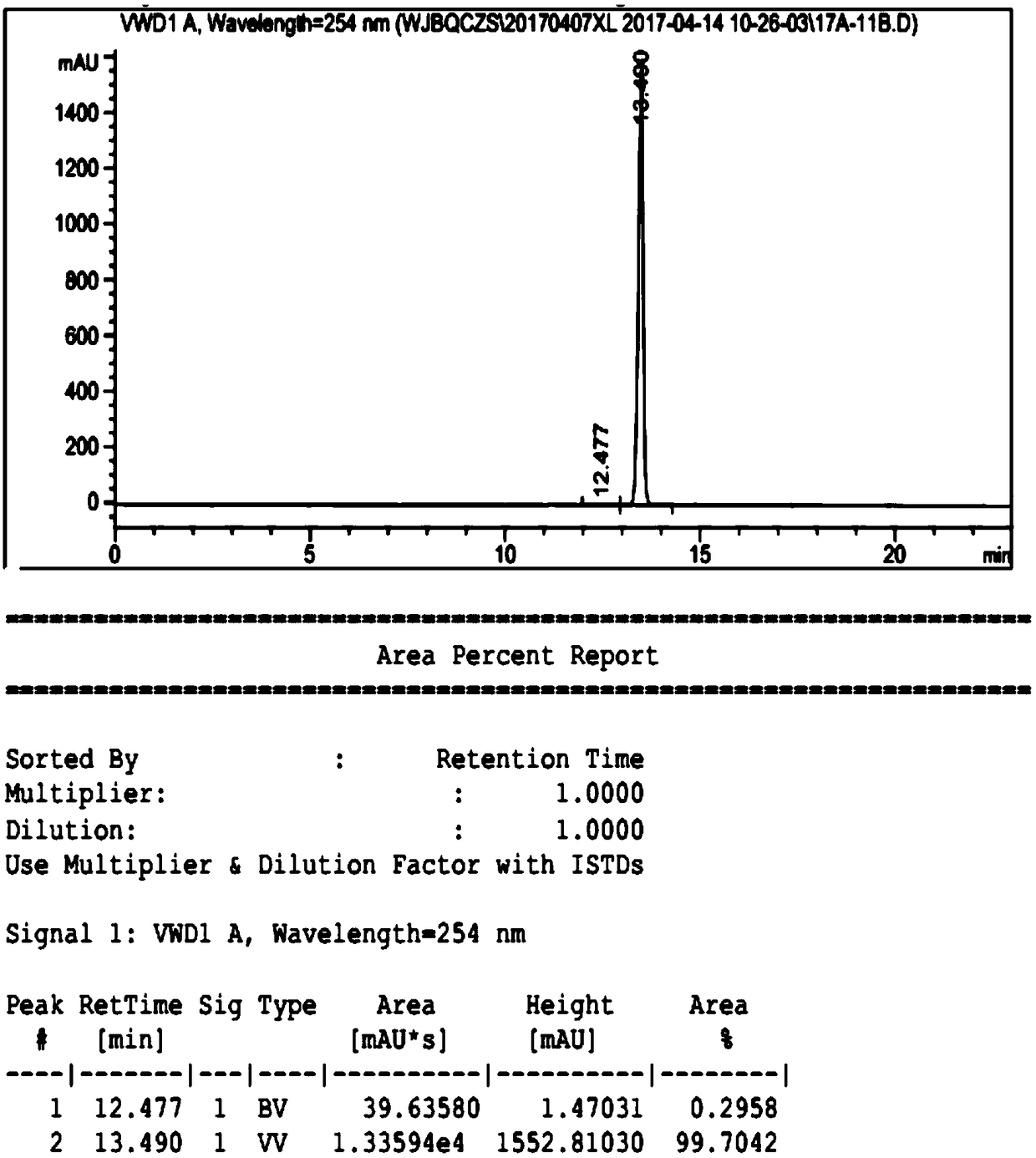
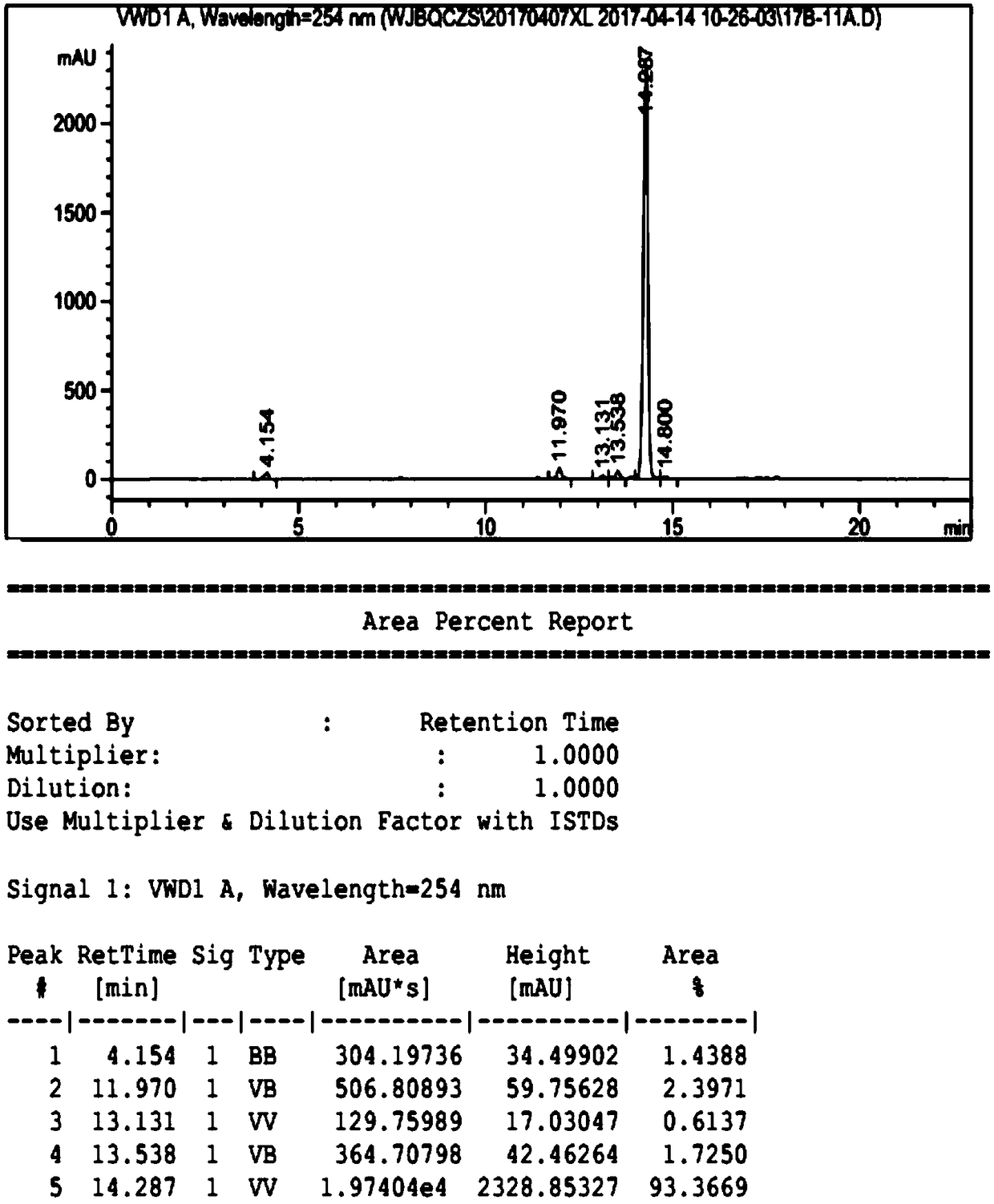
The invention discloses a high-purity ulipristal acetate compound and a preparation method thereof. The preparation method comprises steps of dissolving, cooling, adding a crystal seed, continuing to cool, filtering the crystal, baking to dry and the like. The prepared ulipristal acetate crystal is a high-purity single A-crystal-form product with excellent stability. The preparation method has the advantages of high crystal yield, simple operation, stable technology and the like, and is suitable for industrialized production.
Owner:SICHUAN HAISCO PHARMA CO LTD
Ulipristal acetate related chiral impurities and synthetic preparation method thereof
InactiveCN109369766AQuality improvementEasy to controlComponent separationOrganic chemistry methodsGrignard reactionImpurity
The invention discloses ulipristal acetate related chiral impurities and a synthetic preparation method thereof. Part of chiral impurities can be prepared by the following steps: by taking 3-ketal asan initial raw material, carrying out an addition reaction to obtain an intermediate II; deprotecting the intermediate II in an acidic condition to obtain an intermediate III; carrying out hydrolysisand oxidation to obtain an intermediate IV; and adding protecting groups to obtain the part of chiral impurities; or carrying out a Grignard reaction on a 3,20-diketal oxidative product to obtain thepart of chiral impurities. Structures of all the impurity compounds are confirmed by a magnetic resonance hydrogen spectrum, a high resolution mass spectrum and high performance liquid chromatography.The purities of the ulipristal acetate related chiral impurity compounds prepared by the method are over 95% and can be used as reference substances for quality research.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4 +1
Novel preparation method of ulipristal acetate key intermediate
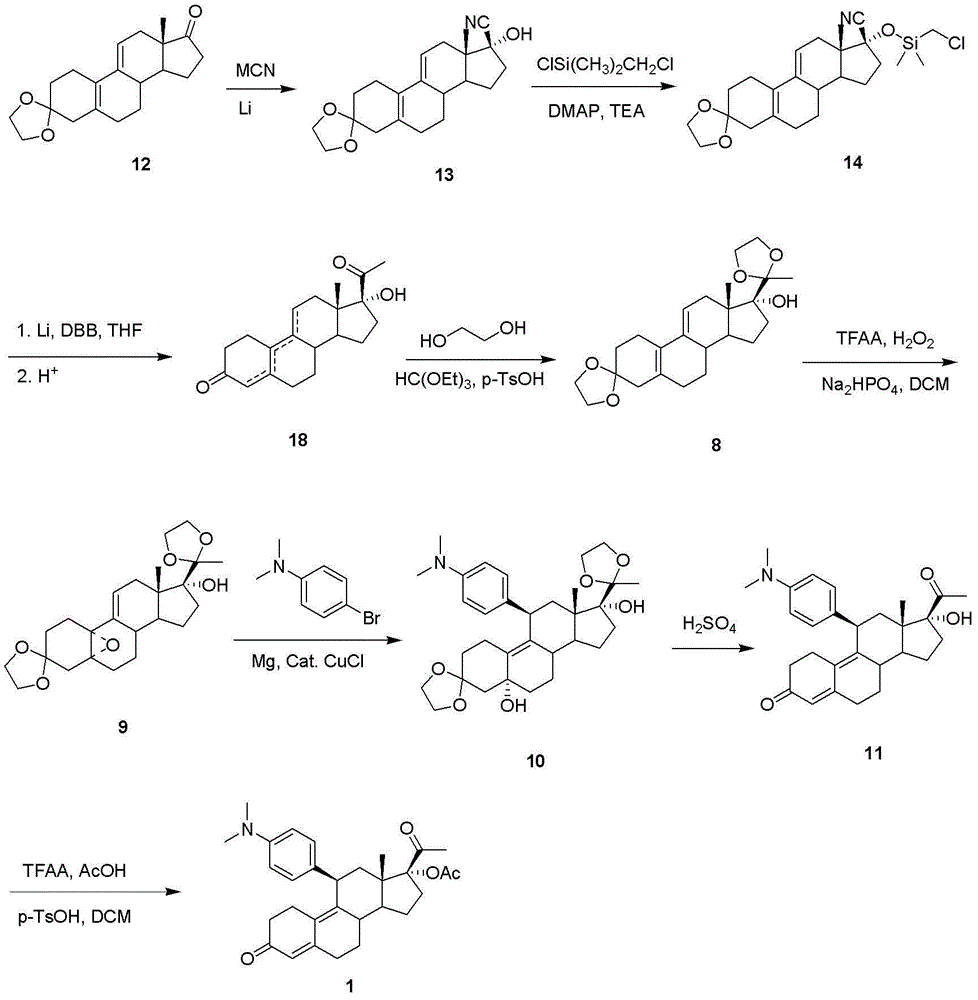
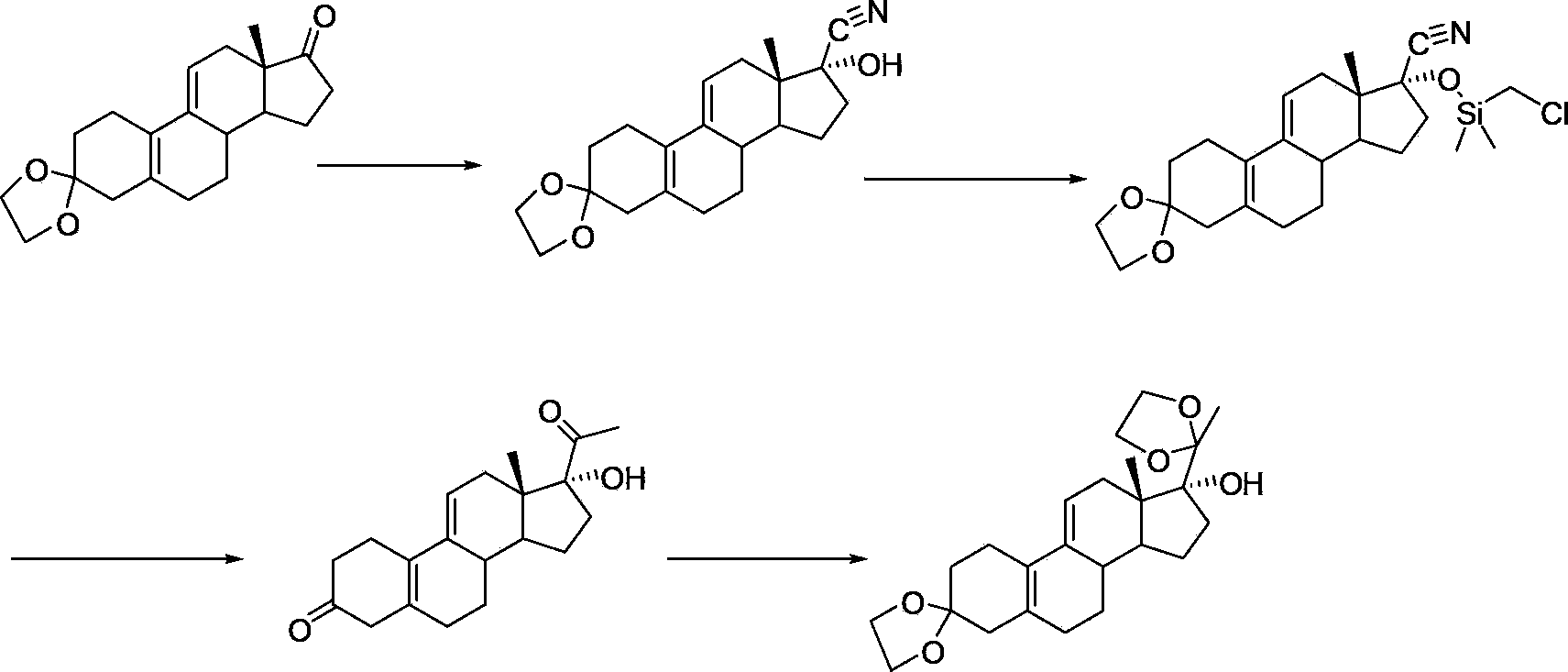
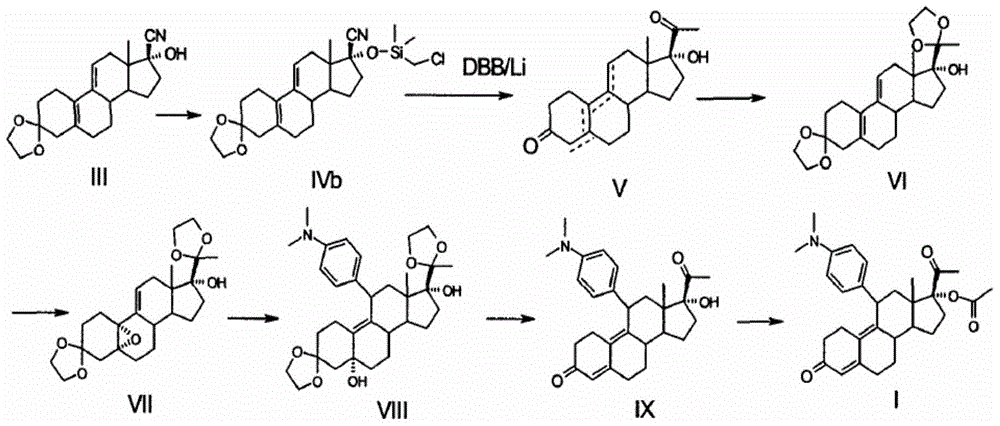
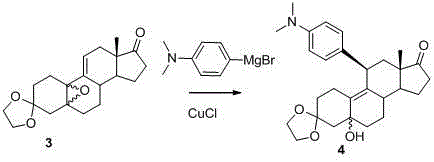
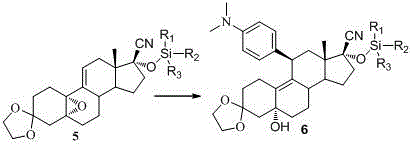
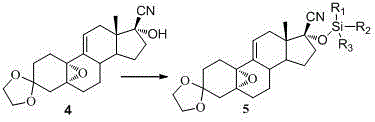
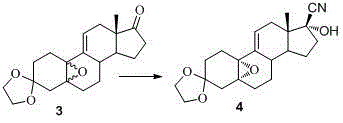
The invention discloses a novel preparation method of ulipristal acetate key intermediate 3,3,20,20-double (ethylenedioxy group)-5 alpha, 17 alpha- dihydroxyl-11 bata-[4-(N,N- dimethyl amidogen)- phenyl]-19-norprogesterone-9(11)-alkene, namely 3,3- ethylenedioxy group-17 beta-cyangroup female steroid-5(10), 9(11) diene-17 alpha-alcohol is used as a raw material, through the protection of hydroxyl, the addition of Grignard reagent and cyangroup, and the protection of ketal, and the target compound is obtained finally through 1,4 addition of alpha, beta unsaturation epoxide under the catalysis of a system of cuprous halides and dimethyl sulfide. The raw materials used in the method are safe and reliable, reaction is easy to control, reaction yield and stereoselectivity are high, and the method is applicable for industrial production.
Owner:CHANGZHOU YABANG PHARMA
Preparation method of key intermediate of Ulipristal acetate
The invention discloses a preparation method of a key intermediate of Ulipristal acetate. The key intermediate of the Ulipristal acetate has a structure of a formula II. A compound IV is prepared through Wittig reaction by using a compound III, a compound V is prepared through selective oxidization, and finally secondary alcohol at a No.20 site is oxidized into carbonyl by using oxidant to obtain the key intermediate of the Ulipristal acetate. The synthesis route and the synthesis steps of the key intermediate of the Ulipristal acetate are simple and convenient, and the operation is easy to conduct; the obtained product has the advantages of low cost, high yield and high purity; and the preparation method is suitable for industrial production.
Owner:SHANDONG UNIV
Method for providing emergency contraception
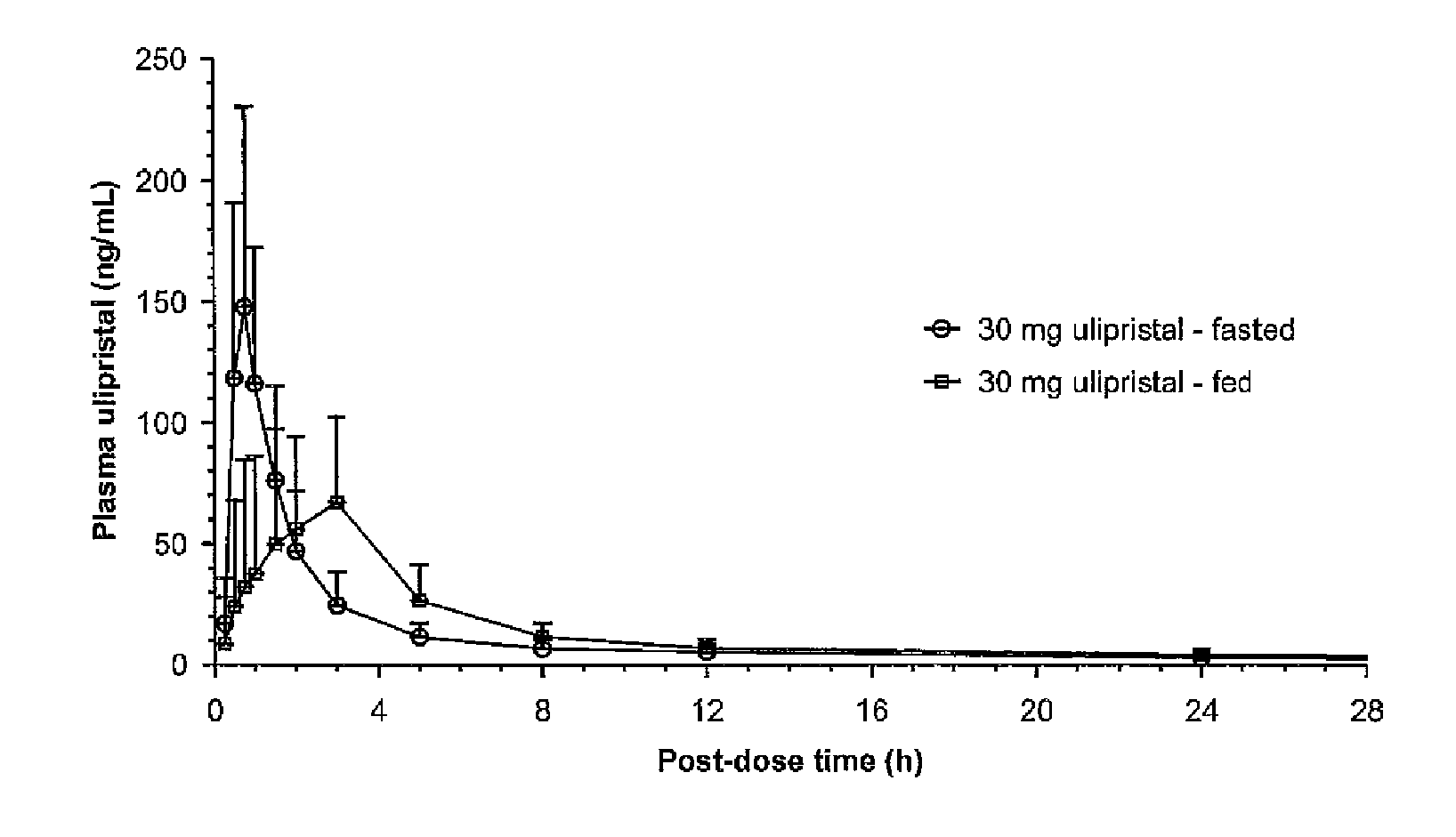
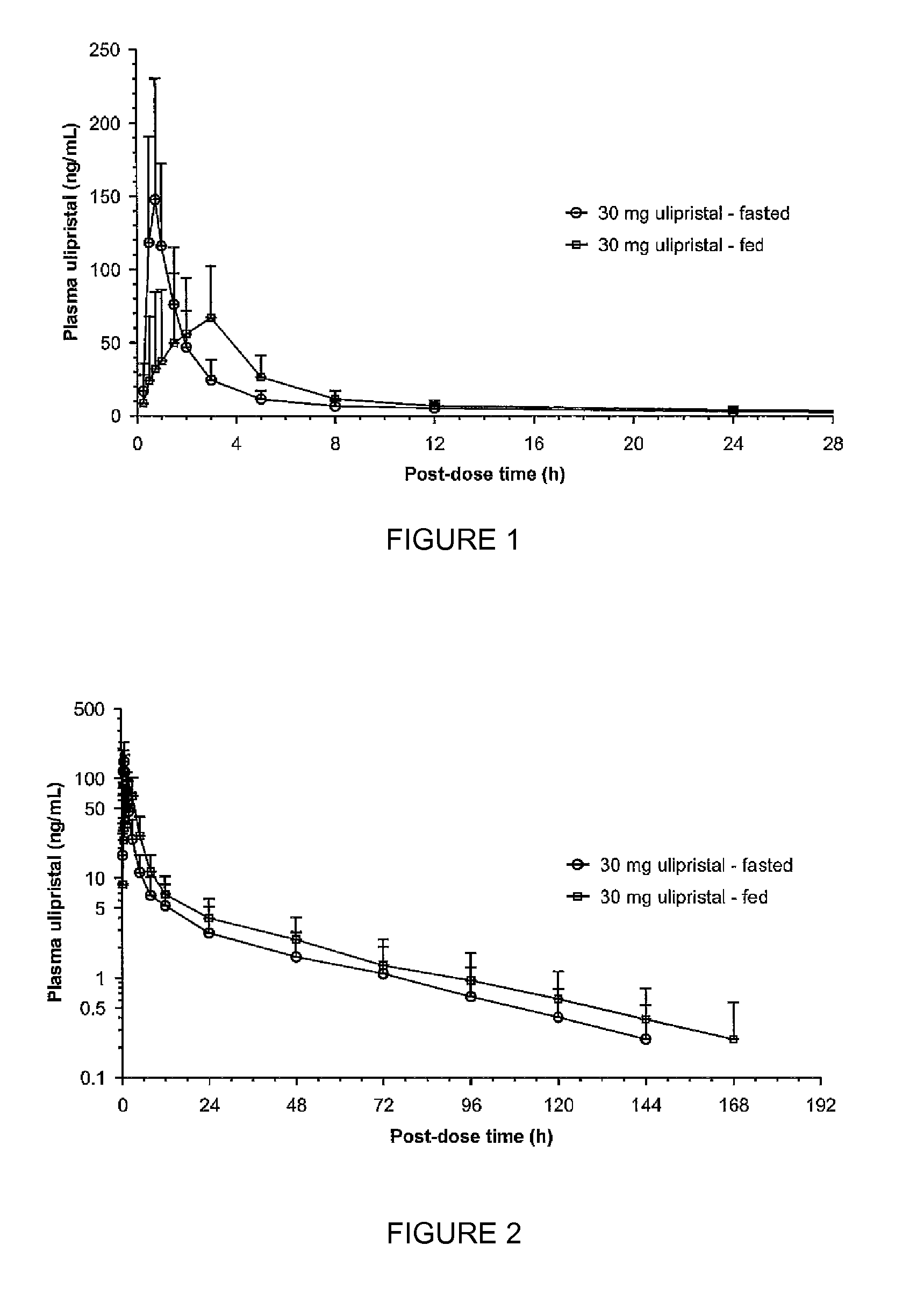
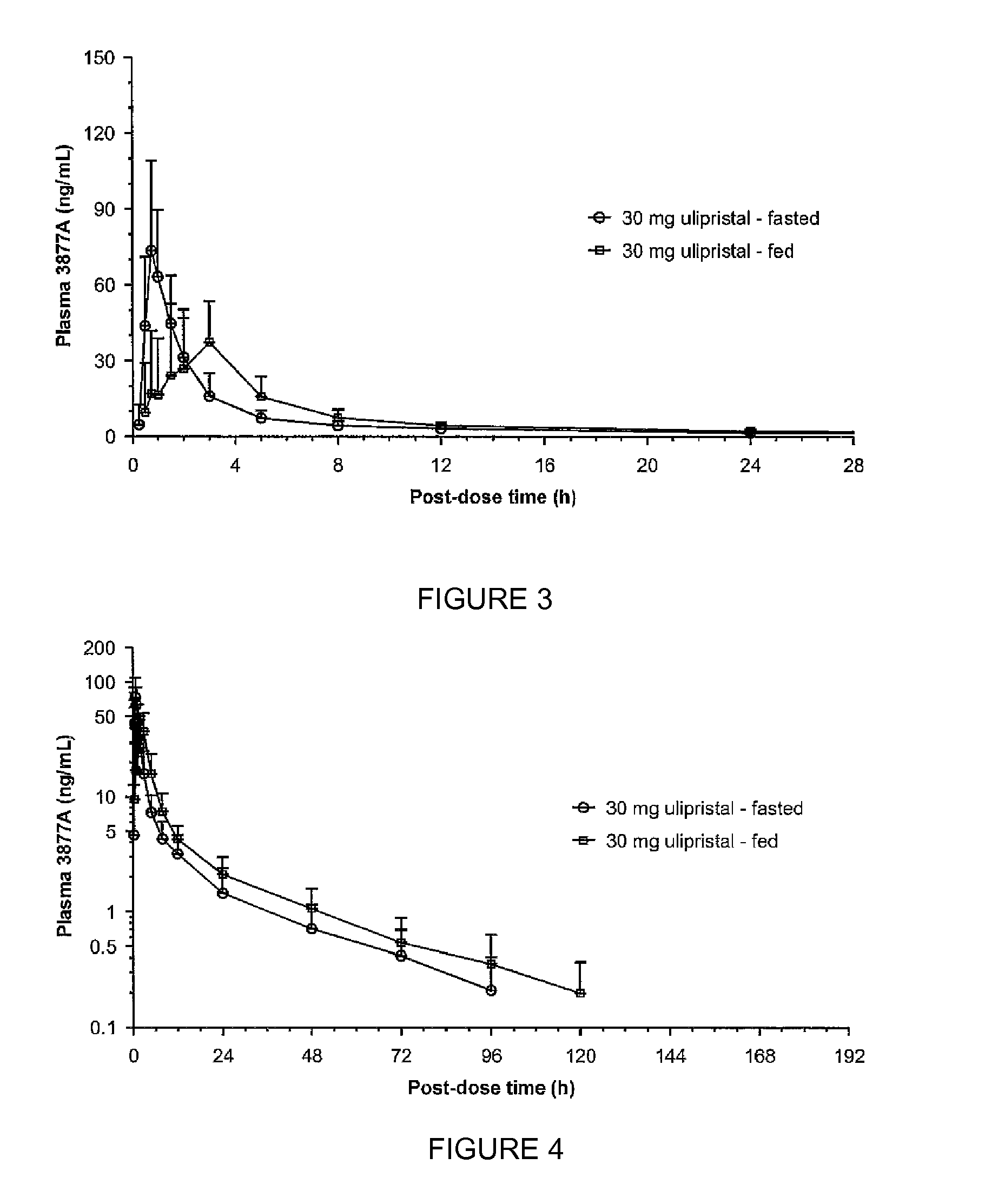
The invention provides a method for providing emergency contraception in a female subject, comprising providing the subject with a therapeutically effective amount of ulipristal acetate in an oral dosage form before, during or after a meal.The invention further provides a kit comprising i) an oral dosage form comprising ulipristal acetate and ii) a printed matter stating that ulipristal acetate may be taken with or without food.
Owner:LAB HRA PHARMA SA
Method for scheduling ovulation
InactiveUS9522154B2Promote fertilizationMaximize chanceOrganic active ingredientsPharmaceutical delivery mechanismGynecologyFollicular phase
The invention relates to a method for scheduling ovulation in a female subject, which method comprises administering ulipristal acetate (UPA) to the female subject during the follicular phase.
Owner:LAB HRA PHARMA SA
Method for using ulipristal acetate with cytochrome isozyme modulators
InactiveUS20120115802A1Reduce concentrationIncrease exposureBiocideCarbohydrate active ingredientsMetaboliteIsozyme
The invention relates to a method of using ulipristal acetate or a metabolite thereof for providing contraception or for treating a patient's condition, comprising providing a patient with ulipristal acetate or a metabolite thereof, and informing the patient or a medical care worker that ulipristal acetate or a metabolite thereof affects activity of a cytochrome p450 isozyme, and that administration of ulipristal acetate or a metabolite thereof with a substance that affects activity of a cytochrome p450 isozyme can affect plasma concentration, safety, efficacy or any combination thereof of ulipristal acetate or a metabolite thereof, the substance, or both.
Owner:LAB HRA PHARMA SA
Preparation method of ulipristal acetate key intermediate
ActiveCN105622702AReduce feed concentrationGuaranteed oxidation effectKetal steroidsIce waterFiltration
The invention provides a preparation method of a ulipristal acetate key intermediate 3,3,20,20-bis(ethylenedioxy)-17alpha-hydroxy-5,10-epoxy-19-norpregn-9(11)ene. The preparation method comprises the following steps: dissolving 3,3,20,20-bis(ethylenedioxy)-17alpha-hydroxy-19-norpregn-5(10),9(11)diene in dichloromethane, carrying out treatment in an ice bath under pyridine conditions, adding anhydrous magnesium sulfate, hexachloroacetone and 30% oxydol, and stirring in the ice bath to react for 1.5 hours, thereby obtaining 3,3,20,20-bis(ethylenedioxy)-17alpha-hydroxy-5,10-epoxy-19-norpregn-9(11)ene; and mixing the 3,3,20,20-bis(ethylenedioxy)-17alpha-hydroxy-5,10-epoxy-19-norpregn-9(11)ene, sodium-thiosulfate-pentahydrate-containing ice water and dichloromethane to react, and carrying out skimming, extraction, washing, drying, filtration and drying by distillation to obtain the 3,3,20,20-bis(ethylenedioxy)-17alpha-hydroxy-5,10-epoxy-19-norpregn-9(11)ene. The concentration of the oxydol used in the preparation method is 30%, so the potential safety hazard is low.
Owner:SUZHOU XINENG ENVIRONMENTAL SCI & TECH CO LTD
Industrial process for the synthesis of ulipristal acetate and its 4'-acetyl analogue
ActiveCN105593236AReduce side effectsOrganic chemistry methodsKetal steroidsMethyl groupUlipristal acetate
Owner:RICHTER GEDEON NYRT
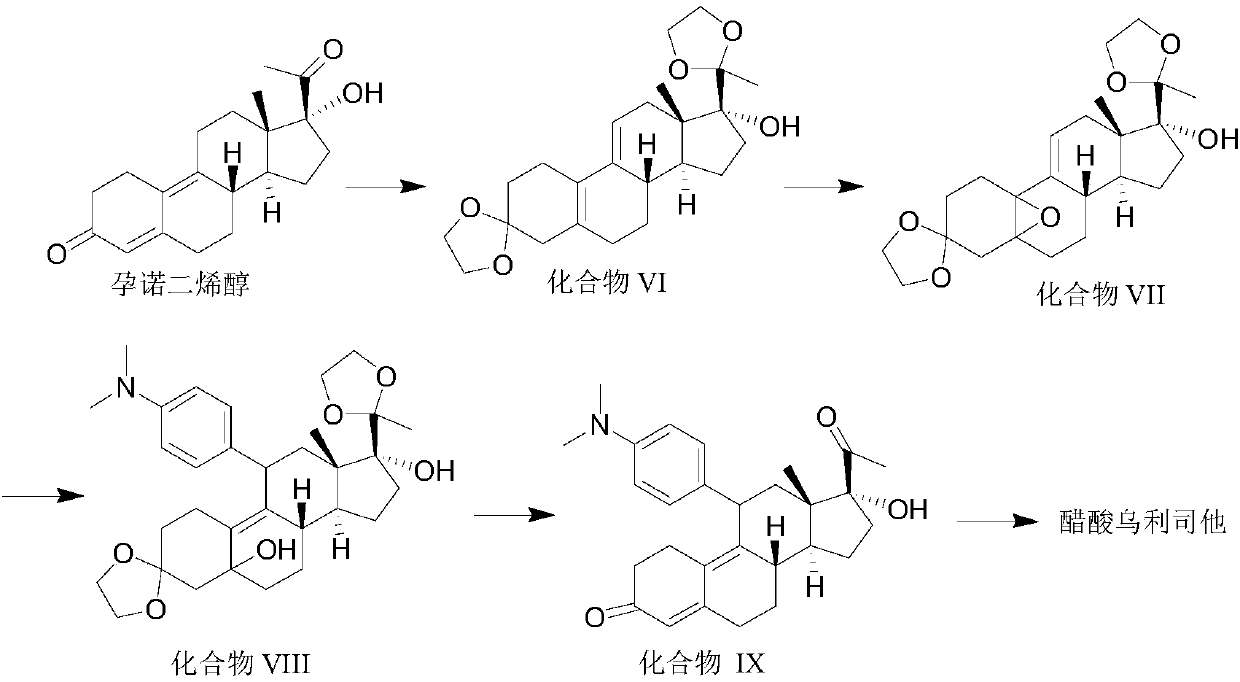
Synthesis method of ulipristal acetate
The invention discloses a synthesis method of ulipristal acetate. The synthesis method comprises the following steps: 1), producing a compound VI from gestadienol under the action of ethylene glycol,p-toluenesulfonic acid and trimethyl orthoformate; 2), oxidizing the compound VI by using hydrogen peroxide to obtain a compound VII; 3), preforming a reaction on the compound VII and a 4-(N, N-dimethylamino) phenylmagnesium bromide Grignard reagent to obtain a compound VIII; 4), hydrolyzing a compound VIII to obtain a compound XI; 5), preforming a reaction on the compound XI, glacial acetic acidand acetic anhydride under the catalytic action of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 4-dimethylaminopyridine and iron perchlorate to obtain the ulipristal acetate. In the synthesis method, reaction conditions for obtaining the ulipristal acetate from ulipristal are relatively mild, demethylated ulipristal or ulipristal oxynitride is unlikely to produce, and the production process is more controllable.
Owner:广州市桐晖药业有限公司
Novel synthesis method of Ulipristal acetate
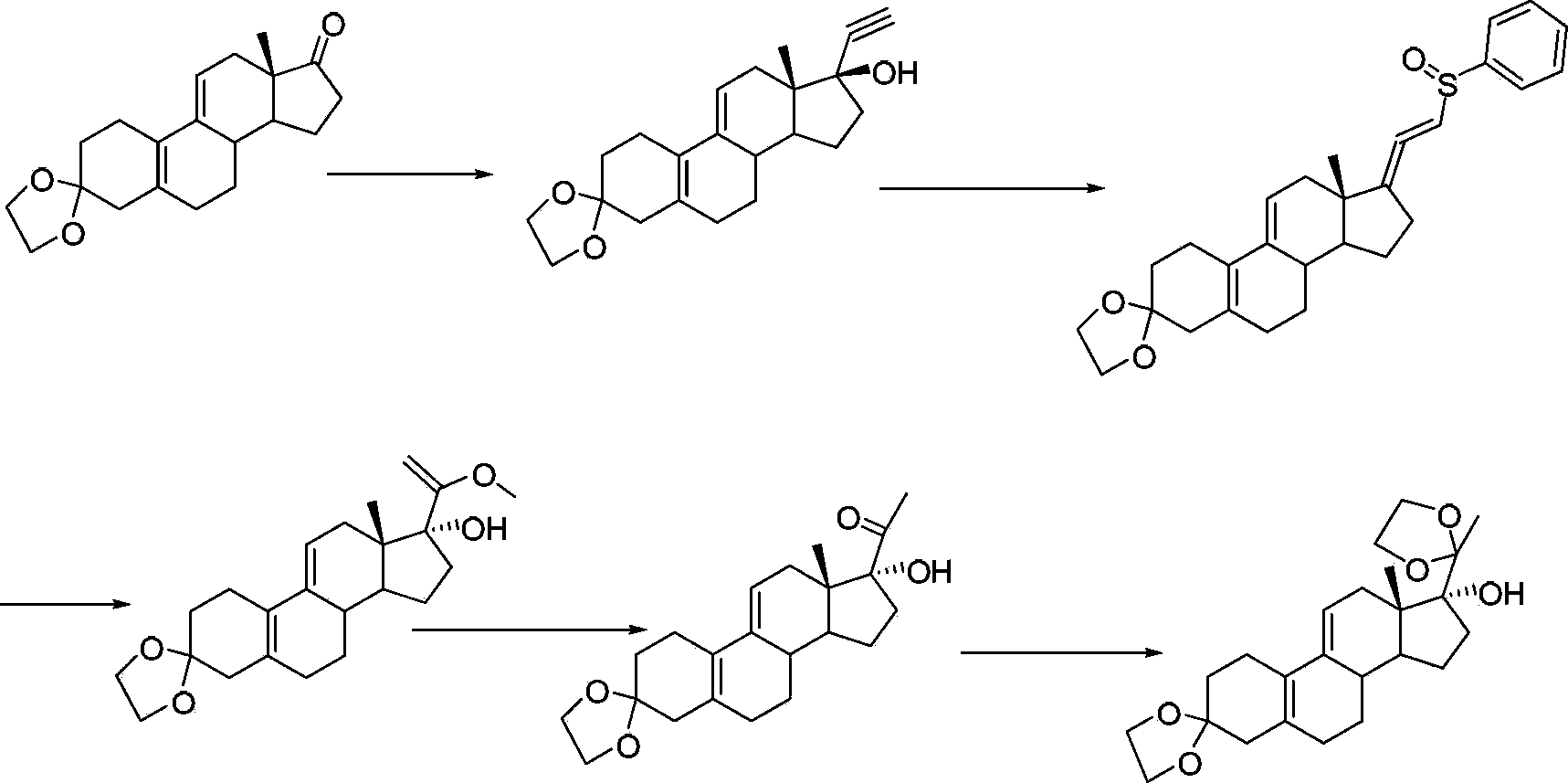
The invention provides a novel method for synthesizing a progesterone receptor regulator, namely Ulipristal acetate (represented by a formula I shown in the specification). According to the method, Ulipristal acetate is prepared from a compound 2 through reaction in six steps, namely epoxidation, cyanation, hydroxyl protecting, Grignard reaction, methylation and acetylation. The method provided by the invention is ingenious in conception and has the characteristics of short steps, simplicity in operation and high product yield and purity, and the cost is reduced greatly, so that the method is applicable to industrial production.
Owner:SICHUAN UNIV
Copper intrauterine device
InactiveUS20150320766A1Inhibits motilityInhibits viabilityBiocideOrganic active ingredientsSide effectGynecology
The invention relates to a copper contraceptive intrauterine system (IUS) with a flexible frame, which system is further capable of releasing a selective progesterone receptor modulator (SPRM), such as ulipristal acetate, for reducing or preventing bleeding side effects.
Owner:LAB HRA PHARMA SA
Solid dispersion and solid preparation of ulipristal acetate
ActiveCN103006603BHigh dissolution rateImprove bioavailabilityOrganic active ingredientsPill deliveryMANNITOL/SORBITOLDissolution
The invention discloses solid dispersion of ulipristal acetate which is prepared from 1 part by weight of ulipristal acetate and 3-20 parts by weight of auxiliary material, wherein the auxiliary material is one or combination of more than two selected from lactose, mannitol, sorbitol and povidone. The invention also discloses a solid preparation of the ulipristal acetate which is prepared from the solid dispersion of the ulipristal acetate and the conventional preparation auxiliary material, wherein the conventional preparation auxiliary material comprises filler, a disintegrating agent, a binding agent, a lubricating agent or flow aid. The solid preparation of the ulipristal acetate disclosed by the invention has higher dissolution rate compared with the ulipristal acetate tablet disclosed by the Chinese patent with the publication number of CN102245173A, the dissolution rate of the ullipristal acetate in a slightly soluble medium can be greatly increased, further bioavailability of the ulipristal acetate is improved and a pharmaceutical effect is guaranteed.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com