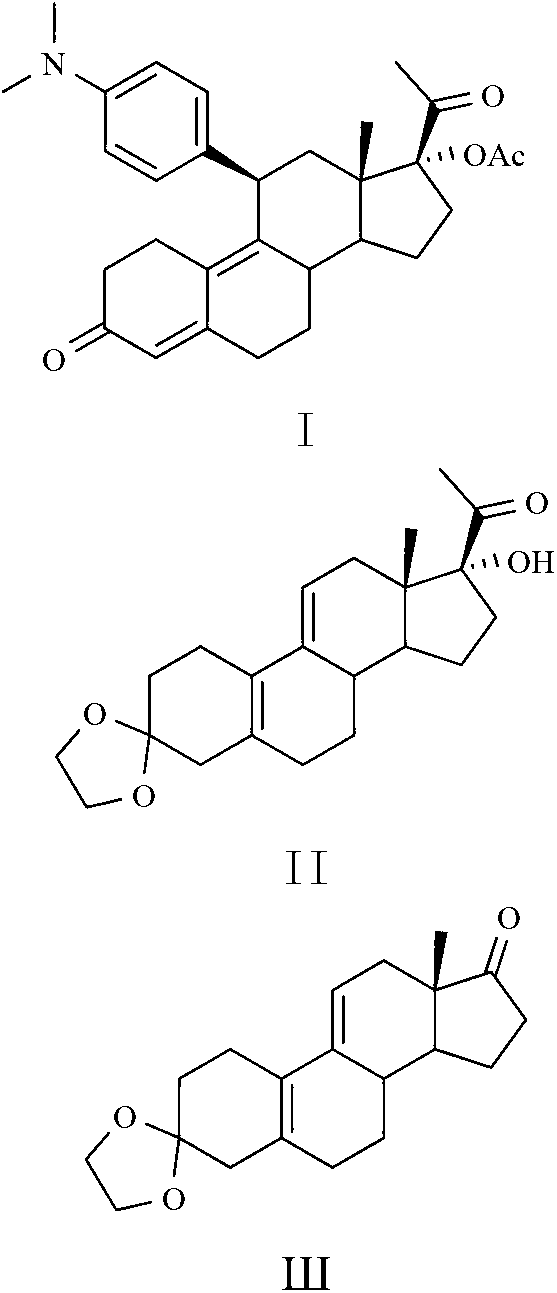
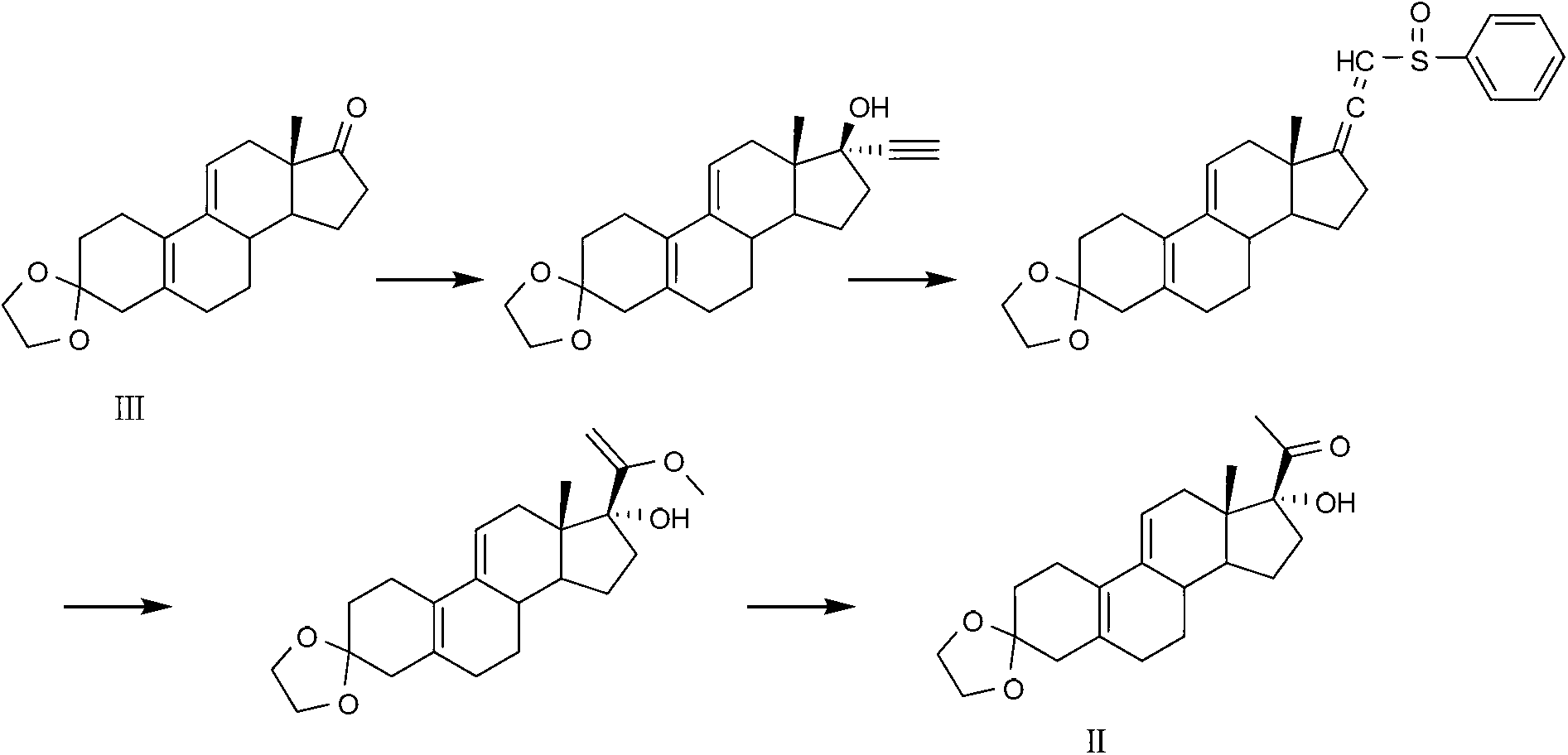
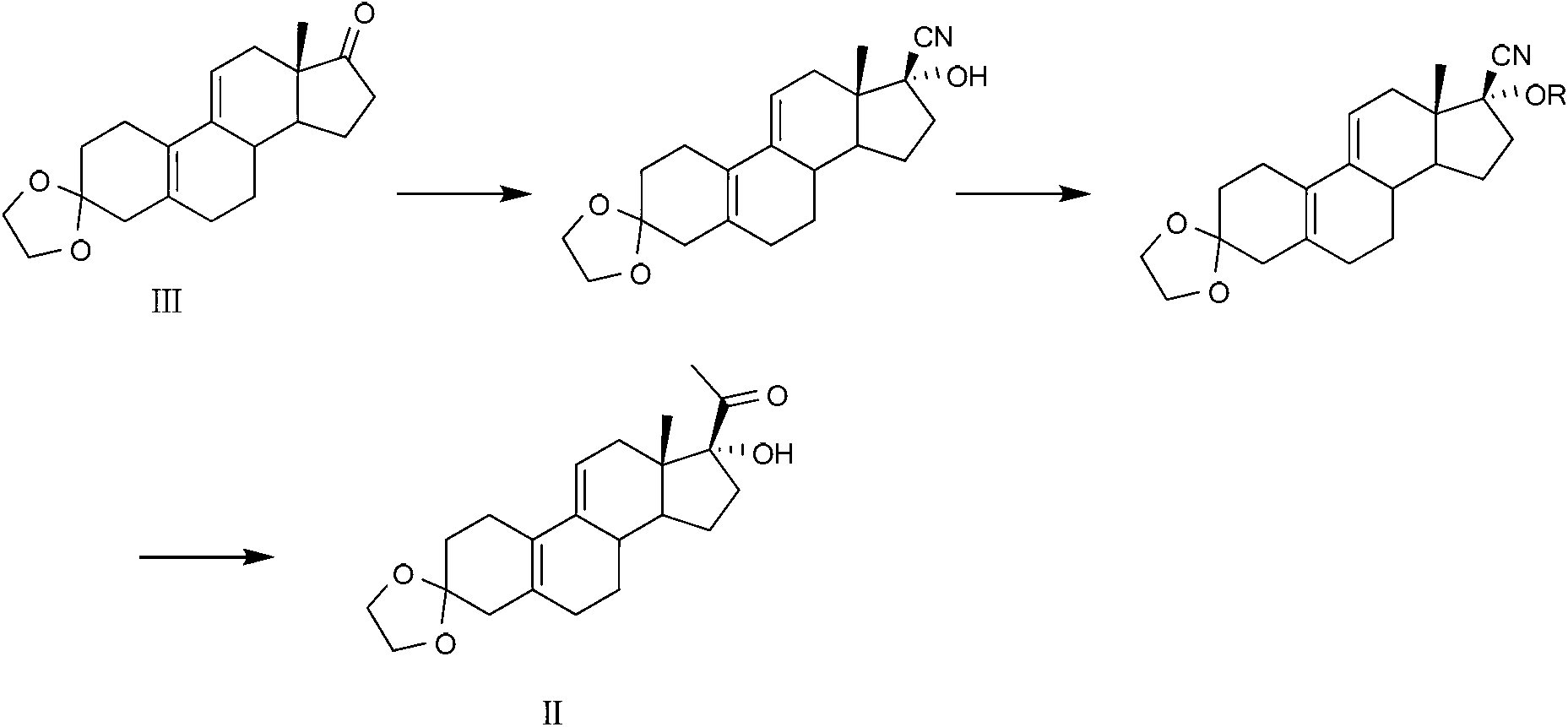
Preparation method of key intermediate of Ulipristal acetate
A technology of ulipristal acetate and an intermediate, applied in the field of medicine, can solve problems such as strict requirements for post-processing methods, increase reaction steps and the like, and achieve the effects of simple steps, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Take a 3-liter one-necked flask, add 39 grams (0.35 moles) of potassium tert-butoxide, 130 grams (0.35 moles) of triphenylethylphosphorus bromide and 1 liter of toluene, and stir at room temperature for 1 hour. 100 g (0.32 mol) of compound III was added, the temperature was raised to 60°C, and the reaction was stirred for 1 hour. Add 200 ml of n-hexane and 100 ml of water, stir for 10 minutes and separate the layers. The aqueous layer was extracted with n-hexane, and the organic layers were combined, washed with 200 ml of saturated brine, and dried over anhydrous sodium sulfate. Concentrate after filtration, add 1 liter of n-hexane, stir for 30 minutes, and filter the precipitate. The filtrate was concentrated to obtain 90 g of a white solid (compound IV), with a yield of 85% and a purity of 98% by HPLC. Compound Ⅳ, melting point: 120-125℃; MS: 327.22(M+1); 1 H NMR (600MHz, CDCl 3 ):5.57(s,1H),5.20(quart,1H),3.99(s,4H),0.84-2.58(m,24H).
Embodiment 2
[0037] Example 2. Take a 3-liter single-necked flask, add 11.5 grams (0.48 moles) of sodium hydride, 178 grams (0.48 moles) of triphenylethylphosphorus bromide and 1 liter of toluene, and stir for 1 hour. 100 g (0.32 mol) of compound III was added, the temperature was raised to 60°C, and the reaction was stirred for 1.5 hours. Add 250 ml of n-hexane and 120 ml of water, and stir for 10 minutes. The layers were separated, the aqueous layer was extracted with n-hexane, the organic layers were combined, washed with 200 ml of saturated brine, and dried over anhydrous sodium sulfate. Concentrate after filtration, add 1 liter of n-hexane, stir for 30 minutes, and then filter the precipitate. The filtrate was concentrated to obtain 88 g of white solid (compound IV), with a yield of 83% and a purity of 96% by HPLC.
Embodiment 3
[0038]Example 3. Take a 3-liter one-necked flask, add 178 grams (0.48 moles) of triphenylethylphosphonium bromide and 1 liter of tetrahydrofuran, cool to -78 ° C, add 48 milliliters (0.48 moles) of n-butyllithium, and stir the reaction 30 minutes. 100 g (0.32 mol) of compound III was added, and the reaction was stirred for 1.5 hours. Add 200 ml of n-hexane and 100 ml of saturated sodium bicarbonate solution, and stir for 10 minutes. The layers were separated, the aqueous layer was extracted twice with 100 ml of n-hexane, the organic layers were combined, washed with 200 ml of saturated brine, and dried over anhydrous sodium sulfate. Concentrate after filtration, add 1 liter of n-hexane, stir for 30 minutes, and then filter the precipitate. The filtrate was concentrated to obtain 95 g of a white solid (compound IV), with a yield of 90% and an HPLC purity of 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com