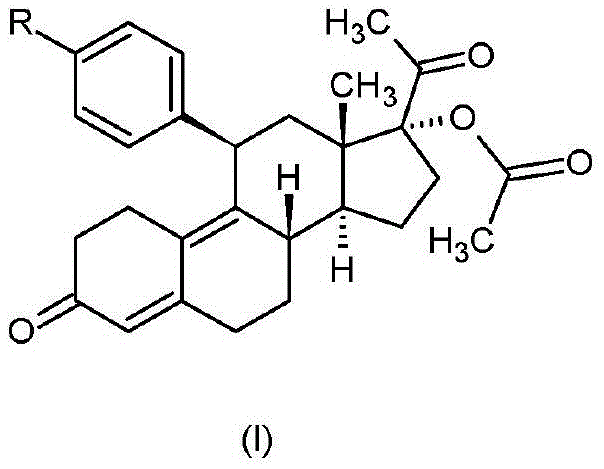
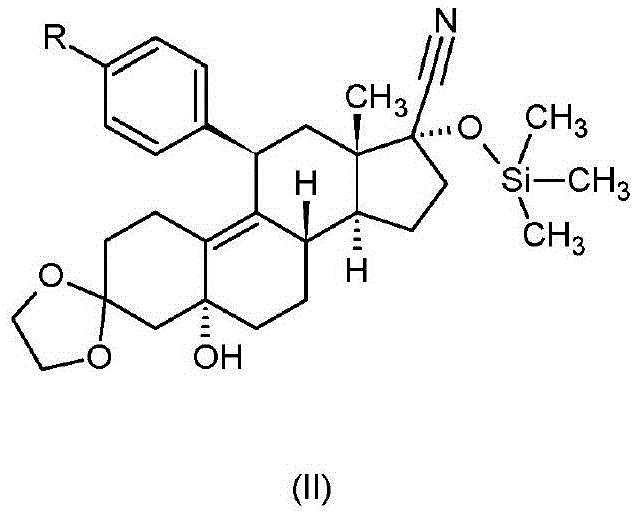
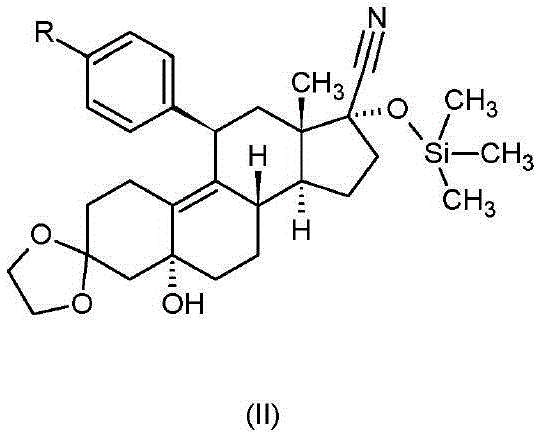
Industrial process for the synthesis of ulipristal acetate and its 4'-acetyl analogue
A technology of acetyl group and compound, applied in the industrialized field for synthesizing ulipristal acetate and its 4'-acetyl analog, can solve problems such as incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 11β-[4-(N,N-dimethylamino)-phenyl]-17-hydroxy-19-norpregna-4,9-diene-3,20-dione
[0048] 8.0g (14.5mM) 11β-[4-(N,N-dimethylamino)-phenyl]-3,3-ethanedioxy-5α-hydroxyl-17α-[(trimethylsilyl) Oxy]-5α-estr-9-ene-17β-carbonitrile was dissolved in 130 ml of tetrahydrofuran, and the solution was cooled to -50°C. First 60 ml (180 mM) of methyl lithium 3.0 M solution in diethoxymethane were added dropwise followed by 27 ml (180 mM) of tetramethylethylenediamine at such a rate that the reaction temperature was maintained below -45°C. The reaction mixture was stirred at a temperature of -45 to (-40)°C for 3 hours, then 70 ml of water was added dropwise very carefully to the reaction mixture while the temperature was raised to +20°C. After stirring for 5 minutes, the phases were separated and the organic phase was washed with 20 ml of water and concentrated under reduced pressure. To the residue were added 80 ml of methanol and 110 ml of 1N sulfuric acid solution, and...
Embodiment 2
[0053] Synthesis of 11β-[4-(N,N-dimethylamino)-phenyl]-17-hydroxy-19-norpregna-4,9-diene-3,20-dione
[0054] 25.0 g (40.1 mM) of 11β-[4-(N,N-dimethylamino)-phenyl]-3,3-ethanedioxy-5,17α-bis[(trimethylsilyl)oxy Base]-5α-estr-9-ene-17β-carbonitrile was dissolved in 500 ml of dimethoxymethane, and the solution was cooled to -50°C. First 66.7 ml (200 mM) of a 3.0 M solution of methyllithium in diethoxymethane was added dropwise, followed by 30 ml (200 mM) of tetramethylethylenediamine, at such a rate that the reaction temperature was maintained below -40°C. The reaction mixture was stirred at a temperature of -45 to (-35)°C for 3 hours, then 210 ml of water were added dropwise to the reaction mixture very carefully while the temperature was raised to +20°C. After stirring for 5 minutes, the phases were separated and the organic phase was washed with 50 ml of water and concentrated under reduced pressure. To the residue were added 220 ml of methanol and 300 ml of 1N sulfuric acid...
Embodiment 3
[0059] 3,3-Ethylenedioxy-11β-[4-(2-methyl-1,3-dioxolan-2-yl)-phenyl]-5,17α-bis[(trimethylsilyl Synthesis of yl)oxy]-5α-estr-9-ene-17β-carbonitrile
[0060] 25.0 g (41.68 mM) of 3,3-ethanedioxy-11β-[4-(2-methyl-1,3-dioxolan-2-yl)-phenyl]-5-hydroxyl-17α -[(trimethylsilyl)oxy]-5α-estr-9-ene-17β-carbonitrile (Example 25 of WO2001 / 74840) was dissolved in 125ml of dichloromethane, and dropped into the solution at 20°C Add 5 g of imidazole, and then dropwise add 8.4 ml of chlorotrimethylsilane. The reaction mixture was stirred at 20-25°C for 1 hour, then diluted with 70ml of dichloromethane and 70ml of water. After vigorous stirring for 10 minutes, the phases were separated. The organic phase was washed with 2 x 50 ml of water, dried over anhydrous sodium sulfate and concentrated. The residue was recrystallized from methanol to afford 22.2 g (80.0%) of the title compound.
[0061] Melting point: 134-135°C
[0062] 1 HNMR (800MHz, CDCl 3 )δ:7.34(m,2H),7.16(m,2H),4.33(m,1H),3.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com