Purification method of ulipristal acetate
A purification method and patented technology, applied in the field of purification of ulipristal acetate, can solve the problems of increased demethylated impurities, large N-demethylated impurities, incomplete drying, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
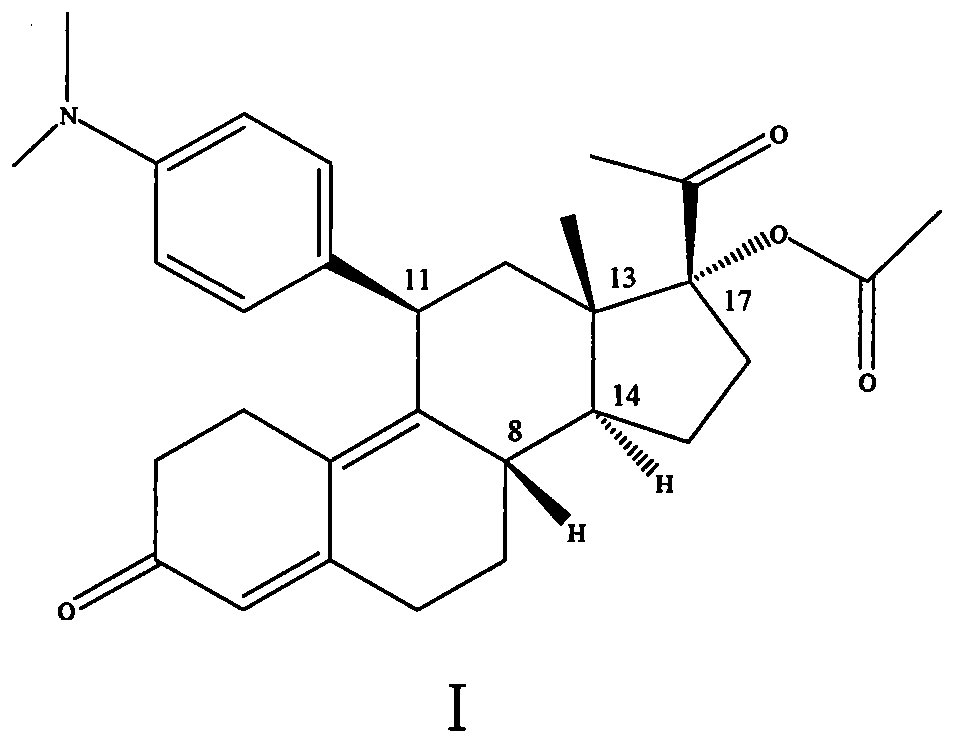
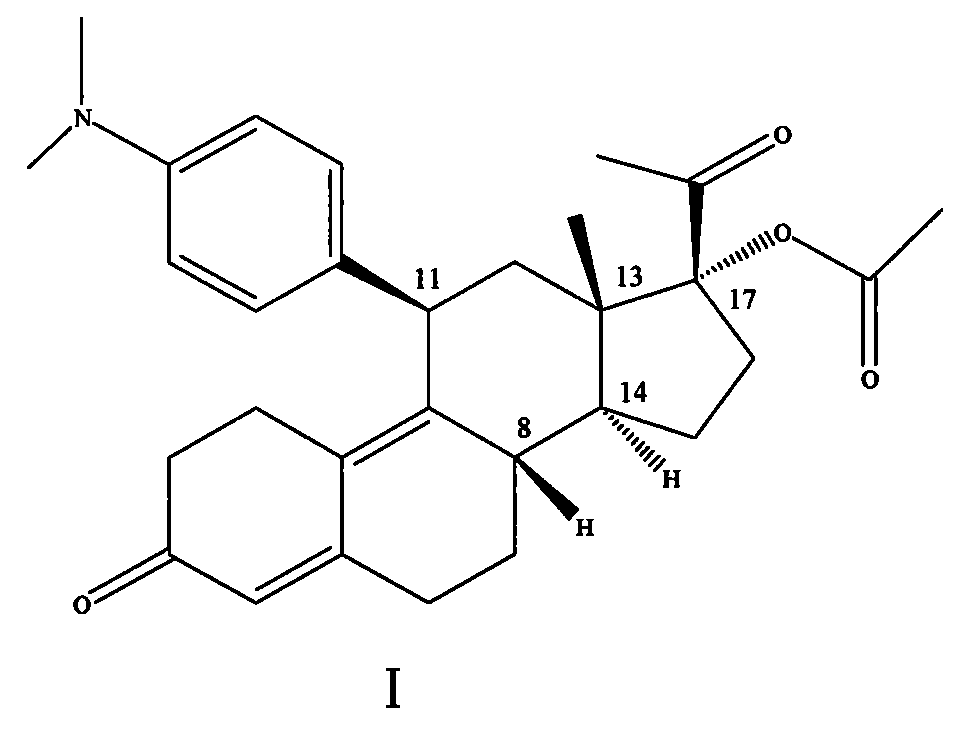
[0011] Purification of compound formula (I) 17α-(acetyloxy)-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione Crude.
[0012] In a 20L glass reactor, dissolve 1.44kg of oily matter with 2.9L (2.61kg) of ethyl acetate, heat to 45°C, slowly add 8.65L (5.88kg) of n-heptane dropwise at this temperature, crystals slowly precipitate out, add After that, cool to room temperature, then crystallize at 5-10°C for 16 hours, filter, and dry the filter cake at 40°C with blast air to obtain 780.11g of light yellow crystalline powder (single-step yield 64%, yield range: 60%- 65%). In a 10L glass reactor, heat 780.11g of light yellow crystals with 3.9L (3.08kg) of absolute ethanol to 65-70°C to dissolve. After adding, cool to room temperature, then crystallize at 5-10°C for 16 hours, filter, The filter cake was air-dried at 40° C. to obtain 665.21 g of light yellow crystalline powder (85% yield in one step), 43%-47%.
[0013] (Based on intermediate 2).
Embodiment 2
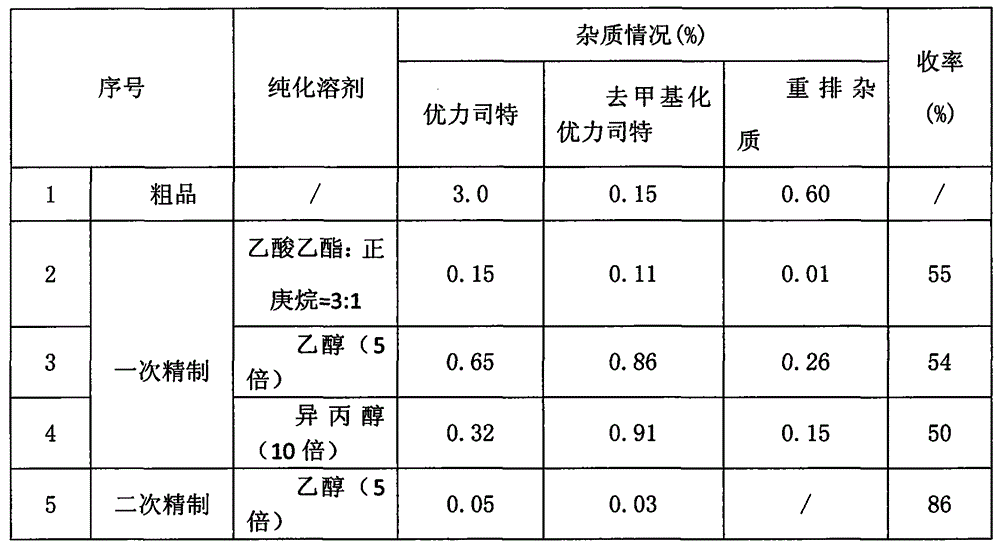
[0015] Most reports in the literature have been purified by column method, which is not suitable for industrial production. Based on the impurity distribution and solubility of the crude product, the solvents were screened, and the purification effects of different solvents are compared in Table 1.
[0016] Table 1 Comparison of purification effects of different solvents
[0017]
[0018] As can be seen from the above table, it can be seen that the impurity content of the product obtained by the refining method of the present invention (ethyl acetate: n-heptane=3: 1) is obviously lower than other refining methods, and the maximum single impurity can be less than 0.05% when secondary refining with ethanol , the total impurity is less than 1.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com