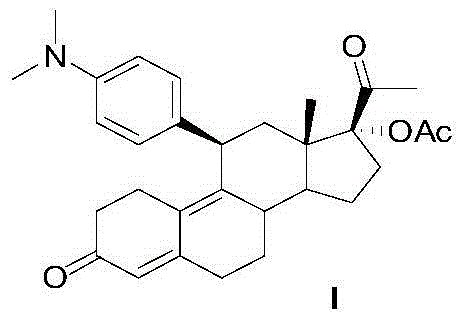
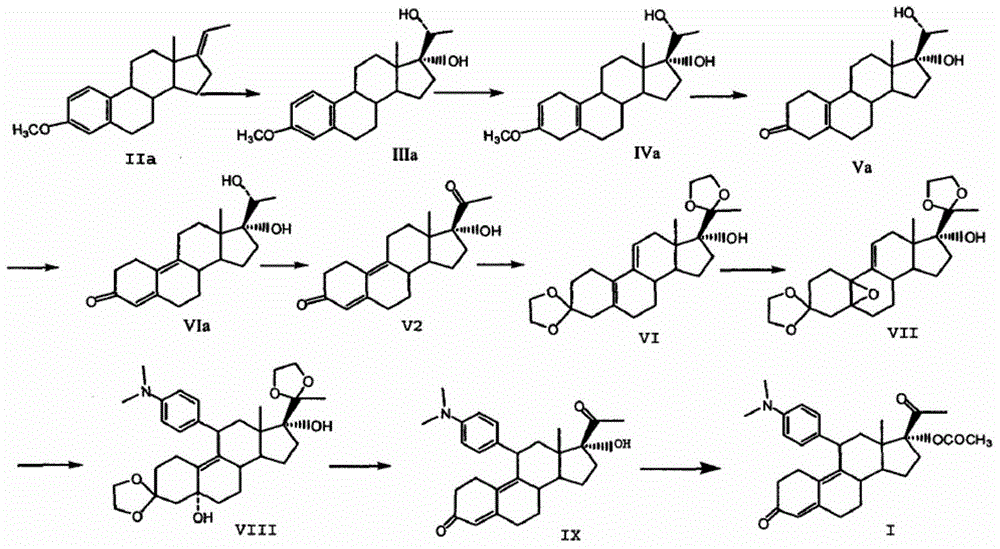
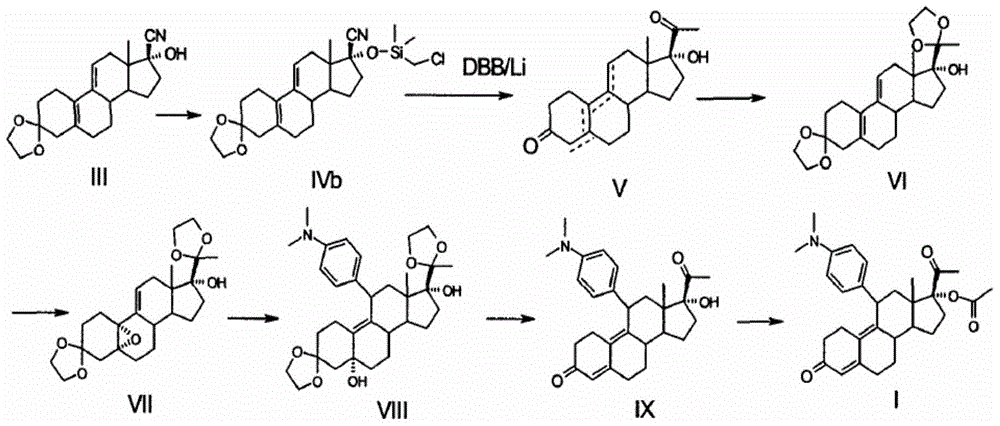
Preparation method of ulipristal acetate and intermediate thereof
A technology for ulipristal acetate and compounds, which is applied in the directions of steroids, organic chemistry, etc., can solve the problems of high cost, unsuitability for industrial production, dangerous large acetylene, etc., and achieves low cost, convenient for industrial scale production, The effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of 3-(ethylenedioxy)-17α-ethynyl-17β-hydroxyl-estra-5(10), 9(11) diene (compound III) mainly comprises the following steps:
[0055] Under nitrogen protection and 0°C, add 3-ketal (5.00Kg, 15.93mol) and anhydrous tetrahydrofuran (63L) into the reaction kettle, stir for 20 minutes, 3-ketal is completely dissolved, add sodium acetylene (1.61Kg , 33.45mol), stirred at -2 to 0°C for 6 hours, then added saturated ammonium chloride solution (30L) and stirred the reaction mixture for 30 minutes, separated the organic layer, and extracted the aqueous phase with tetrahydrofuran (4L×3) , combined organic layers;
[0056] The obtained organic layer was washed with saturated ammonium chloride solution (6L), the washed product was concentrated to 2.5L and then poured into ice water (16L), and the resulting mixture was stirred at -2 to 0°C for 3 hours, and then filtered into the The resulting precipitate was filtered and dried at 45°C to obtain 5.36 Kg (yield 99%, HP...
Embodiment 2
[0059] Preparation of 3-(ethylenedioxy)-17α-ethynyl-17β-hydroxy-estra-5(10),9(11) diene (compound III):
[0060] Under nitrogen protection and -2°C, add 3-ketal (5.00Kg, 15.93mol) and 2-methyltetrahydrofuran (50L) into the reaction kettle, stir for 30 minutes, 3-ketal is completely dissolved, and add potassium acetylide (2.05Kg, 31.86mol), stirred at -2 to 0°C for 8 hours, then added saturated ammonium chloride solution (30L) and stirred the reaction mixture for 40 minutes, separated the organic layer, and washed with 2-methyltetrahydrofuran (content 99%) after extracting the aqueous phase, combine the organic layers;
[0061] The obtained organic layer was washed with saturated ammonium chloride solution (5.5L), the washed product was concentrated to 2.2L and then poured into ice water (15.6L), the resulting mixture was stirred at -2 to 0°C for 3 hours, and then filtered The precipitate produced in the mixture was filtered and dried at 45° C. to obtain 5.33 Kg (yield 98%, HP...
Embodiment 3
[0064] Preparation of 3-(ethylenedioxy)-5α,10α-epoxy-17α-ethynyl-17β-hydroxy-19-norpregna-9(11)-ene (compound IV):
[0065] At 4°C, compound III (5.03Kg, 14.78mol) was dissolved in 50L of dichloromethane, and 1,1,1-trifluoroacetone (2.20L, 25mol), disodium hydrogen phosphate (8.75Kg, 62.5 mol) and 50% hydrogen peroxide (3.84L, 62.5mol), stirred and reacted at constant temperature for 18 hours, added 15L of saturated sodium sulfite solution, and continued to stir for 2 hours, separated the organic layer, and extracted with dichloromethane (5L×2) The organic layer in the water layer, each organic layer of gain is combined;
[0066] After the combined organic layer was washed with 20L saturated sodium chloride solution, the washed product was dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated to 3L, then 12L of isopropyl ether was added, stirred slowly for 15 hours and then filtered, and the filtered precipitate was heated at 50°C Drying under low te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com