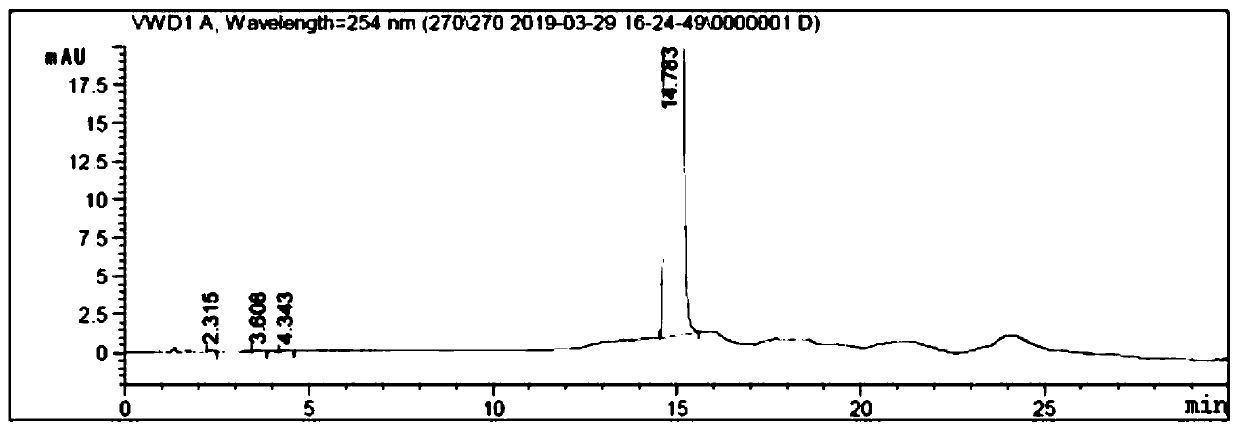
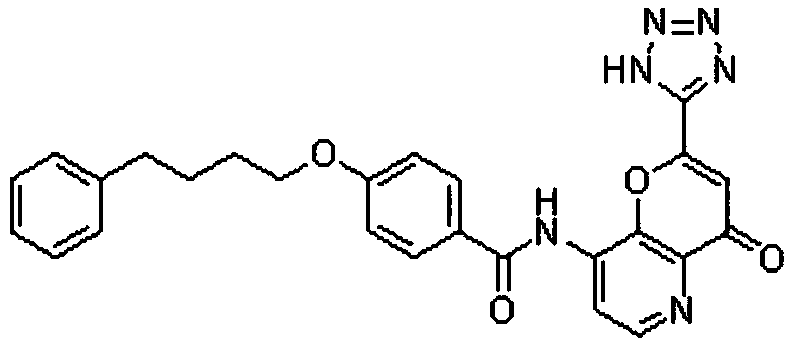
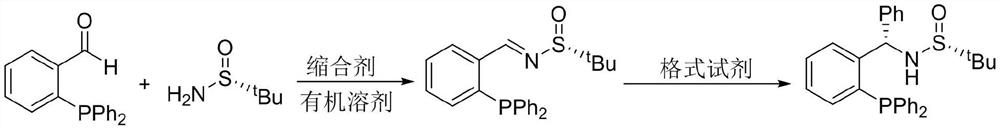
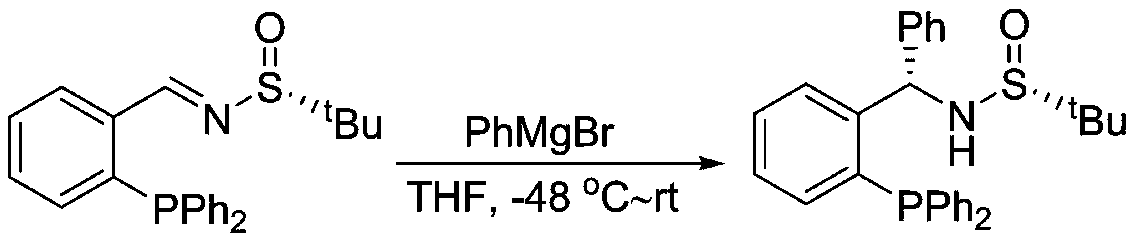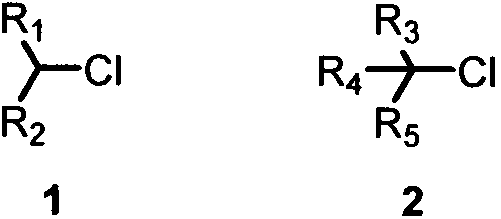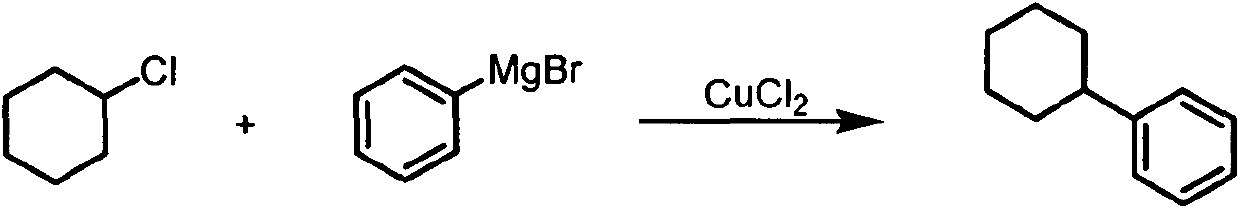Patents
Literature
30 results about "Phenylmagnesium bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenylmagnesium bromide, with the simplified formula C 6H 5MgBr, is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is often used as a synthetic equivalent for the phenyl "Ph⁻" synthon.
Preparation method for Sacubitril intermediate
InactiveCN105566194AHigh purityHigh yieldOrganic chemistry methodsPhenylmagnesium bromideMethyl methanesulfonate
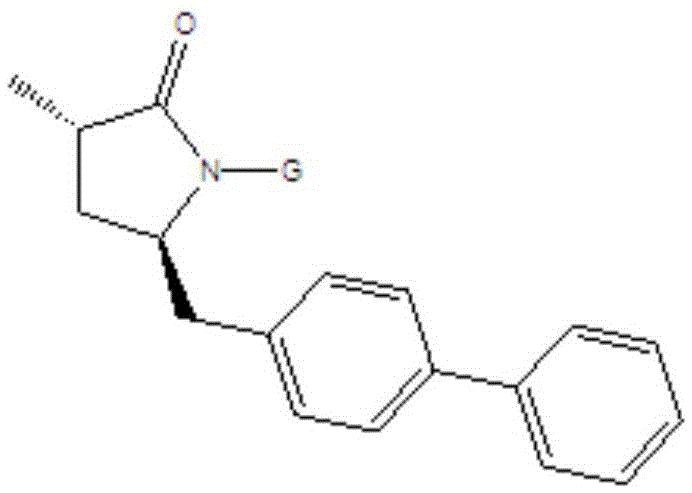
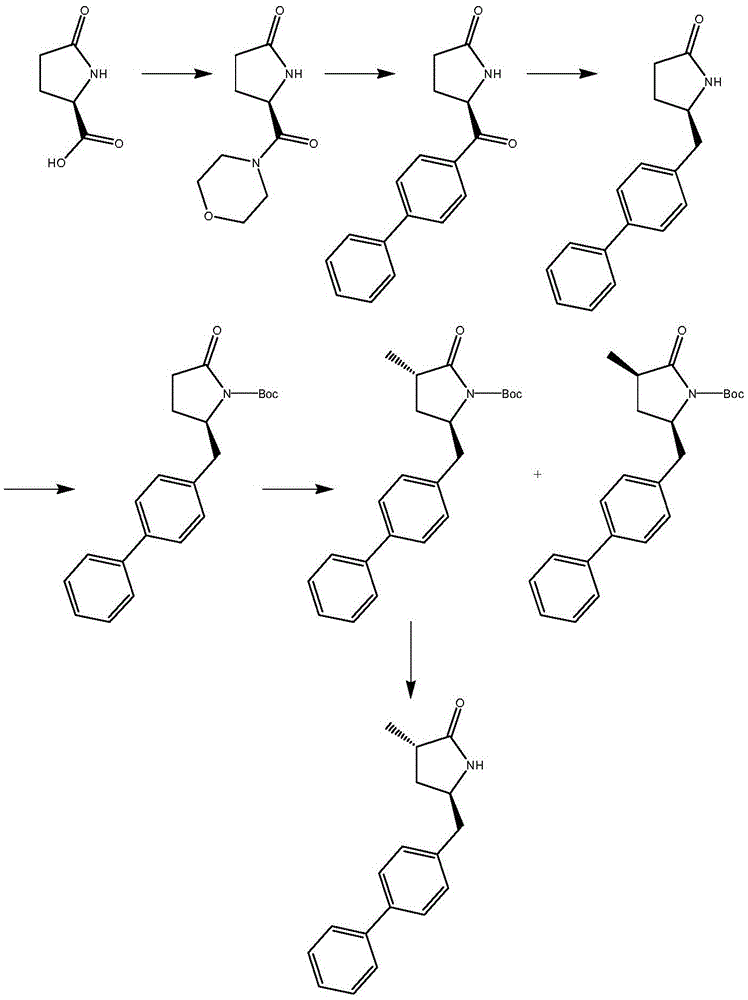
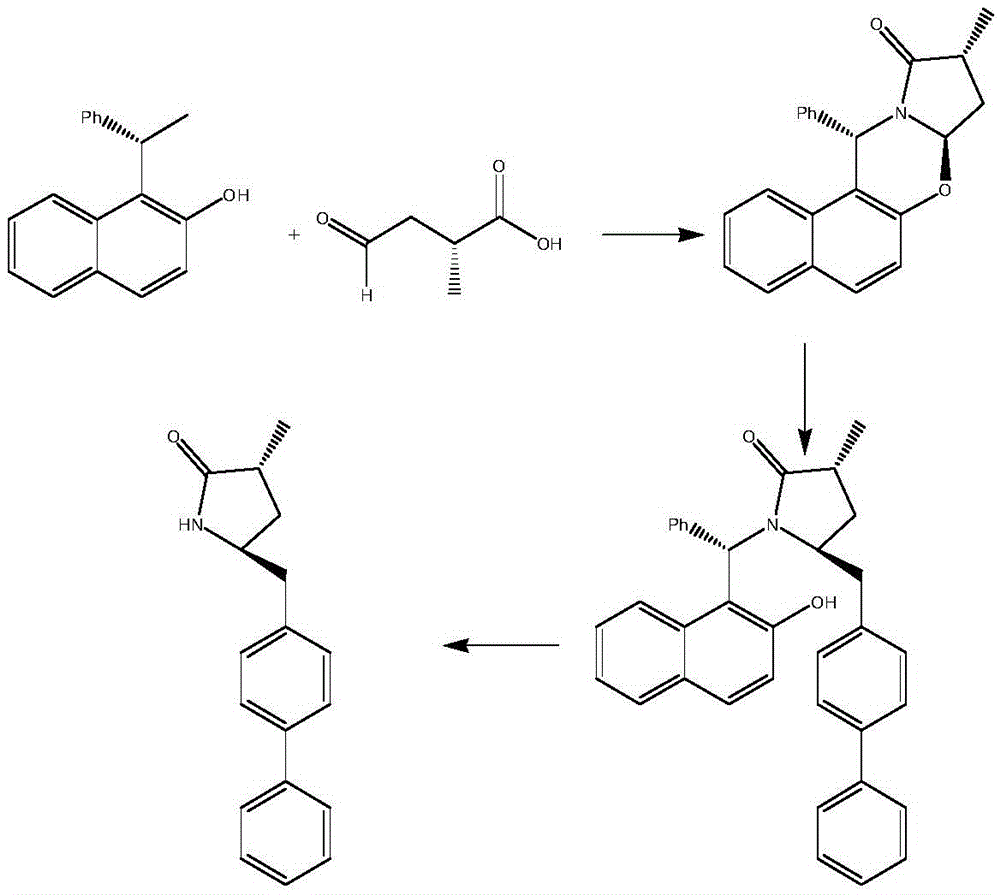
The invention relates to a preparation method for a Sacubitril intermediate. The preparation method comprises the following steps that (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone is esterified with toluene sulfochloride or methanesulfonyl chloride to obtain (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone; (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone is coupled with 4-diphenylmagnesium bromide or 4-diphenylmagnesium chloride to obtain (3R,5S)-3-methyl-5-(1,1'-diphenyl-4-yl-methyl)-2-pyrrolidinone. According to the preparation method, the method is novel, the raw materials are easy to obtain, the technology is simple, and the purity and yield of the product are both very high.
Owner:张伯引
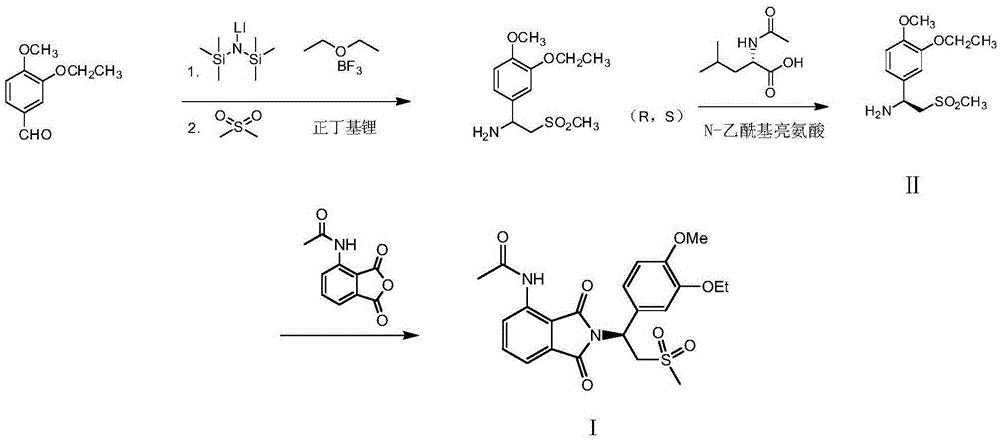
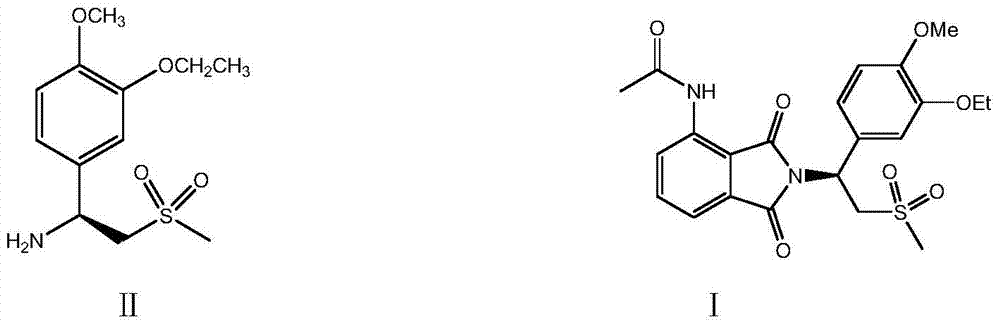
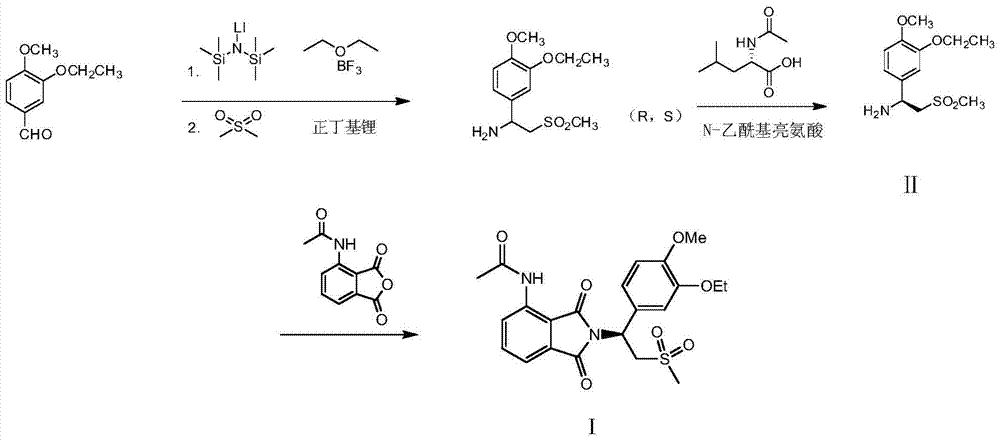
Preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine and preparation method of apremilast
ActiveCN105348172AHigh yieldEmission reductionOrganic chemistryOrganic compound preparationPhenylmagnesium bromideGrignard reaction
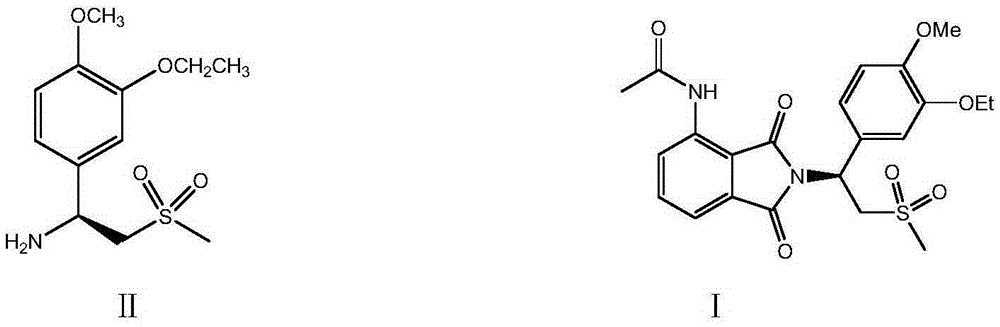
The invention relates to preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine and a preparation method of apremilast. 4-methoxy-3-ethoxy phenylmagnesium bromide is prepared from 4-methoxy-3-ethoxy bromobenzene through Grignard reaction; 4-methoxy-3-ethoxy phenylmagnesium bromide is in addition reaction with methylsulfonyl acetonitrile, and then hydrolysis and reduction are carried out, so that (R,S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine (III) is obtained; separation and filtering are carried out, so that (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine N-acetyl-L-leucine salt (IV) is obtained; through neutralization, (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine (II) is obtained; the compound (II) is subjected to imidization, so that apremilast (1) is obtained. The mother liquor obtained after separation is recycled and converted into a compound IV, so that discharge of waste liquid is reduced, environment friendliness is realized and the cost is reduced.
Owner:XINFA PHARMA
2-substituted rubrene compound and its synthesis method and application
InactiveCN1911904AEnhanced fluorescence emission intensityOrganic chemistryLuminescent compositionsQuantum efficiencyAcetic acid
The present invention is one kind of 2-substituted rubrene compound as shown and its synthesis process. The synthesis process includes the first synthesis of 2-formoxyl rubrene, and the subsequent oxidation, reduction and condensation with 2-formoxyl rubrene to obtain other 2-substituted rubrene compound. The synthesis process of 2-formoxyl rubrene under protection of inert gas includes the first reaction of 2-formoxyl -6, 11-diphenyl-5, 12-tetracene quinine material with glycol and catalyst at 120-160 deg.c; the subsequent reflux reaction with phenyl magnesium bromide and anhydrous tetrafuran; and final reflux reaction with iron powder and glacial acetic acid under heliophobic condition to obtain 2-formoxyl rubrene. The 2-substituted rubrene compound of the present invention as new type of red fluorescent material has high fluorescence quantum efficiency and may be used as the luminous material in electroluminescent device.
Owner:辽宁奥克华辉新材料有限公司 +1
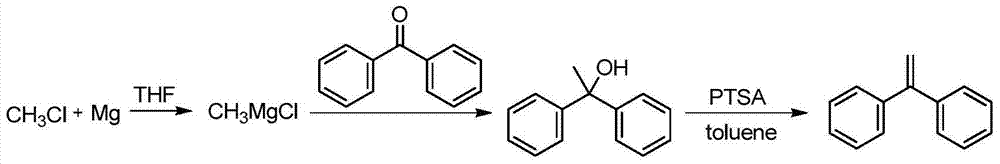
Preparation method of 1,1-diphenylethylene
ActiveCN103755516AModerate boiling pointStrong Lewis alkalineHydrocarbonsBulk chemical productionPhenylmagnesium bromideGrignard reagent
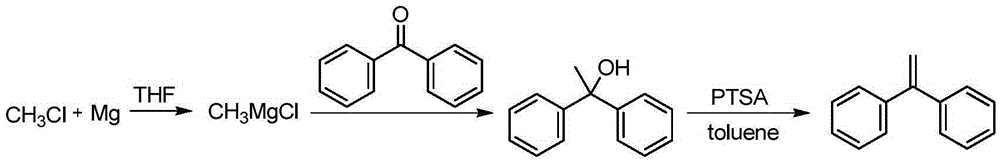
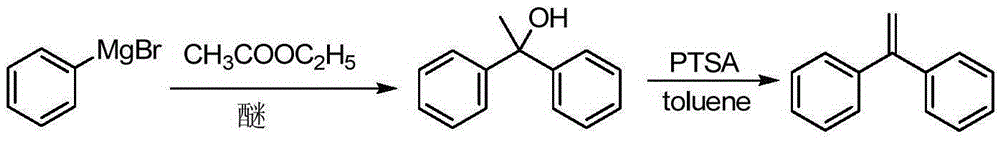
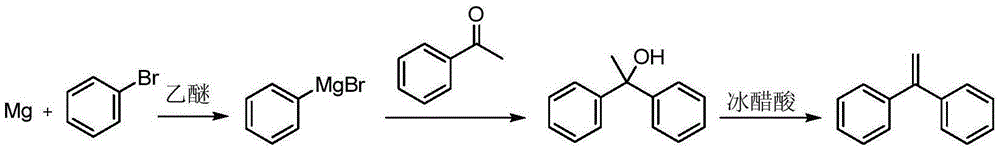
The invention discloses a preparation method of 1,1-diphenylethylene shown in a formula (V). The method comprises the steps of firstly carrying out reaction on bromobenzene and magnesium chips in anhydrous 2-methyltetrahydrofuran to obtain a phenylmagnesium bromide Grignard reagent, and then dripping acetophenone into the phenylmagnesium bromide Grignard reagent to react, so as to generate 1,1-diphenylethanol; finally, dewatering 1,1-diphenylethanol in the presence of a sulfoacid functional ionic liquid catalyst, so as to obtain 1,1-diphenylethylene shown in the formula (V). The preparation method disclosed by the invention is short in reaction step, mild in condition, simple and convenient to operate, high in product yield, low in production cost, friendly to environment, and applicable to industrial production.
Owner:ZHEJIANG UNIV OF TECH +1
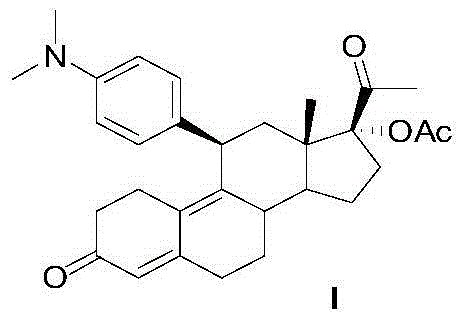
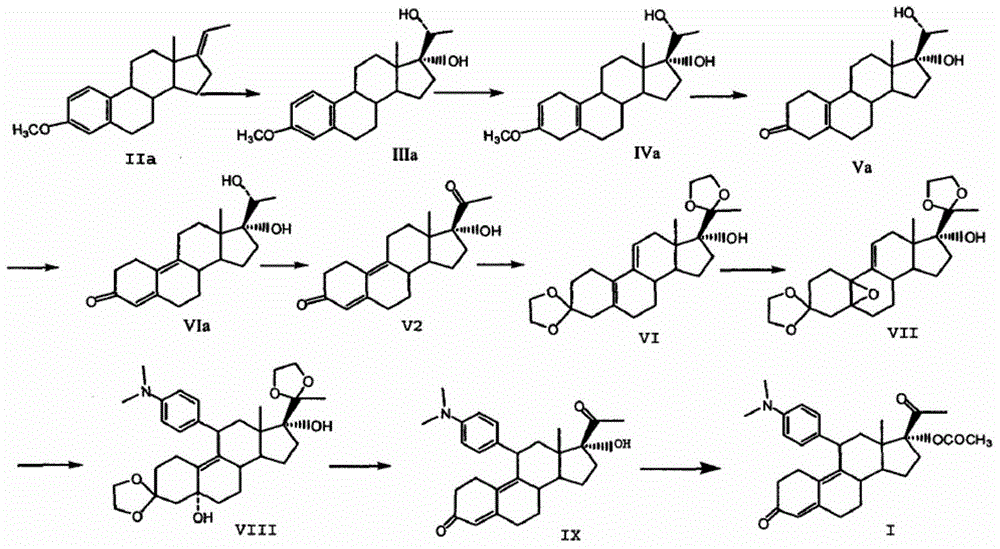
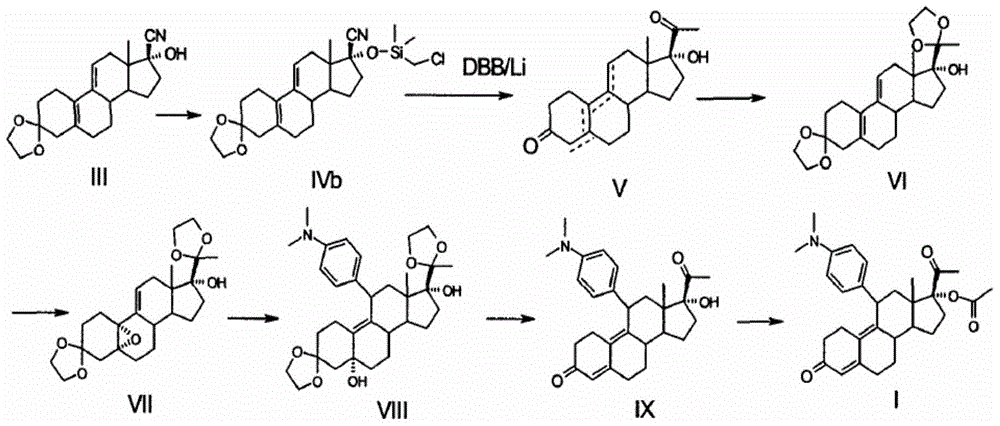
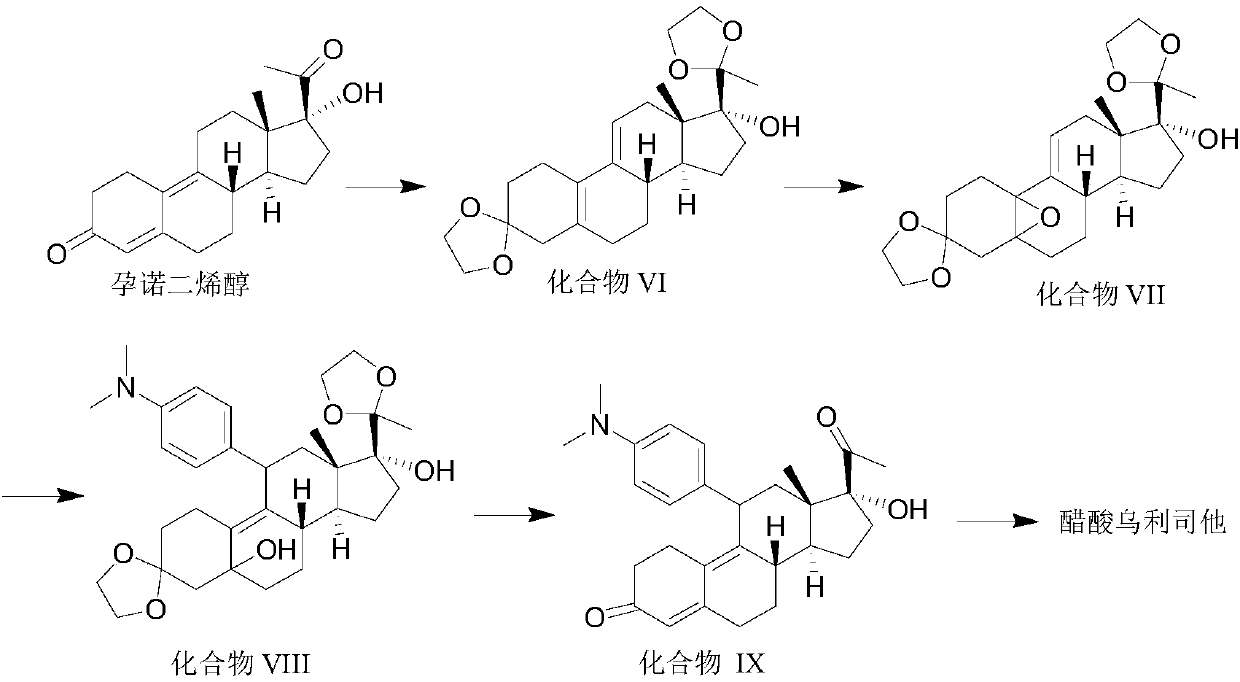
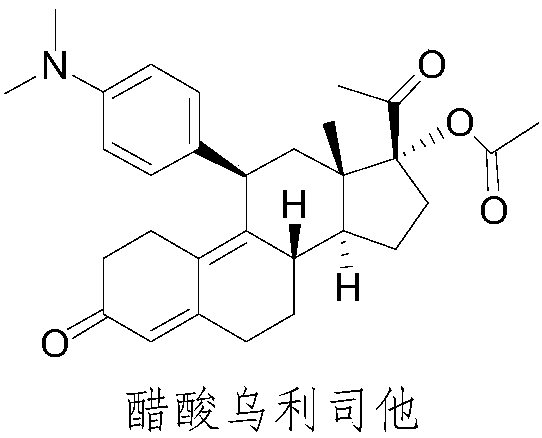
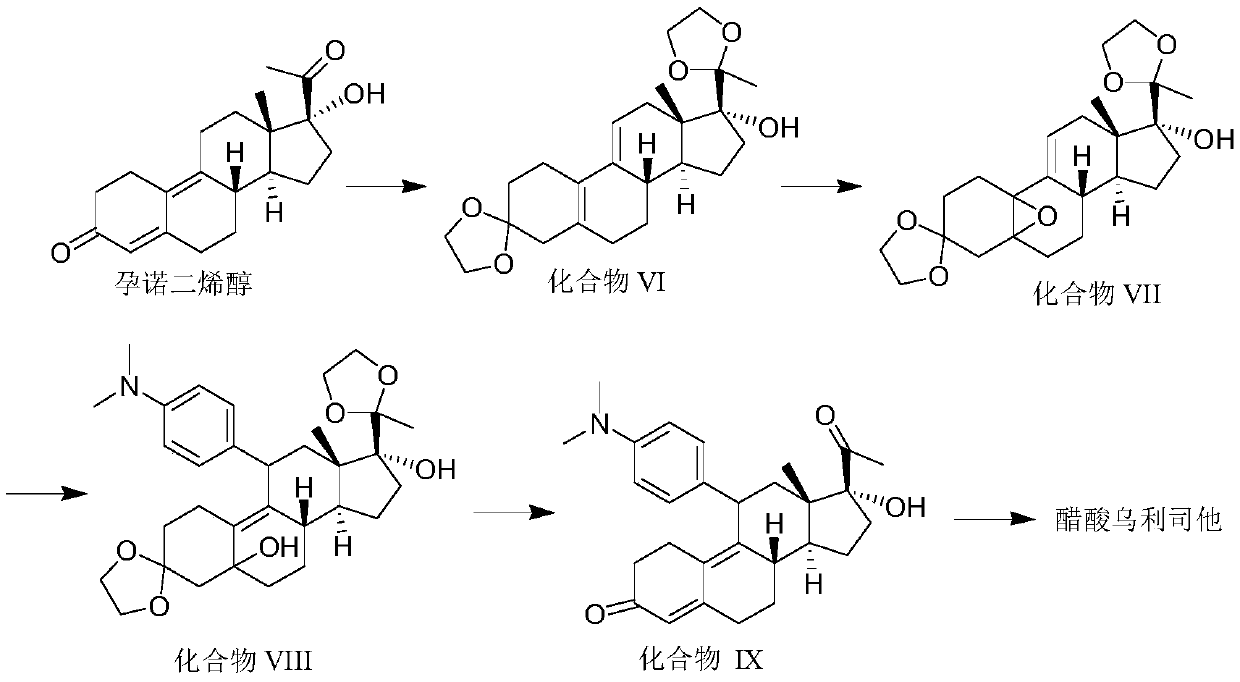
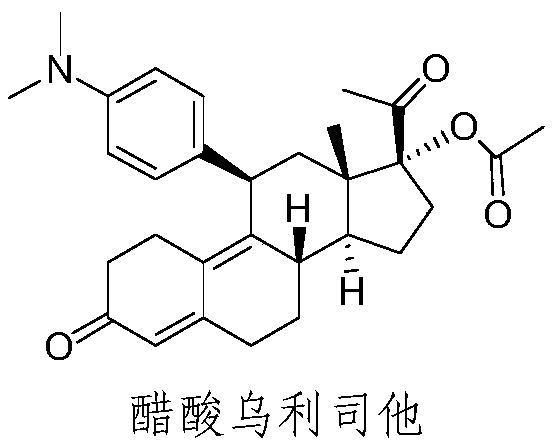
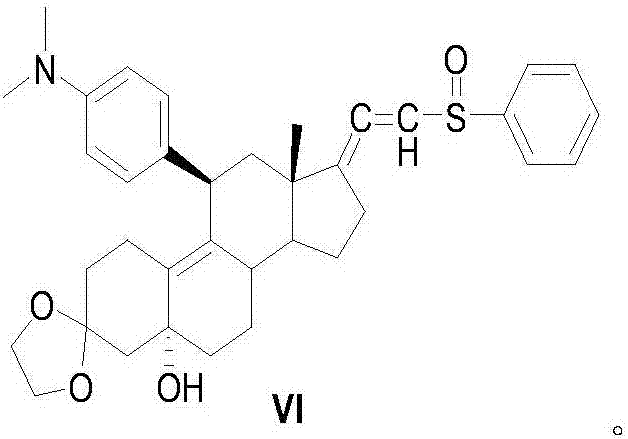
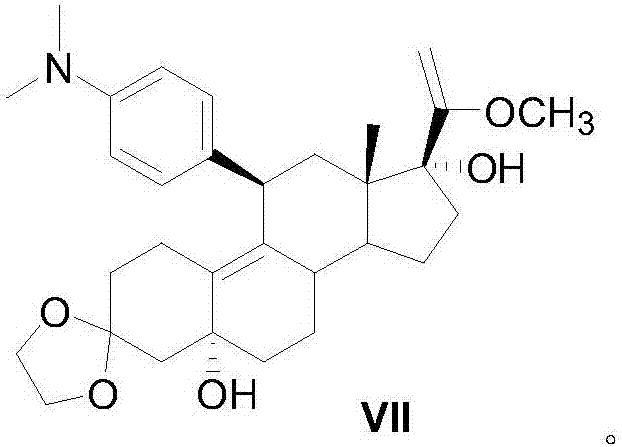
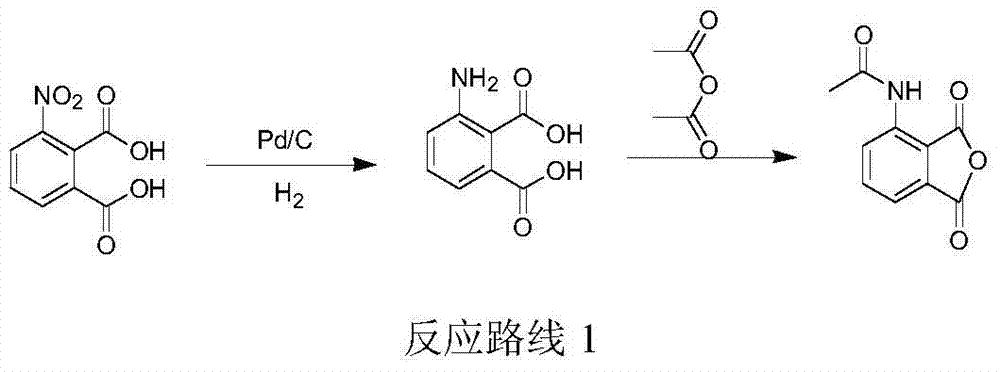
Preparation method of ulipristal acetate and intermediate thereof
The invention discloses a preparation method of ulipristal acetate and an intermediate thereof, and belongs to the field of pharmaceutical synthesis. The preparation method of the ulipristal acetate comprises the following steps: by taking 3,3-(ethylenedioxy)-19-methylestra-5(10),9(11)-diene-3,17-diketone as raw material, enabling the raw material to react with sodium acetylide or potassium acetylide to obtain a compound III, carrying out high-selectivity epoxidation by oxide to obtain a compound IV, subsequently enabling the compound IV to react with 4-(N,N-dimethyl amino) phenyl magnesium bromide Grignard reagent to obtain a compound V, then enabling the compound V to react with phenyl sulfonic acid chloride to obtain a compound VI, enabling the compound VI to respectively react with sodium methoxide and trimethyl phosphate to obtain a compound VII, hydrolyzing and removing a protection group to obtain a compound VIII, finally carrying out acetylation reaction to obtain the ulipristal acetate, wherein the reaction formulae are as shown in the description. The method is short in synthetic route, mild in reaction conditions, high in yield and purity of products, low in cost, stable and controllable in quality, and is suitable for industrial production.
Owner:CHENGDU ORGANOCHEM CO LTD
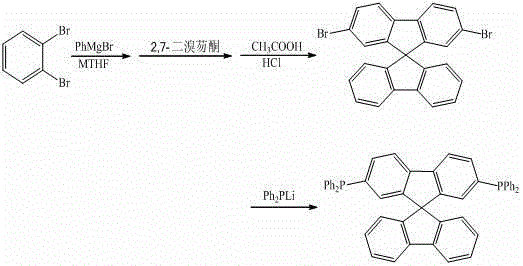
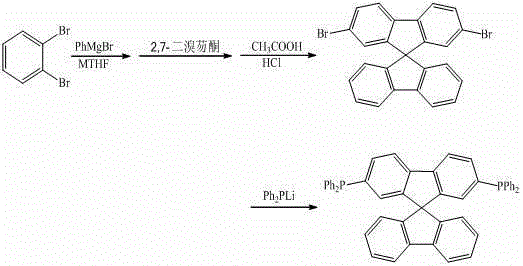
Synthesis method of 9,9'-spirobifluorene derivative
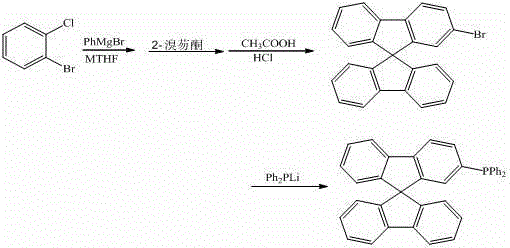
ActiveCN103333204AExcellent reaction timeGroup 5/15 element organic compoundsChemical synthesisLithium diphenylphosphide
The invention discloses a synthesis method of a 9,9'-spirobifluorene derivative, and belongs to the field of organic chemical synthesis. The method comprises the steps that 2-bromochlorobenzene as a raw material acts with phenylmagnesium bromide and then reacts with bromofluorenone at 50-100 DEG C in a methyltetrahydrofuran solvent, hydrolysis and filtration are performed, solid performs closed-loop synthesis under acid catalysis to produce bromo-9,9'-spirobifluorene, and bromo-9,9'-spirobifluorene reacts with lithium diphenylphosphide to synthesize the 9,9'-spirobifluorene diphenylphosphine derivative. The synthesis method is simple in process; raw materials are low in price and easy to obtain; the production cost of series products is obviously lowered during synthesis; and the application of the fluorene derivative in design and synthesis of organic photoelectric materials as an intermediate is extended.
Owner:PUYANG HUICHENG ELECTRONICS MATERIAL +1
Preparing method of 3-alkoxy phenylcarbinol
InactiveCN105085194ALow priceMild reaction conditionsMagnesium organic compoundsGroup 2/12 organic compounds without C-metal linkagesPhenylmagnesium bromideGrignard reagent
The invention discloses a preparing method of 3-alkoxy phenylcarbinol, comprising the steps: (1) dissolving 3-bromobenzene alkyl ether into a solvent, thus generating a Grignard reagent (3-alkoxy) phenyl magnesium bromide with Mg under the action of an initiator; (2) adding paraformaldehyde into the Grignard reagent at 50-85 DEG C to generate a substitution product; (3) performing acid hydrolyzing on the substitution product to obtain 3-alkoxy phenylcarbinol. Raw materials adopted in the invention are lower in price, the preparing method is mild in reaction conditions, only post-treatment of one time is required after three steps of reaction, and operation time is short; the purity of the obtained 3-alkoxy phenylcarbinol is high and completely accords with the requirements on use as a medicine intermediate.
Owner:JIANGXI RV PHARMA
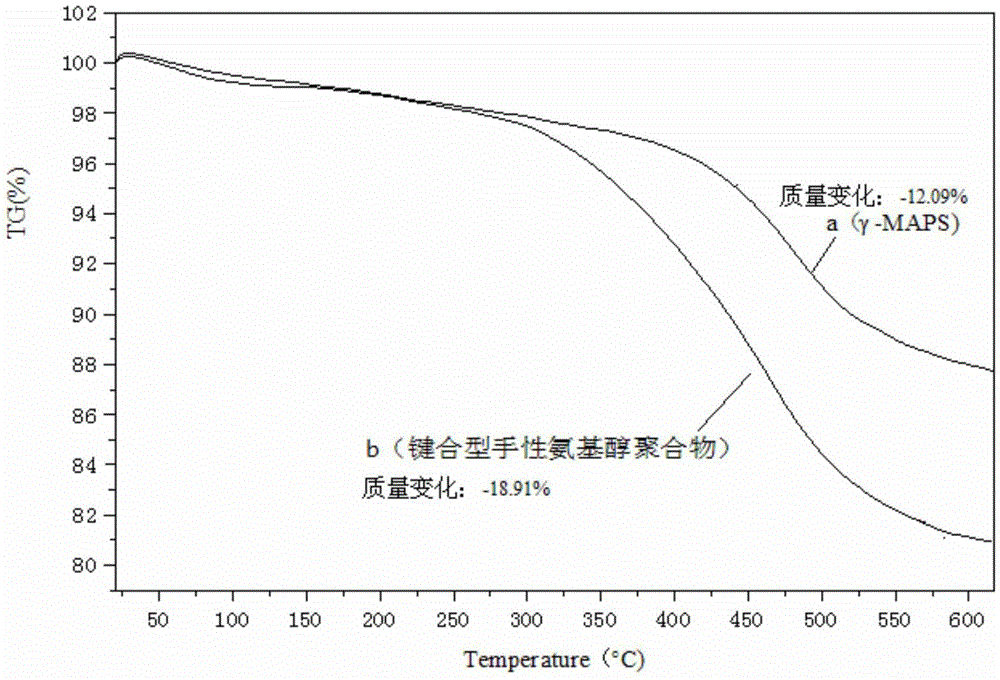
Bonded chiral amino alcohol polymer and preparation method and application thereof
InactiveCN105504159AAvoid churnGood physical and chemical stabilityOrganic compound preparationCarboxylic acid amides preparationPhenylmagnesium bromideDouble bond
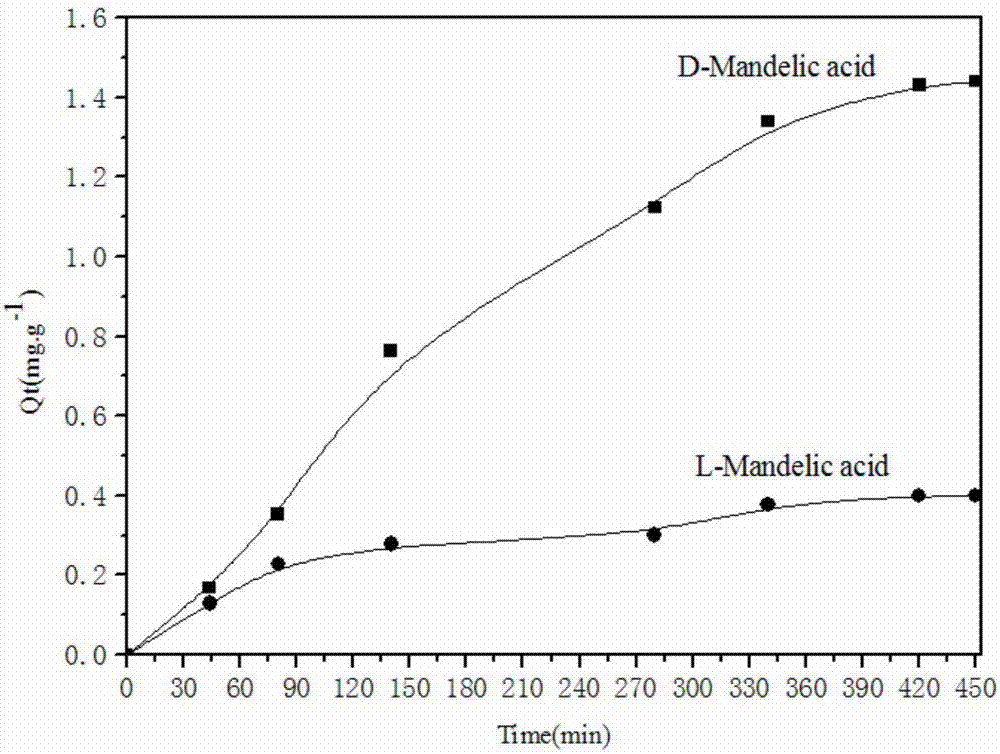
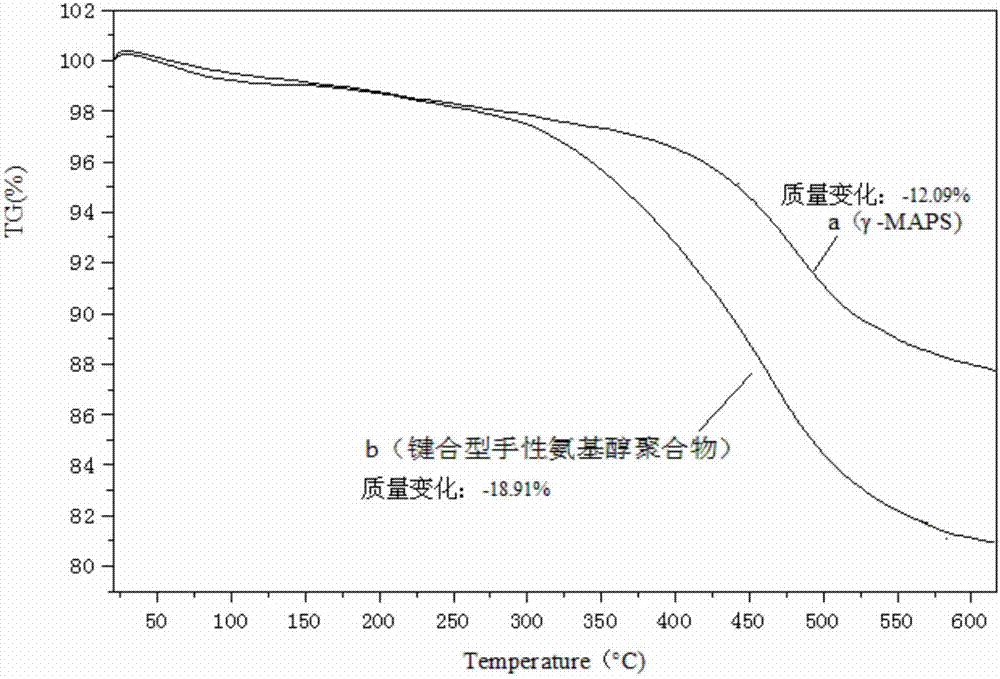
The invention discloses a bonded chiral amino alcohol polymer and a preparation method and application thereof. The bonded chiral amino alcohol polymer is composed of chiral amino acid derivative monomers on modified silica gel by virtue of amide group bonding. The preparation method comprises the following steps: taking L-amino acid as a raw material, and sequentially performing methyl esterification and phenylmagnesium bromide addition; introducing double bonds through acryloyl chloride, thereby obtaining a chiral monomer; polymerizing the chiral monomer and modified silica gel introduced with double bonds through free radicals, thereby obtaining the product. The process is simple in method, wide in raw material source and low in cost and is beneficial to industrial production; and moreover, the bonded chiral amino alcohol polymer has organic solvent resistance and high chiral resolution performance, can serve as a novel HPLC chiral stationary phase material applied to chiral compound identification or resolution and overcomes the defect that the existing chiral resolution material is limited by mobile phase.
Owner:CENT SOUTH UNIV
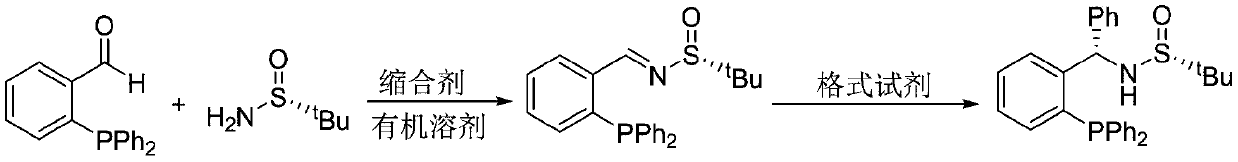
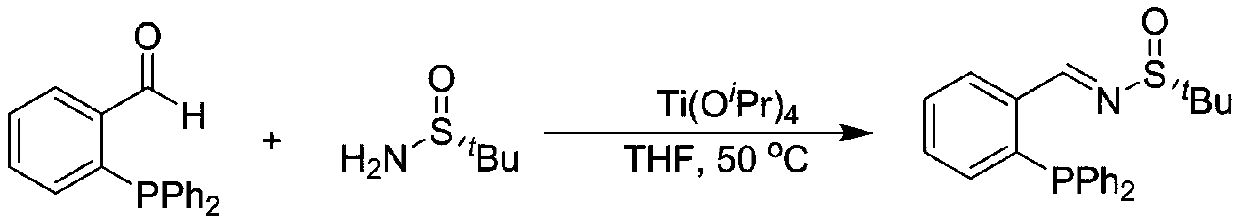
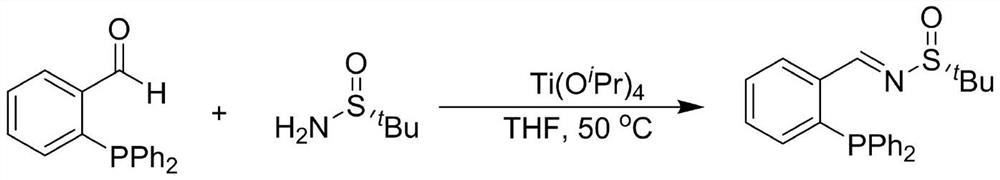
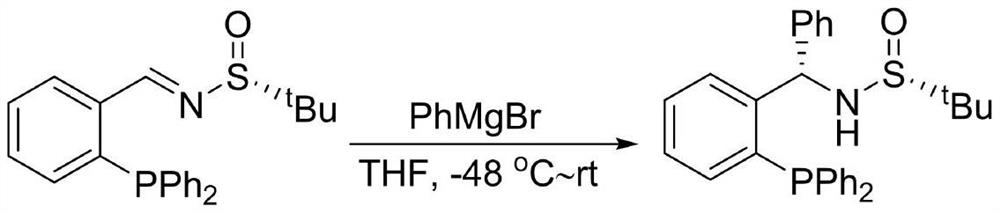
Large-scale preparation method of chiral sulfinamide monophosphine ligand
ActiveCN110615811AHigh selectivityHigh synthesis efficiencyGroup 5/15 element organic compoundsOrganic chemistry methodsImideBenzaldehyde
The invention discloses a large-scale preparation method of a chiral sulfinamide monophosphine ligand. The method includes condensing chiral sulfinamide and 2-(diphenylphosphino)benzaldehyde which areadopted as raw materials to obtain an intermediate that is chiral sulfinyl imide, and carrying out a nucleophilic addition reaction with a phenylmagnesium bromide reagent to generate the chiral sulfinamide monophosphine ligand. The intermediate imine product and a final product chiral sulfinamide monophosphine ligand are purified by adopting a crystallization method, and column chromatography isnot needed. The method has the characteristics of cheap and accessible raw materials, simple synthesis steps, large-scale synthesis and preparation, a specific three-dimensional structure of the product and the like. The method is mild in condition, and is of important significance for future process and commercialization development of chiral sulfinamide monophosphine ligand products.
Owner:EAST CHINA NORMAL UNIV
Method for synthesizing AHU377 calcium salt
InactiveCN108299226ALow costMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationPhenylmagnesium bromideTert-Butyloxycarbonyl protecting group
The invention discloses a method for synthesizing an AHU377 calcium salt. The method comprises the following steps: reacting 4-bromo-D-phenylalanine with thionyl chloride, reacting obtained methyl 4-bromo-D-phenylalaninate hydrochloride with BOC acid anhydride, reacting the obtained reaction product with phenylmagnesium bromide to obtain N-tert-butyloxycarbonyl-amino-4,4-biphenyl-R-alanine methylester, reacting the N-tert-butyloxycarbonyl-amino-4,4-biphenyl-R-alanine methyl ester with sodium borohydride, reacting the obtained reaction product with ethyl 2-(triphenylphosphoranylidene)propionate to obtain ethyl (4R)-5-[1,1'-biphenyl]-4-yl-4-[[tert-butoxycarbonyl]amino]-2-methyl-2-pentenoate, reacting the ethyl (4R)-5-[1,1'-biphenyl]-4-yl-4-[[tert-butoxycarbonyl]amino]-2-methyl-2-pentenoatewith lithium hydroxide, performing catalytic hydrogenation, reacting the obtained catalytic hydrogenation product with thionyl chloride to obtain ethyl (2R, 4S)-5- ([1,1-biphenyl)-4-amino-2-methylpentenoate hydrochloride, and stirring and reacting the ethyl (2R, 4S)-5- ([1,1-biphenyl)-4-amino-2-methylpentenoate hydrochloride, calcium chloride and succinic anhydride to obtain the target product.The method has the advantages of simple steps, mild reaction conditions, high purity and high yield.
Owner:NANJING REDWOOD FINE CHEM CO LTD
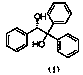
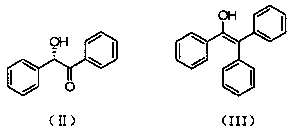
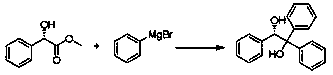
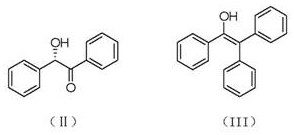
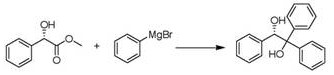
Preparation method of (S)-(-)-1,1,2-triphenyl-1,2-ethanediol
ActiveCN108250042AShort reaction timeImprove reaction efficiencyOrganic compound preparationOrganic chemistry methodsPhenylmagnesium bromideSolvent
The invention relates to a preparation method of (S)-(-)-1,1,2-triphenyl-1,2-ethanediol. The preparation method is characterized by comprising the following steps: respectively dissolving methyl mandelate and phenyl magnesium bromide in solvents to prepare a methyl mandelate solution and a phenyl magnesium bromide solution; feeding the prepared methyl mandelate solution and the prepared phenyl magnesium bromide solution into a first micro-channel reactor respectively through a metering pump so as to perform main reaction, and enabling the reaction solution obtained after the reaction to directly flow into a second micro-channel reactor; feeding an acidic aqueous solution into the second micro-channel reactor through a metering pump so as to perform quenching reaction while the reaction solution flows into the second micro-channel reactor, layering the reaction solution obtained after the quenching reaction, and taking an organic phase; drying the organic phase, and then, performing vacuum concentration; and performing recrystallization with toluene to obtain the finished product (S)-(-)-1,1,2-triphenyl-1,2-ethanediol. The preparation method provided by the invention has the advantages of high yield and fast reaction.
Owner:常州沃腾化工科技有限公司
Synthesis method of ulipristal acetate
The invention discloses a synthesis method of ulipristal acetate. The synthesis method comprises the following steps: 1), producing a compound VI from gestadienol under the action of ethylene glycol,p-toluenesulfonic acid and trimethyl orthoformate; 2), oxidizing the compound VI by using hydrogen peroxide to obtain a compound VII; 3), preforming a reaction on the compound VII and a 4-(N, N-dimethylamino) phenylmagnesium bromide Grignard reagent to obtain a compound VIII; 4), hydrolyzing a compound VIII to obtain a compound XI; 5), preforming a reaction on the compound XI, glacial acetic acidand acetic anhydride under the catalytic action of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 4-dimethylaminopyridine and iron perchlorate to obtain the ulipristal acetate. In the synthesis method, reaction conditions for obtaining the ulipristal acetate from ulipristal are relatively mild, demethylated ulipristal or ulipristal oxynitride is unlikely to produce, and the production process is more controllable.
Owner:广州市桐晖药业有限公司
Preparation method for p-phenylbutoxybenzoic acid
InactiveCN110357772AAvoid security issuesAvoid environmental pollutionOrganic compound preparationCarboxylic acid esters preparationSolubilityPhenylmagnesium bromide
The invention provides a preparation method for p-phenylbutoxybenzoic acid, belonging to the field of organic synthesis. According to the invention, palladium-based catalytic coupling is adopted, andthe Grignard reaction and the Friedel-Craft reaction are avoided, thereby avoiding the production of blue-green copper ion wastewater and generation of a large amount of acidic wastewater due to usageof aluminum trichloride; the preparation method of the invention is friendly to environment, simple in synthesis route and high in the yield of each step; and halogeno-benzene is used for replacing more expensive phenylmagnesium bromide and used as a starting material, so the preparation cost of p-phenylbutoxybenzoic acid is lowered. The p-phenylbutoxybenzoic acid obtained in the invention has good crystal form, high purity and good solubility. The data of embodiments of the invention show that the total yield of p-phenylbutoxybenzoic acid prepared in the invention is 60% or above, and the HPLC purity of p-phenylbutoxybenzoic acid is 99.9% or above.
Owner:HANGZHOU HUANGSEN BIOLOGICAL TECH CO LTD
One-pot synthesis method for substituted indane compounds
PendingCN112574011AMethod route shortShort routeGroup 4/14 element organic compoundsOrganic compound preparationChromatographic separationPhenylmagnesium bromide
The invention discloses one-pot synthesis method for a substituted indane compound. The method comprises the following steps: slowly and dropwise adding a mixed solution dissolved with far-end double-bond aryl halide and PEPPSI into a mixed solution of a phenylmagnesium bromide Grignard reagent and iodoisopropane, and stirring the reaction solution at a room temperature for 5-15 minutes; and quenching the reaction solution with a saturated NH4Cl solution, conducting extracting with ethyl acetate, washing the organic phase with saturated edible salt water, carrying out drying with anhydrous sodium sulfate, concentrating the organic phase, and carrying out column chromatography separation to obtain the substituted indane compound. According to the method, the transition metal salt PEPPSI andiodoisopropane in a catalytic system are commercial products; a catalytic reaction is conducted under mild conditions and is easy to operate; the related reaction can synthesize the target compound by using a one-pot process, so material resources and human resources can be further saved, and the social necessary labor time for synthesizing the target compound is shortened; and the cyclized coupling product synthesized by using the method has potential pharmaceutical activity and also has important reference value for the industry.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
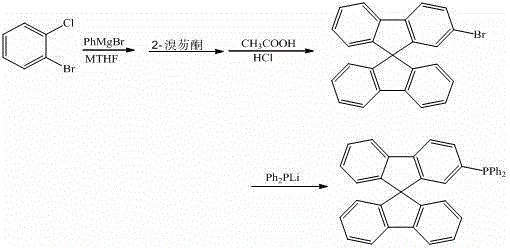
Synthetic method of a class of 9,9'-spirobifluorene derivatives
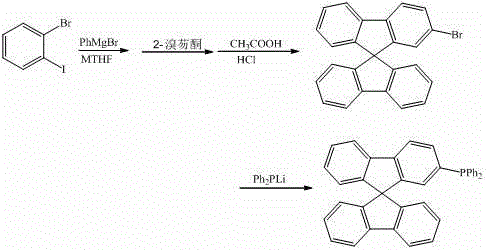
ActiveCN103333204BEasy to operateEasy to use raw materialsGroup 5/15 element organic compoundsChemical synthesisPhenylmagnesium bromide
The invention discloses a synthesis method of a class of 9,9'-spirobifluorene derivatives, which belongs to the field of organic chemical synthesis. Realized by the following method: under the protection of inert gas, use o-bromohalobenzene as raw material in methyl tetrahydrofuran solvent, react with phenylmagnesium bromide at 50-100°C, react with bromofluorenone, and then undergo hydrolysis, Filtration, the solid is closed under acid catalysis to synthesize bromo-9,9'-spirobifluorene; then bromo-9,9'-spirobifluorene reacts with diphenylphosphine lithium to synthesize 9,9'-spirobifluorene Phenylphosphine derivatives. The synthesis method of the invention has simple process, low price and easy availability of raw materials, significantly reduces the production cost of series products in the synthesis process, and expands the application of fluorene derivatives as intermediates in the design and synthesis of organic photoelectric materials.
Owner:PUYANG HUICHENG ELECTRONICS MATERIAL +1
A kind of bonded chiral aminoalcohol polymer and its preparation method and application
InactiveCN105504159BAvoid churnGood physical and chemical stabilityOrganic compound preparationCarboxylic acid amides preparationPhenylmagnesium bromideDouble bond
The invention discloses a bonded chiral amino alcohol polymer and a preparation method and application thereof. The bonded chiral amino alcohol polymer is composed of chiral amino acid derivative monomers on modified silica gel by virtue of amide group bonding. The preparation method comprises the following steps: taking L-amino acid as a raw material, and sequentially performing methyl esterification and phenylmagnesium bromide addition; introducing double bonds through acryloyl chloride, thereby obtaining a chiral monomer; polymerizing the chiral monomer and modified silica gel introduced with double bonds through free radicals, thereby obtaining the product. The process is simple in method, wide in raw material source and low in cost and is beneficial to industrial production; and moreover, the bonded chiral amino alcohol polymer has organic solvent resistance and high chiral resolution performance, can serve as a novel HPLC chiral stationary phase material applied to chiral compound identification or resolution and overcomes the defect that the existing chiral resolution material is limited by mobile phase.
Owner:CENT SOUTH UNIV
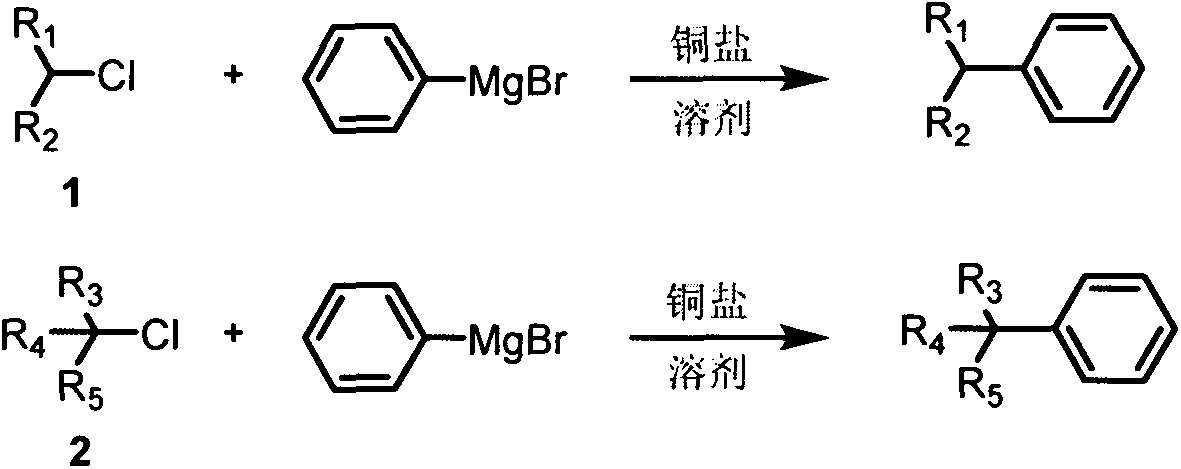
Cross-coupling method of alkyl chloride and phenyl magnesium bromide
InactiveCN107805181ACross-couplingHydrocarbonsOrganic substitutionNatural productPhenylmagnesium bromide
The invention provides a cross-coupling method of an alkyl chloride and phenyl magnesium bromide, wherein a copper salt is used as a catalyst, the 2-methyltetrahydrofuran solution of phenyl magnesiumbromide is used as a coupling reagent, and the corss-coupling of the inactive secondary / tertiary alkyl chloride and the phenyl magnesium bromide is achieved. According to the present invention, the method has the high yield, does not require the addition of the ligand, is simple and easy to perform, and has important significance in the synthesis of complex molecules such as natural products, chiral drugs, and the like.
Owner:JIANGSU MARINE RESOURCES DEV RES INST LIAN YUNGANG
A kind of synthetic method of ulipristal acetate
The invention discloses a synthesis method of ulipristal acetate. The synthesis method comprises the following steps: 1), producing a compound VI from gestadienol under the action of ethylene glycol,p-toluenesulfonic acid and trimethyl orthoformate; 2), oxidizing the compound VI by using hydrogen peroxide to obtain a compound VII; 3), preforming a reaction on the compound VII and a 4-(N, N-dimethylamino) phenylmagnesium bromide Grignard reagent to obtain a compound VIII; 4), hydrolyzing a compound VIII to obtain a compound XI; 5), preforming a reaction on the compound XI, glacial acetic acidand acetic anhydride under the catalytic action of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 4-dimethylaminopyridine and iron perchlorate to obtain the ulipristal acetate. In the synthesis method, reaction conditions for obtaining the ulipristal acetate from ulipristal are relatively mild, demethylated ulipristal or ulipristal oxynitride is unlikely to produce, and the production process is more controllable.
Owner:广州市桐晖药业有限公司
A kind of preparation method of 1,1-diphenylethylene
ActiveCN103755516BModerate boiling pointStrong Lewis alkalineHydrocarbonsBulk chemical productionPhenylmagnesium bromideGrignard reagent
The invention discloses a preparation method of 1,1-diphenylethylene shown in a formula (V). The method comprises the steps of firstly carrying out reaction on bromobenzene and magnesium chips in anhydrous 2-methyltetrahydrofuran to obtain a phenylmagnesium bromide Grignard reagent, and then dripping acetophenone into the phenylmagnesium bromide Grignard reagent to react, so as to generate 1,1-diphenylethanol; finally, dewatering 1,1-diphenylethanol in the presence of a sulfoacid functional ionic liquid catalyst, so as to obtain 1,1-diphenylethylene shown in the formula (V). The preparation method disclosed by the invention is short in reaction step, mild in condition, simple and convenient to operate, high in product yield, low in production cost, friendly to environment, and applicable to industrial production.
Owner:ZHEJIANG UNIV OF TECH +1
The preparation method of (1s,2r)-2-phenylcyclohexanol
ActiveCN111138243BHighlight substantiveHighlight substantive featuresOrganic compound preparationOrganic chemistry methodsPhenyl cyclohexanolPhenylmagnesium bromide
The invention provides a method for preparing (1S,2R)-2-phenylcyclohexanol, which specifically comprises the steps of mixing epoxycyclohexane solution, tetrahydrofuran solution of phenylmagnesium bromide, cuprous chloride or cuprous bromide Perform a mixed reaction for 1h to 3h, then quench with saturated ammonium chloride or saturated ammonium sulfate aqueous solution; collect the upper organic layer and conduct concentration, distillation, and recrystallization to obtain the racemic solution of chiral 2-phenylcyclohexanol The chiral 2-phenylcyclohexanol racemate is resolved to obtain (1S,2R)-2-phenylcyclohexanol. The method can make the ee value of the obtained product reach more than 98%, the process is simple, and the product has high purity.
Owner:ZHENGZHOU INST OF CHIRAL DRUGS RES CO LTD
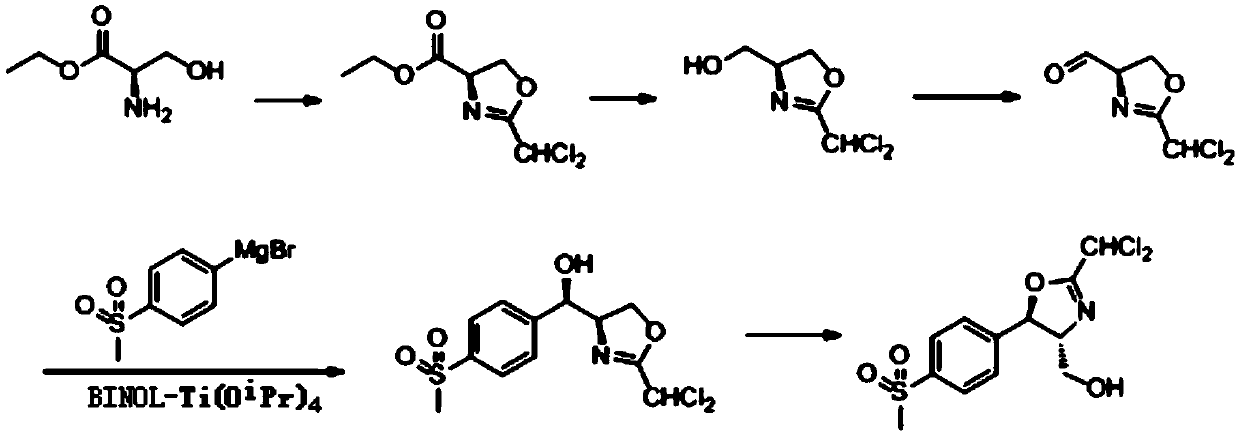
A kind of asymmetric preparation method of florfenicol intermediate cyclic compound
ActiveCN109678811BPreferably low toxicityEasy to follow upOrganic chemistryIsomerizationPtru catalyst
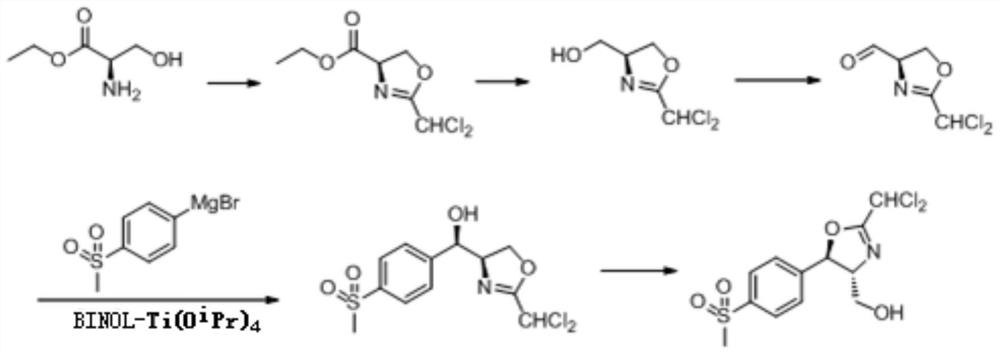
The invention discloses an asymmetric preparation method of a florfenicol intermediate cyclocompound. An optically pure cyclocompound is prepared by adopting D-ethyl serinate, dichloroacetonitrile, methylsulfonyl bromobenzene and the like as main raw materials, and implementing the five steps of cyclization, reduction, oxidation, asymmetric addition and isomerization reaction. The asymmetric addition reaction in the method has mild conditions; in an organic solvent and under the catalysis of a BINOL chiral ligand-Ti complex catalyst, an addition reaction between 4-methylsulfonyl phenylmagnesium bromide and (4R)-2-(dichloromethyl)-4,5-dihydro-4-oxazole formaldehyde is carried out to complete key steps, and the reaction yield and the selectivity are high.
Owner:HUBEI ZHONGMU ANDA PHARMACEUTICAL CO LTD
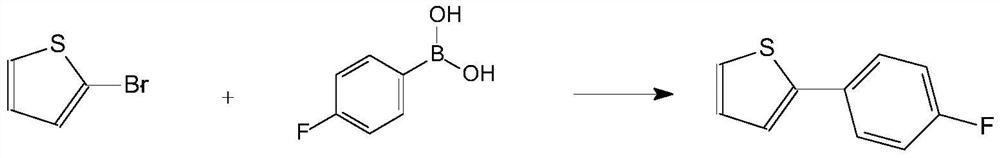
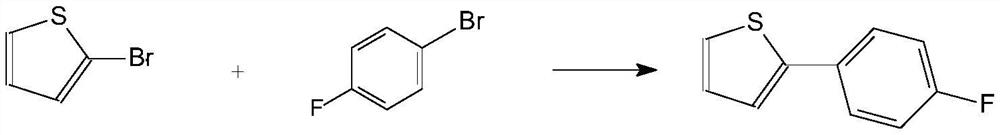
A kind of preparation method of high-purity 2-(4-fluorophenyl)thiophene
The invention relates to a preparation method of high-purity 2-(4-fluorophenyl)thiophene. The method comprises the following steps: performing a kumada coupling reaction between a Grignard reagent 4-fluorophenyl magnesium bromide and 2-bromo-thiophene under the action of a catalyst 1,2-bis(diphenylphosphine)ethane nickel chloride, to obtain high-purity 2-(4-fluorophenyl)thiophene. The preparationmethod provided by the invention has the following advantages: the particle size and appearance of the catalyst DPPE.NICl2 are adjusted, then the DPPE.NICl2 in a specific particle size range has a better catalysis effect, and the dosage of the catalyst is greatly reduced; moreover, the reaction efficiency is appropriately increased, and the nickel residue in the product is perfectly controlled; meanwhile, the impurity 2,2'-bithiophene in the system is inhibited quite well. The product quality is remarkably improved while the cost is lowered. The preparation method is more suitable for industrial production.
Owner:宁波人健化学制药有限公司
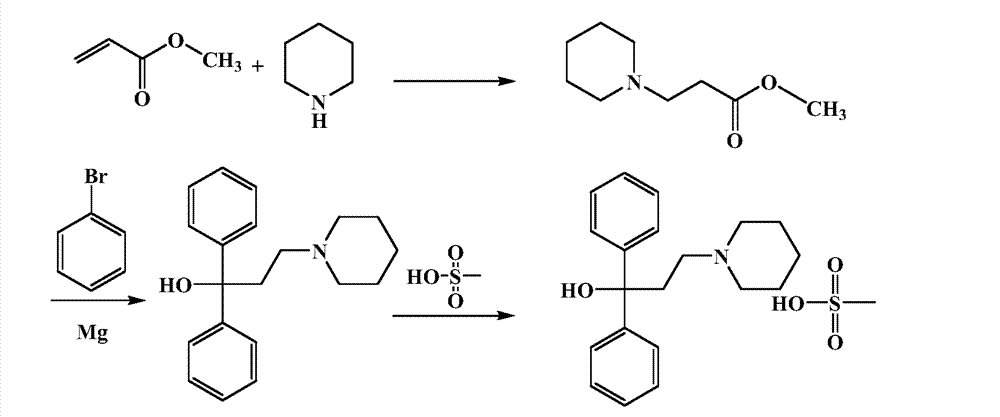
Preparation method of pridinol mesylate
InactiveCN104262290ALow costShort synthetic routeSulfonic acids salts preparationPropionatePhenylmagnesium bromide
The invention discloses a preparation method of pridinol mesylate. The preparation method comprises the following steps: carrying out reaction on methyl acrylate and piperidine to obtain methyl 3-(1-piperidyl) propionate, carrying out reaction on methyl 3-(1-piperidyl) propionate and phenyl magnesium bromide (as a Grignard reagent) to obtain pridinol, and forming a salt by pridinol and methanesulfonic acid in a solvent such as an ether solvent to obtain pridinol mesylate as a final product. The process route is simple in synthetic steps, is short in reaction route, is high in yield and is economical and practical.
Owner:HUBEI UNIV OF TECH
A large amount of preparation method of chiral sulfinamide monophosphine ligand
ActiveCN110615811BHigh selectivityHigh synthesis efficiencyOrganic chemistry methodsGroup 5/15 element organic compoundsPhenylmagnesium bromidePhenylphosphine
The invention discloses a mass preparation method of a chiral sulfinamide monophosphine ligand. The chiral sulfinamide and 2-phenylphosphine benzaldehyde are used as raw materials to condense to obtain an intermediate chiral sulfenimide. Then it undergoes nucleophilic addition reaction with phenylmagnesium bromide reagent to generate chiral sulfinamide monophosphine ligand. Both the intermediate imine product and the final chiral sulfinamide monophosphine ligand are purified by crystallization without column chromatography. The method has the characteristics of cheap and easy-to-obtain raw materials, simple synthesis steps, large-scale synthesis and preparation, and a product with a specific three-dimensional structure. The method has mild conditions and is of great significance for the future technological and commercial development of chiral sulfinamide monophosphine ligand products.
Owner:苏州凯若利新材料科技有限公司
Asymmetric preparation method of florfenicol intermediate cyclocompound
ActiveCN109678811APreferably low toxicityEasy to follow upOrganic chemistryIsomerizationOrganic solvent
The invention discloses an asymmetric preparation method of a florfenicol intermediate cyclocompound. An optically pure cyclocompound is prepared by adopting D-ethyl serinate, dichloroacetonitrile, methylsulfonyl bromobenzene and the like as main raw materials, and implementing the five steps of cyclization, reduction, oxidation, asymmetric addition and isomerization reaction. The asymmetric addition reaction in the method has mild conditions; in an organic solvent and under the catalysis of a BINOL chiral ligand-Ti complex catalyst, an addition reaction between 4-methylsulfonyl phenylmagnesium bromide and (4R)-2-(dichloromethyl)-4,5-dihydro-4-oxazole formaldehyde is carried out to complete key steps, and the reaction yield and the selectivity are high.
Owner:HUBEI ZHONGMU ANDA PHARMACEUTICAL CO LTD
A kind of preparation method of ulipristal acetate and its intermediate
The invention discloses a preparation method of ulipristal acetate and an intermediate thereof, and belongs to the field of pharmaceutical synthesis. The preparation method of the ulipristal acetate comprises the following steps: by taking 3,3-(ethylenedioxy)-19-methylestra-5(10),9(11)-diene-3,17-diketone as raw material, enabling the raw material to react with sodium acetylide or potassium acetylide to obtain a compound III, carrying out high-selectivity epoxidation by oxide to obtain a compound IV, subsequently enabling the compound IV to react with 4-(N,N-dimethyl amino) phenyl magnesium bromide Grignard reagent to obtain a compound V, then enabling the compound V to react with phenyl sulfonic acid chloride to obtain a compound VI, enabling the compound VI to respectively react with sodium methoxide and trimethyl phosphate to obtain a compound VII, hydrolyzing and removing a protection group to obtain a compound VIII, finally carrying out acetylation reaction to obtain the ulipristal acetate, wherein the reaction formulae are as shown in the description. The method is short in synthetic route, mild in reaction conditions, high in yield and purity of products, low in cost, stable and controllable in quality, and is suitable for industrial production.
Owner:CHENGDU ORGANOCHEM CO LTD
The preparation method of (s)-(-)-1,1,2-triphenyl-1,2-ethanediol
ActiveCN108250042BShort reaction timeImprove reaction efficiencyOrganic compound preparationOrganic chemistry methodsPhenylmagnesium bromidePhenylethylene glycol
The present invention relates to a preparation method of (S)-(-)-1,1,2-triphenyl-1,2-ethylene glycol, which is characterized in that methyl mandelate and phenylmagnesium bromide are respectively dissolved Make methyl mandelate solution and phenylmagnesium bromide solution after solvent; The methyl mandelate solution that makes and phenylmagnesium bromide solution are imported into the first microchannel reactor by a metering pump and carry out main reaction respectively, The reaction solution obtained after the reaction directly flows into the second microchannel reactor, and when the reaction solution flows into the second microchannel reactor, the acidic aqueous solution is input into the second microchannel reactor through a metering pump for quenching reaction, and the quenching reaction ends After obtaining the reaction solution, the reaction solution was separated into layers to take the organic phase, dried and concentrated under reduced pressure, and then recrystallized with toluene to obtain the finished product (S)-(-)-1,1,2-triphenyl-1 ,2‑ethylene glycol. The invention has the advantages of high yield and rapid reaction.
Owner:常州沃腾化工科技有限公司
Novel method for preparing penehyclidine hydrochloride
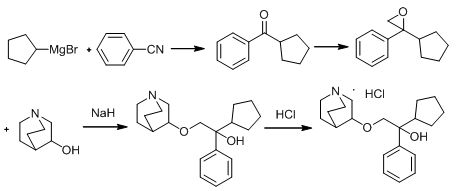
PendingCN114805338AEmission reductionSimple reaction conditionsOrganic chemistryMeth-Phenylmagnesium bromide
The invention discloses a novel method for preparing penehyclidine hydrochloride, which comprises the following steps: taking quinuclidin-3-ol as a raw material, carrying out chloromethylation on the quinuclidin-3-ol, trioxymethylene and hydrochloric acid to obtain an intermediate 1, preparing the intermediate 1 into a Grignard reagent to obtain an intermediate 2, carrying out addition reaction on the intermediate 2 and N-methoxyl-N-methylcyclopentanecarboxamide to obtain an intermediate 3, and preparing the intermediate 3 into the penehyclidine hydrochloride. And reacting the intermediate 3 with a phenylmagnesium bromide Grignard reagent to obtain penehyclidine, and salifying the penehyclidine through hydrochloric acid to obtain the penehyclidine hydrochloride. According to the method, the reaction conditions are simple, the steps 2-4 are a one-pot shrinkage reaction, the operation is simple and easy to implement, a large amount of operations such as distillation and crystallization are omitted, and the emission of volatile organic gases (VOCs) and organic waste liquid is greatly reduced; the preparation raw materials are cheap and easy to obtain, the production cost is low, and industrial production prospects are achieved.
Owner:南京亿华药业有限公司
Preparation of (s)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonylethylamine and preparation method of apremilast
ActiveCN105348172BProcess environmental protectionLow costOrganic chemistryOrganic compound preparationPhenylmagnesium bromideGrignard reaction
The invention relates to preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine and a preparation method of apremilast. 4-methoxy-3-ethoxy phenylmagnesium bromide is prepared from 4-methoxy-3-ethoxy bromobenzene through Grignard reaction; 4-methoxy-3-ethoxy phenylmagnesium bromide is in addition reaction with methylsulfonyl acetonitrile, and then hydrolysis and reduction are carried out, so that (R,S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine (III) is obtained; separation and filtering are carried out, so that (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine N-acetyl-L-leucine salt (IV) is obtained; through neutralization, (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine (II) is obtained; the compound (II) is subjected to imidization, so that apremilast (1) is obtained. The mother liquor obtained after separation is recycled and converted into a compound IV, so that discharge of waste liquid is reduced, environment friendliness is realized and the cost is reduced.
Owner:XINFA PHARMA
Synthesis method of benzylisofuryl alcohol
The invention relates to a synthesis technology of an insecticidal pesticide intermediate, and in particular relates to a synthesis method of a benzfuryl alcohol compound. The preparation method is characterized by comprising the following steps: (1) deprotonating acetone at the temperature of-50 to-70 DEG C, and reacting the deprotonated acetone with oxetanone to obtain a 3-oxetanol derivative; (2) carrying out ring-opening reaction on the product obtained in the step (1) under the action of acid to obtain 2-methyl-3-furanol; (3) protecting hydroxyl in the product obtained in the step (2) by using chlorosilane; (4) brominating methyl in the product obtained in the step (3); (5) carrying out substitution reaction on the product obtained in the step (4) and phenyl lithium or phenyl magnesium bromide to obtain a benzfuryl silyl ether compound; and (6) carrying out silicon-based deprotection on the benzfuryl silyl ether obtained in the step (5) by using fluoride to obtain the benzfuryl alcohol compound. The synthesis method of the benzyl furofuryl alcohol is convenient to operate, the raw materials are simple and easy to obtain, and the overall yield is obviously improved compared with the prior art.
Owner:YANCHENG TEACHERS UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com