2-substituted rubrene compound and its synthesis method and application
A synthesis method and compound technology, applied in the field of 2-substituted rubrene compounds and their synthesis, can solve the problems of inactive properties and low effects, and achieve the effect of broadening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
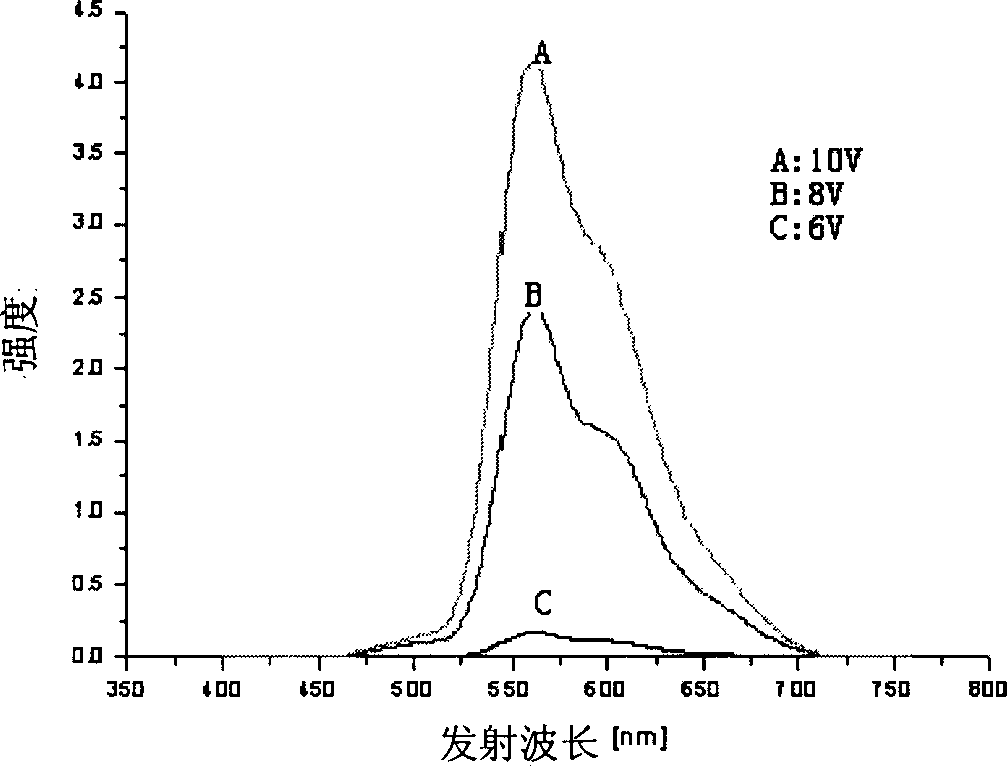
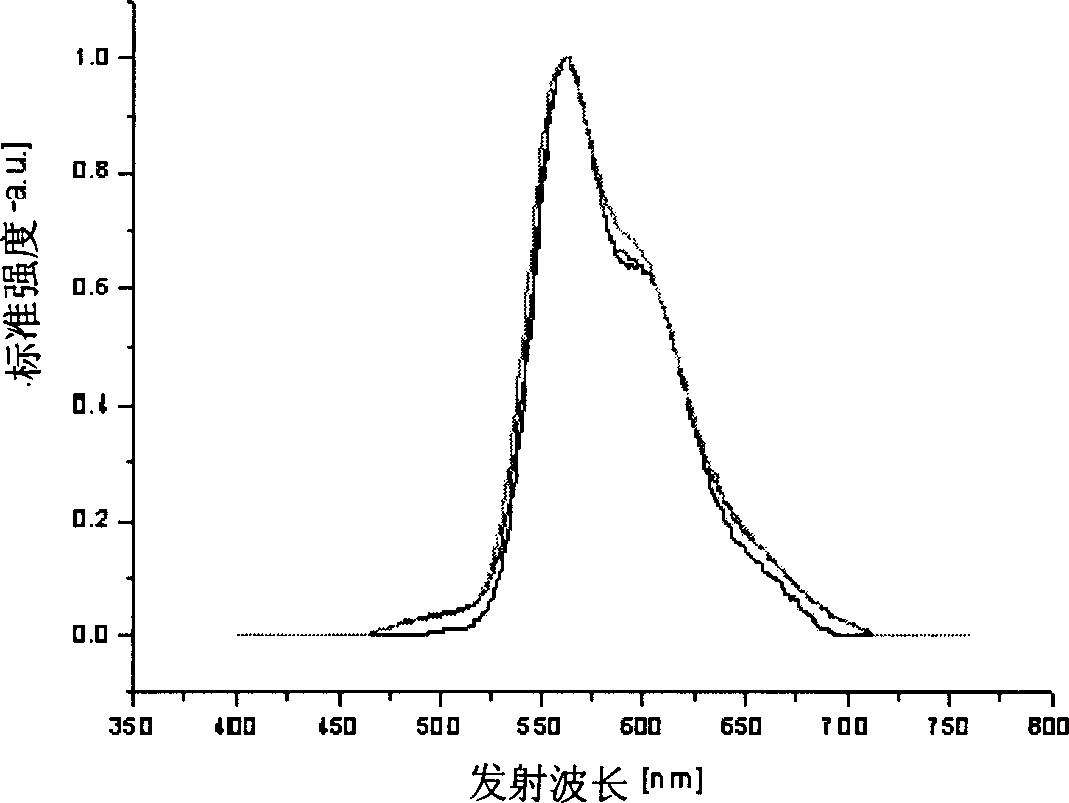
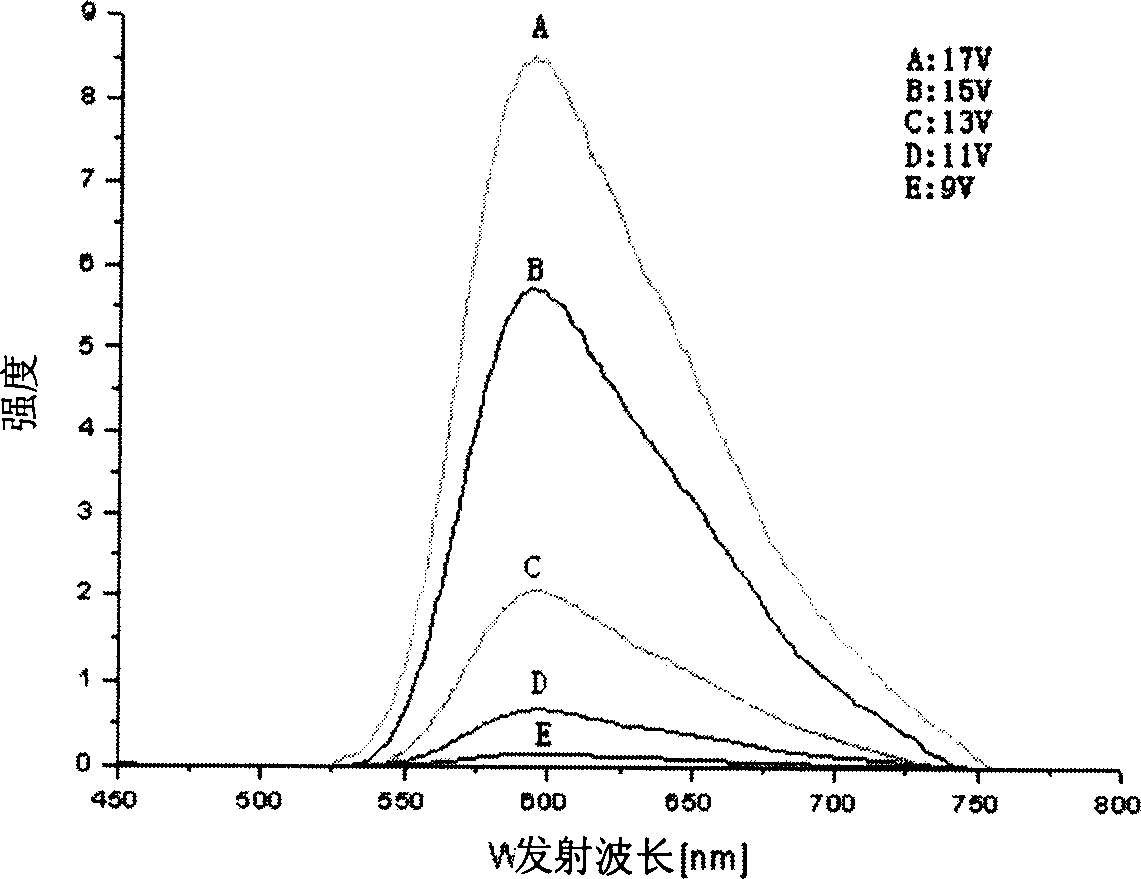
Image
Examples
Embodiment 1
[0038] Example 1: Synthesis of 2-formylrubrene (2-formyl-5,6,11,12-tetraphenyltetracene)
[0039] (1) Synthesis of intermediate 2-(1',3'-dioxolane)-6,11-diphenyl-5,12-tetracenequinone
[0040]
[0041] Add 0.964 g (2.00 mmol) of 2-formyl-6,11-diphenyl-5,12-tetraphenoquinone in a 100 ml two-necked flask, 60 ml of ethylene glycol, 28.9 mg of p-toluenesulfonic acid , heated to 160° C. for about 8 hours under the protection of nitrogen. During this period, HPLC (methanol: water = 9: 1) and TLC (n-hexane: ethyl acetate = 8: 3) were used to track the entire reaction process, and the reaction was stopped when the raw materials were basically consumed. The reaction solution was poured into an appropriate amount of saturated aqueous sodium bicarbonate solution and then filtered, and the filtered product was separated by column chromatography (silica gel 500-800 mesh, eluent was n-hexane:ethyl acetate=30:1), 2-(1',3'-dioxolane)-6,11-diphenyl-5,12-tetraphenoquinone was obtained as a...
Embodiment 2
[0045] Embodiment 2: the synthesis of 2-hydroxymethyl rubrene
[0046]
[0047] In a dark room, 56.1 mg (0.1 mmol) of 2-formylrubrene and 11.4 mg (0.3 mmol) of NaBH 4 Add in the mortar, mix thoroughly and grind, grind for about 30 min, add 10 ml of water to dissolve excess sodium borohydride, extract the product with 20 ml of chloroform, evaporate the solvent, and then process with the solvent method to obtain the target product (51.6 mg, the yield is 91.9%), and the melting point is greater than 300°C. IR (KBr, cm -1 ): 1020, 2928, 2956, 3070, 3620; mass spectrometry (MS - )562.0 (product molecular weight is 562.7); 1 H NMR (500MHz, CD 2 Cl 2 ): δ (ppm): 7.32-7.34 (m, 2H), 7.25 (s, 1H), 7.04-7.11 (m, 16H), 6.85-6.86 (m, 8H), 4.51-4.53 (m, 2H); Product liquid ultraviolet maximum absorption wavelength is 303.35, 462.16, 492.16, 526.35nm respectively (solvent is CH 2 Cl 2 ); the maximum emission wavelength of liquid fluorescence is 561.68nm (the solvent is CH 2 Cl 2...
Embodiment 3
[0048] Embodiment 3: the synthesis of N-(2'-methylene rubrenyl) aniline
[0049]
[0050] Add 56.1 mg (0.1 mmol) of 2-formylrubrene to a 20-milliliter two-port reactor wrapped in tin foil and vacuumize the dry and closed reaction system with an oil pump for 2 hours, during which four weeks are replaced with nitrogen. Once, add 9.2 microliters (0.1 mmol) of aniline with a micro-syringe, then add 5 milliliters of tetrahydrofuran that has just undergone anhydrous treatment with a syringe, and heat to reflux for 4 hours, during which HPLC (mobile phase is pure methanol) and TLC (n-hexane Alkane:ethyl acetate=10:1) followed the whole reaction process, and stopped the reaction when the relative peak of the target product reached the highest. The reaction solution was evaporated to dryness, and then treated by solvent method to obtain the target product (52.3 mg, yield 82.2%). The melting point is greater than 300°C. IR (KBr, cm-1): 1380, 1616; HRMS (EI): M + , found 636.2609.C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com