Fluorescent probe for detecting viscosity, preparation method and application thereof
A fluorescent probe and viscosity technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of limited penetration depth, unfavorable biological imaging, etc., and achieve improved detection sensitivity and large Stokes shift changes , the effect of rapid detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
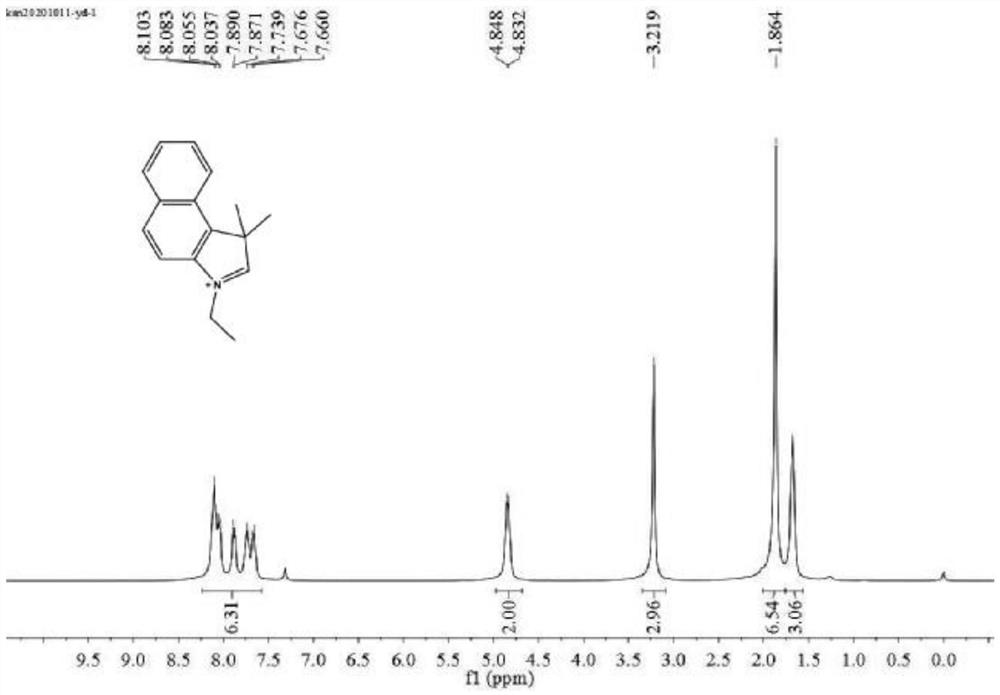
[0046] Synthesis of 3-ethyl-1,1,2-trimethyl-1H-benzo(E)indole
[0047] Weigh 1g (4.8mmol) of 1,1,2-trimethyl-1H-benzo(E)indole, add 463μL (5.8mmol, 1.2equiv) of ethyl iodide in 10mL of anhydrous acetonitrile, 85 Reflux at °C for 12 hours, cool to room temperature after the reaction, filter with suction and wash with anhydrous ether to obtain compound 1, compound 1 is 0.95 g of lavender solid, yield 83.2%. Compound 1 is 3-ethyl-1,1,2-trimethyl-1H-benzo(E)indole.
[0048] see figure 1, is the H NMR spectrum of compound 1 in the preparation method of the fluorescent probe. The results of NMR test are as follows: 1H NMR (400MHz, CDCl3) δ8.24–7.57 (m, 6H), 4.84 (d, J = 6.2Hz, 2H), 3.22 (s, 3H), 1.86 (s, 7H), 1.68(s,3H).
Embodiment 2
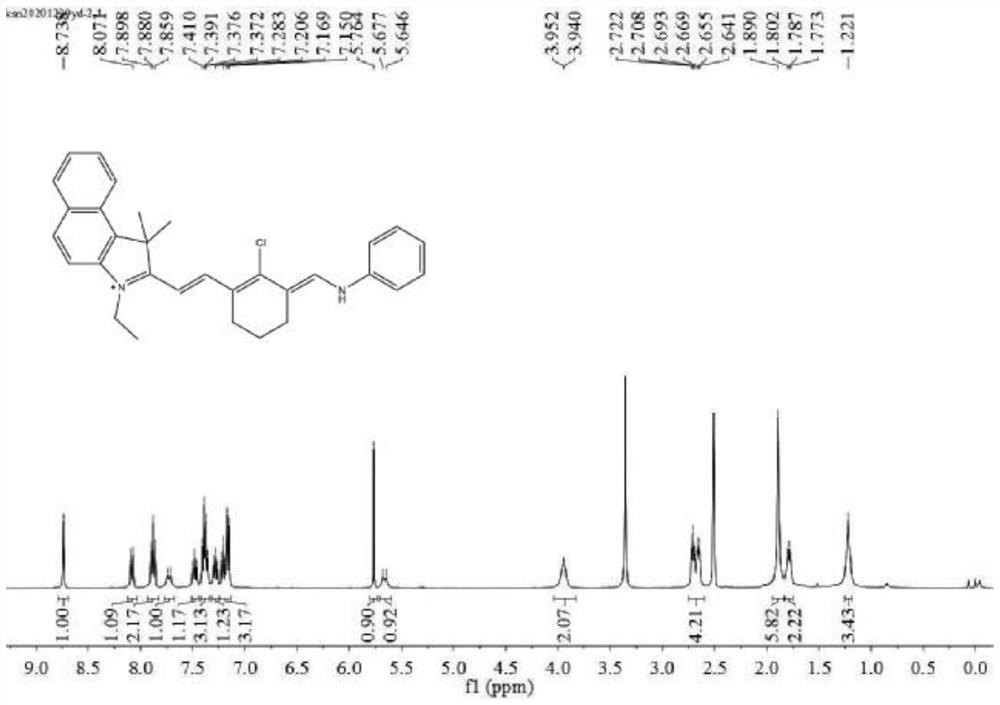
[0050] Synthesis of fluorescent probes
[0051] Take 0.36g (1mmol) of N-[(3-(anilino methylene)-2-chloro-1-cyclohexen-1-yl) methylene] aniline hydrochloride, 0.24g (1mmol) 3-Ethyl-1,1,2-trimethyl-1H-benzo(E)indole, 81 mg (1 mmol) of anhydrous sodium acetate, dissolved in 15 mL of ultra-dry ethanol, set the oil bath temperature to 80°C, and reflux 8h, after the reaction was completed, cooled to room temperature, neutralized with phosphate buffer solution, extracted with dichloromethane several times to obtain an organic phase (purple), then dried with anhydrous magnesium sulfate, concentrated and separated by silica gel column chromatography, developed The solvent was methanol / dichloromethane (volume ratio 1:200) to obtain 61.8 mg of green solid compound 2 with a yield of 13%.
[0052] Please refer to figure 2 , is the H NMR spectrum of compound 2 in the preparation method of the fluorescent probe. The results of NMR test are as follows: 1H NMR (400MHz, DMSO) δ8.74(s, 1H), ...
Embodiment 3
[0060] In order to explore the response of fluorescent probe P-1 to different solvent polarities, at a concentration of 1×10 -5 Under the condition of mol / L, the fluorescent probe P-1 was tested in dichloromethane (CH 2 Cl 2 ), methanol (MeOH), ethanol (EtOH), acetonitrile (Acetonitrile), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) in six different solvents UV-Vis absorption spectra and Changes in the fluorescence emission spectrum.
[0061] Please refer to Figure 4 , are the ultraviolet absorption spectra of the fluorescent probe P-1 in different solvents. Depend on Figure 4 It can be seen that the absorption peaks of ultraviolet in different solvents are between 640 and 670, so 650nm is selected as the excitation wavelength, and 10×10cm is used as the slit width. -5 mol / L The fluorescence emission of fluorescent probes in six different solvents was tested respectively. Please refer to Figure 5 , is the fluorescence emission spectrum of fluorescent probe ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com