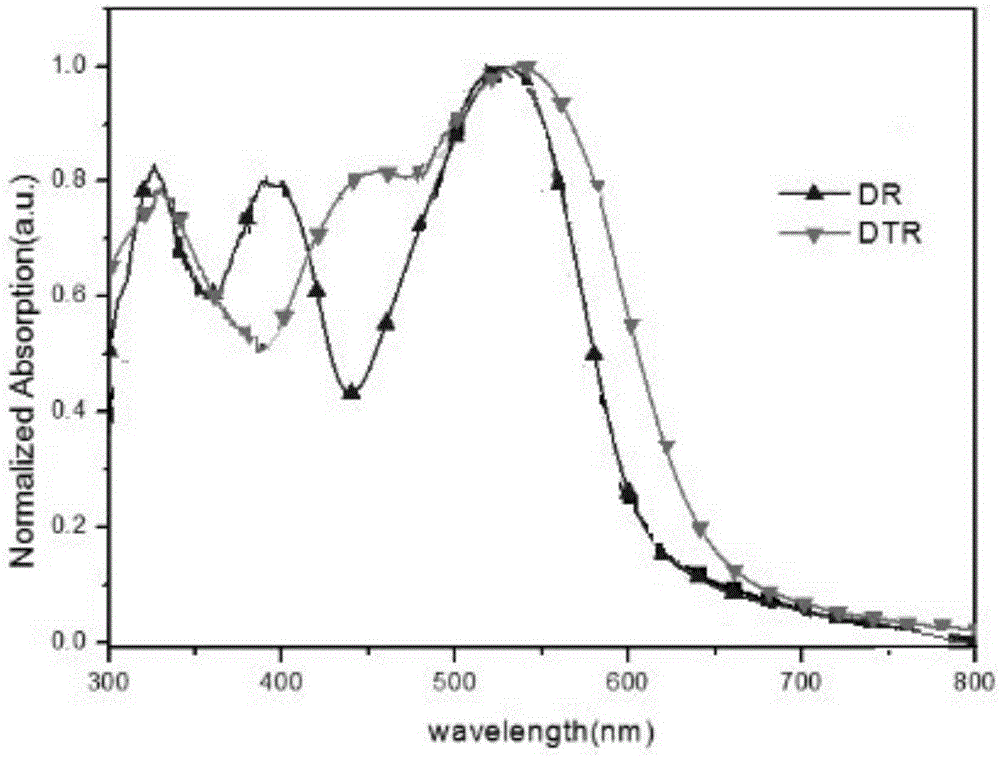
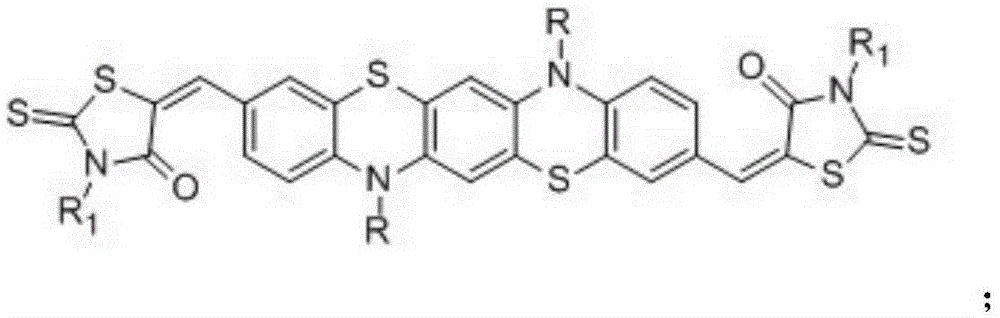
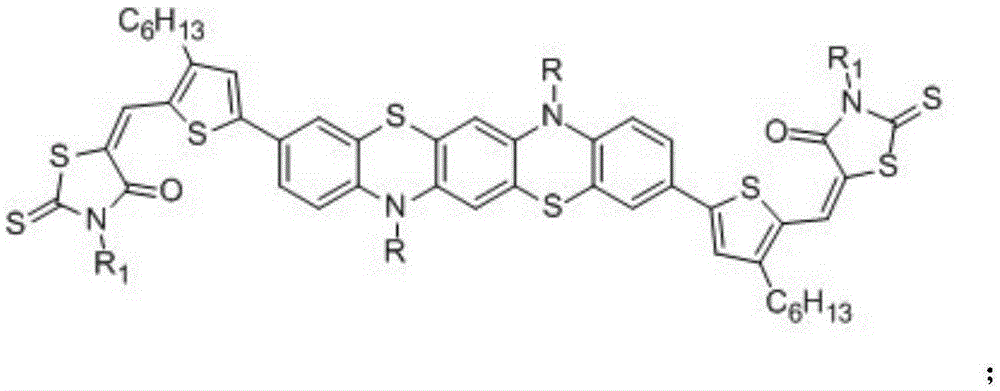
Phenothiazine organic small molecule donor and preparation method thereof
A technology of small molecule donor and phenothiazine, which is applied in the field of phenothiazine organic small molecule donor and its preparation, can solve the problems of rare application and low efficiency of solar cells, and achieves narrow band gap and solubility The effect of good sex and high matching degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation process of the phenothiazine organic small molecule donor of the present invention is as follows:
[0030] 1) Substitution of aldehyde groups at positions 3 and 10 of the core unit by Vilsmeier reaction: N 2 Add POCl to N,N-dimethylformamide (DMF) under atmosphere 3 Stir the reaction; add 7,14-dialkyl-benzo[1,4]thiazino[2,3-b]phenothiazine in DMF solution, heat up to reflux reaction, wash with water and filter to remove DMF and POCl 3 , and separated by column chromatography after drying to obtain intermediate 1 yellow powder 7,14-dialkyl-benzo[1,4]thiazino[2,3-b]phenothiazine-3,10-dialdehyde; DMF with POCl 3 The molar ratio is 1:(0.9-1.1); 7,14-dialkyl-benzo[1,4]thiazino[2,3-b]phenothiazine and POCl 3 The molar ratio of DMF and POCl3 is 1: (15-25); the condition of stirring reaction between DMF and POCl3 is 0-20°C and stirring for half an hour, then return to 25-35°C and stir. After 1-3 hours, return to 0-20°C; heat up and reflux reaction temperatur...
Embodiment 1
[0037] N 2 Under atmosphere and stirring, POCl was added dropwise to DMF (20eq, 80mmol, 6.2mL) at 0°C 3 (20eq, 80mmol, 7.4mL), after the addition, stirred at 0°C for half an hour, then returned to room temperature and stirred. After 2h, return to 0°C, add intermediate 7,14-di(2-octyldodecyl)-benzo[1,4]thiazino[2,3-b]phenothiazine to the reaction system (4mmol, 3.53g) of DMF solution (30mL), after the addition, the temperature of the reaction system was raised to 90°C, and after reflux reaction for 24h, the reaction solution was poured into ice water, a small amount of ammonium acetate was added to promote hydrolysis, stirred, and after the precipitation was complete , suction filtration, and drying the obtained solid crude sample to conduct column chromatography separation with dichloromethane / n-hexane (v / v=2 / 1) as the eluent, and finally obtain intermediate 1, yellow powder 7,14-dialkyl -Benzo[1,4]thiazino[2,3-b]phenothiazine-3,10-dialdehyde 1.45 g, yield 38.7%.
[0038] T...
Embodiment 2
[0041] The intermediate 7,14-bis(2-octyldodecyl)-benzo[1,4]thiazino[2,3-b]phenothiazine (4mmol, 3.53g) was dissolved in chloroform / In acetic acid (100mL / 100mL) solution, down to 0°C, N 2 Under a protective atmosphere, NBS (3eq, 12mmol, 2.14g) was added in five batches to the stirred reaction system. After the addition, it was gradually raised to room temperature, and reacted for 24h. The reaction solution was extracted three times with water / dichloromethane system, and finally the combined Anhydrous MgSO for organic phase 4 Dry overnight, filter, and spin dry the organic phase. The resulting crude sample is separated by column chromatography using n-hexane as the eluent to finally obtain intermediate 2, a yellow oily substance 3,10-dibromo-7,14-dialkyl - Benzo[1,4]thiazino[2,3-b]phenothiazine 2.07 g, yield 49.8%.
[0042] Intermediate 2 (2mmol, 2.07g) and 2-(4-hexyl-2-thienyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (3eq, 6mmol, 1.77g) into the 25ml two-necked flask, with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com