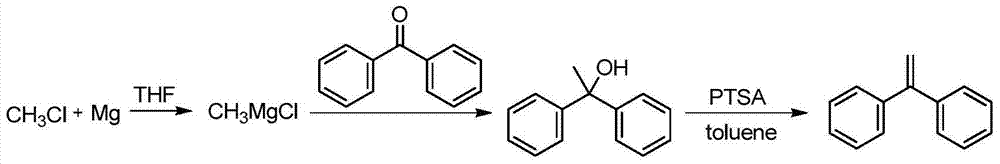
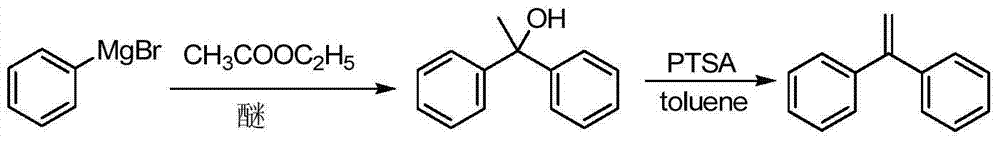
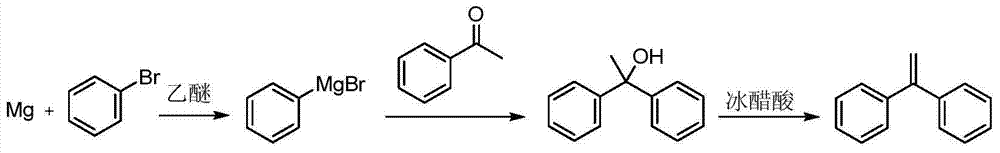
Preparation method of 1,1-diphenylethylene
A technology of diphenylethylene and acetophenone, which is applied in the field of preparation of 1,1-diphenylethylene, can solve the problems of loss of 1,1-diphenylethanol, unsuitability for industrialization, product loss, etc. The effect of obvious layer, wide practical value and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of phenylmagnesium bromide Grignard reagent
[0035]In a 500mL round bottom flask equipped with mechanical stirring, reflux condenser, dropping funnel, and thermometer, add 9.95g (0.41mol) of magnesium chips, 1.47g (0.008mol) of 1,2-dibromoethane, 65mL of anhydrous 2-Methyltetrahydrofuran, blow nitrogen, seal the device, raise the temperature to initiate the reaction, slowly add the 2-methyltetrahydrofuran solution of bromobenzene (containing 61.23g bromobenzene, 60mL of anhydrous 2-methyltetrahydrofuran), and raise the temperature to 60 °C, react for 4h. Take 1mL of the reaction solution and dilute it with 3mL of methanol as a sample. After GC detection: 0.9% bromobenzene, 98.7% benzene, and 0.2% biphenyl. GC detection conditions: gas phase column: SE-54 (50m), inlet sample temperature: 280°C, detector temperature: 280°C, carrier gas N 2 The flow rate is 2mL / min, and the temperature of the column oven is programmed: 100°C for 3 minutes, th...
Embodiment 2
[0036] Embodiment 2: the synthesis of phenylmagnesium bromide Grignard reagent
[0037] In a 500mL round bottom flask equipped with mechanical stirring, reflux condenser, dropping funnel, and thermometer, add 10.43g (0.43mol) of magnesium chips, 2.93g (0.016mol) of 1,2-dibromoethane, 65mL of anhydrous 2-Methyltetrahydrofuran, blow nitrogen, seal the device, raise the temperature to initiate the reaction, slowly add 2-methyltetrahydrofuran solution of bromobenzene (containing 61.23g bromobenzene, 60mL of anhydrous 2-methyltetrahydrofuran), and raise the temperature to 80 ℃ reaction 2h. A small amount of reaction solution was diluted with methanol and tested by GC: 0.5% bromobenzene, 99.1% benzene, and 0.2% biphenyl. The reaction solution dilution method and GC detection conditions are the same as in Example 1.
Embodiment 3
[0038] Embodiment 3: the synthesis of phenylmagnesium bromide Grignard reagent
[0039] In a 500mL round bottom flask equipped with mechanical stirring, reflux condenser, dropping funnel, and thermometer, add 9.95g (0.41mol) of magnesium chips, 1.22g (0.008mol) of bromobenzene, and 65mL of anhydrous 2-methyltetrahydrofuran, Nitrogen was blown, the device was sealed, and the temperature was raised to initiate the reaction. The solution of bromobenzene in 2-methyltetrahydrofuran (containing 60.01 g of bromobenzene and 60 mL of anhydrous 2-methyltetrahydrofuran) was slowly added dropwise, and the temperature was raised to 60°C for 4 hours after the drop. A small amount of the reaction solution was diluted with methanol and tested by GC: 0.8% bromobenzene, 98.8% benzene, and 0.2% biphenyl. The reaction solution dilution method and GC detection conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com