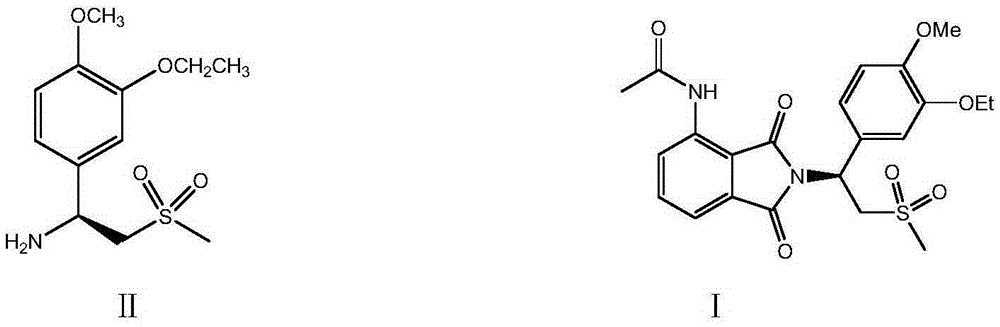
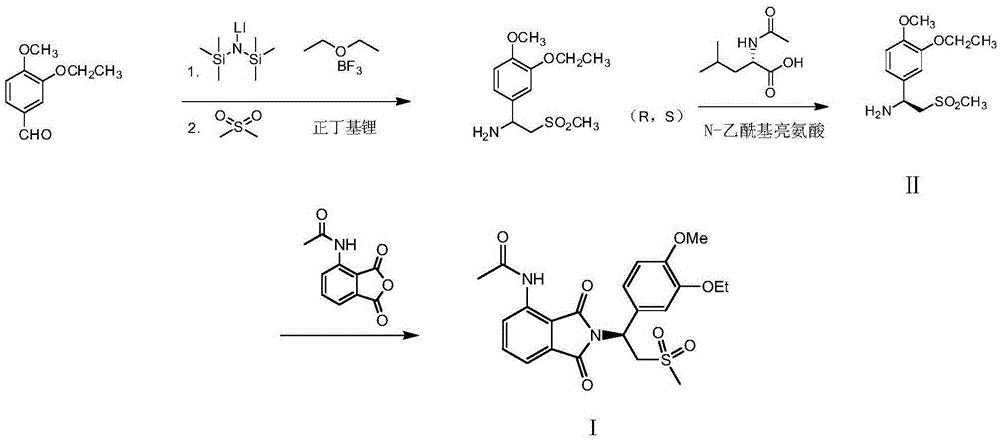
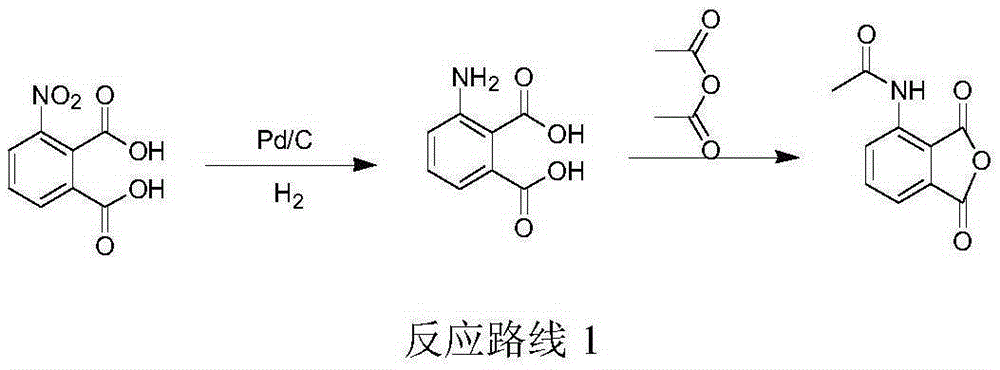
Preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine and preparation method of apremilast
A technology of methylsulfonylethylamine and methylsulfonylacetonitrile, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of "three wastes" such as large discharge, high price, and unfavorable environmental protection, and achieve waste liquid Low emissions, low product cost, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of racemate III (R, S)-1-(4-methoxy-3-ethoxy)phenyl-2-methanesulfonylethylamine (Ⅲ)
[0053] Add 100 grams of tetrahydrofuran, 3.0 grams of magnesium, 1 grain of iodine (about 10-20 mg), 0.5 grams of 4-methoxy-3-ethoxybromobenzene to a dry 500-ml glass flask, and initiate at 45-50 ° C. Reaction, then dropwise add a solution of 22.6 grams (0.1 moles in total) of 4-methoxy-3-ethoxybromobenzene and 150 grams of tetrahydrofuran between 45-50°C, and drop it for about 50 minutes. After that, 55-60°C The reaction time between them was 3 hours.
[0054] After cooling to -5-0°C, 12.0 g (0.11 mole) of methanesulfonyl acetonitrile was added dropwise for about 40 minutes, and then reacted at -5-0°C for 3 hours. Add 20 grams of 30% ammonium chloride aqueous solution to adjust the pH value of the system to 6-7, and transfer it to a stainless steel pressure kettle after reacting at 20-25 ° C for 3 hours, then add 1.0 grams of 50% Raney nickel (water content 50%...
Embodiment 2
[0055] Example 2: Preparation of (R,S)-1-(4-methoxy-3-ethoxy)phenyl-2-methanesulfonylethylamine (Ⅲ)
[0056] Into a dry 500 ml glass flask, add 100 g of ethoxymethyl ether, 3.0 g of magnesium, 1 grain of iodine (about 10-20 mg), 0.5 g of 4-methoxy-3-ethoxybromobenzene, Initiate the reaction at 50-55°C, then add 22.6 grams (0.1 moles in total) of 4-methoxy-3-ethoxybromobenzene and 160 grams of ethoxymethyl ether solution dropwise between 55-60°C for about 50 minutes After dropping, the reaction time is 2 hours at 55-60°C. Cool to -5-0°C, add 12.0 g (0.11 mol) methanesulfonyl acetonitrile dropwise, and drop it in about 40 minutes, then react at 0-5°C for 3 hours. Add 20 grams of 30% ammonium chloride aqueous solution to adjust the pH value of the system to 6-7, and transfer it to a stainless steel pressure kettle after reacting at 20-25 ° C for 3 hours, then add 0.6 grams of 5% palladium carbon, and replace it with nitrogen for 3 times. The inner temperature is 20-25°C, and th...
Embodiment 3
[0057] Example 3: Preparation of (R,S)-1-(4-methoxy-3-ethoxy)phenyl-2-methanesulfonylethylamine (Ⅲ)
[0058] Add 100 grams of cyclopentyl methyl ether, 3.1 grams of magnesium, 1 grain of iodine, and 0.5 grams of 4-methoxy-3-ethoxybromobenzene to a dry 500-milliliter glass flask, and initiate the reaction at 45-50 ° C, then A solution of 22.6 grams (0.1 moles in total) of 4-methoxy-3-ethoxybromobenzene and 150 grams of cyclopentyl methyl ether was added dropwise between 45-50 ° C, and the drop was completed in about 40 minutes. After that, 55-60 The reaction time between °C was 2 hours. After cooling to -5-0°C, 12.0 g (0.11 mole) of methanesulfonyl acetonitrile was added dropwise for about 40 minutes, and then reacted at -5-0°C for 3 hours. Add 20 grams of 30% ammonium chloride aqueous solution to adjust the pH value of the system to 6-7, and transfer it to a stainless steel pressure kettle after reacting at 20-25 ° C for 3 hours, then add 1.0 grams of 50% Raney nickel (water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com