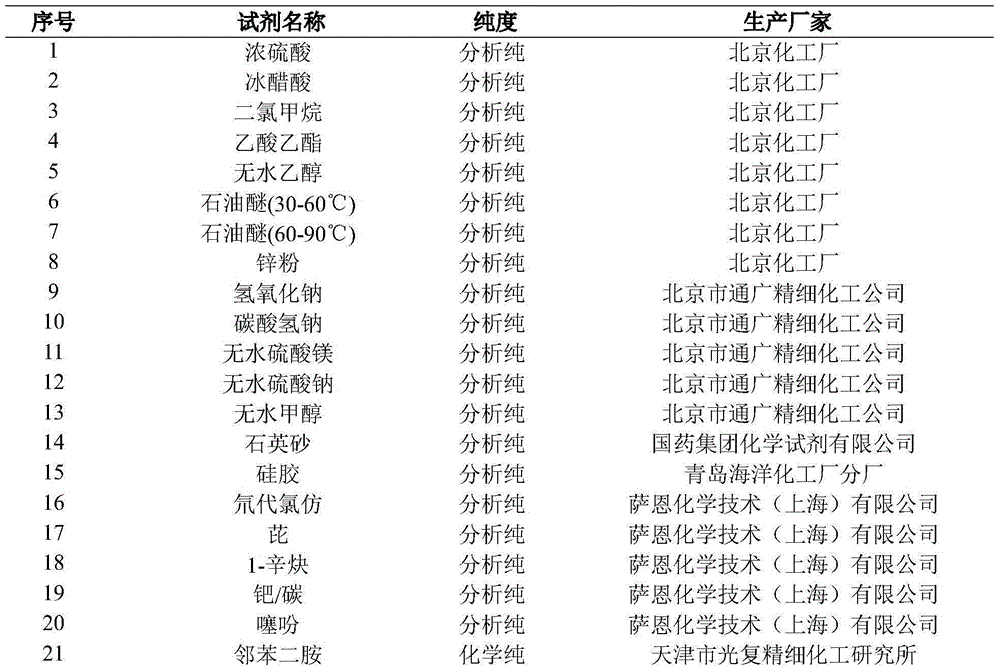
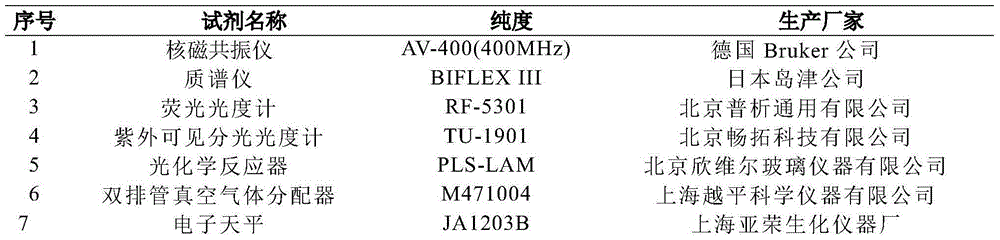
Patents
Literature
742 results about "Bromobenzenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivatives of benzene in which one or more hydrogen atoms on the benzene ring are replaced by bromine atoms.
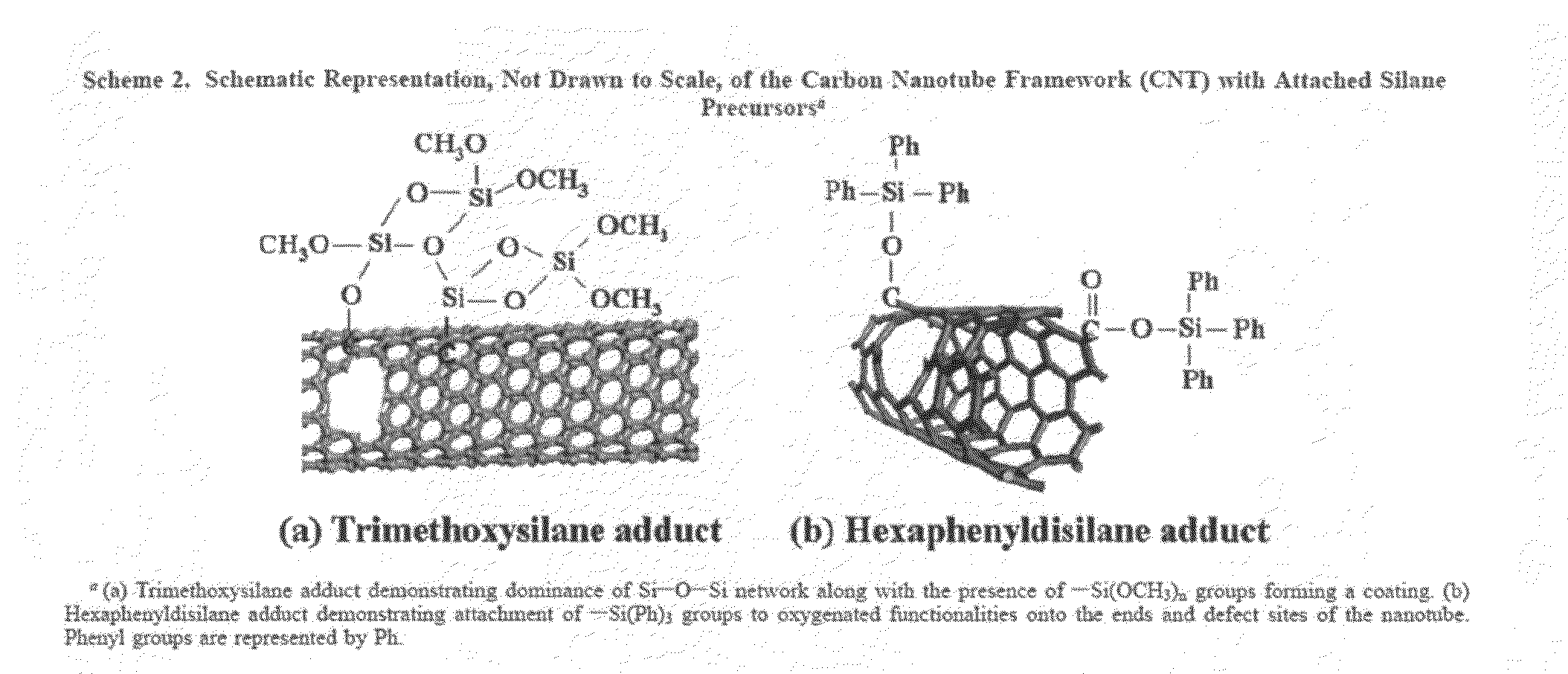
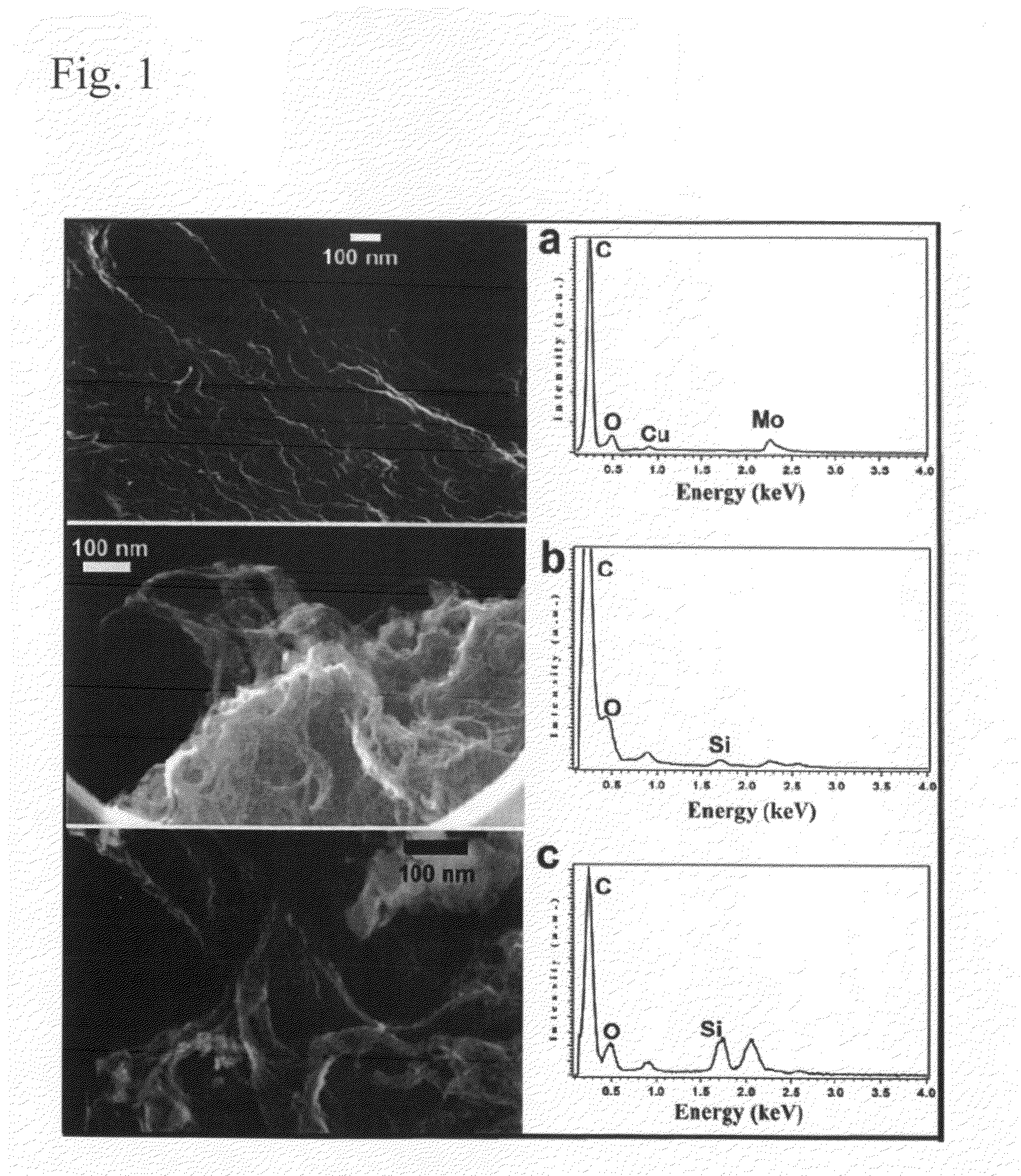
Silylated carbon nanotubes and methods of making same
InactiveUS20090060815A1Silicon organic compoundsMaterial nanotechnologyTert-butyldimethylsilaneBis(trimethylsilyl)amine
The invention provides adducts comprising a carbon nanotube with covalently attached silane moieties, and methods of making such adducts. Examples of silane moieties include trimethoxysilane; hexaphenyldisilane; silylphosphine; 1,1,1,3,5,5,5-heptamethyltrisiloxane; polydimethylsiloxane, poly(N-bromobenzene-1,3-disulfonamide); N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide; hexamethyldisilazane (HMDS); chlorotrimethylsilane (TMCS); trichloromethylsilane (TCMS); an alkyl(alkylamino)silane; a tri(alkoxy)silane; tert-butyldimethylsilane; monochloroaminosilane; dichloroaminosilane; trichloroaminosilane; and dimethylaminosilane.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
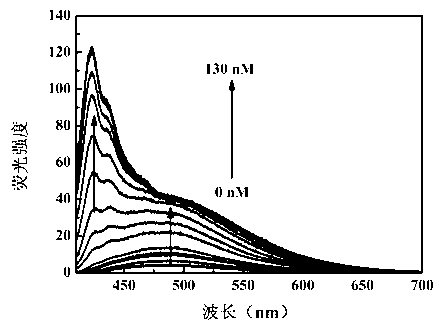
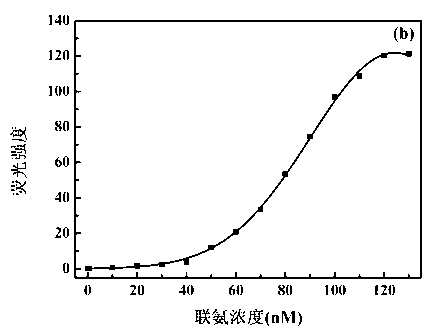
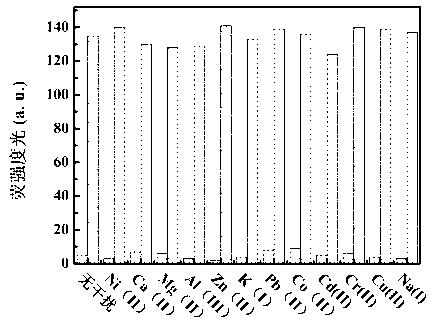
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
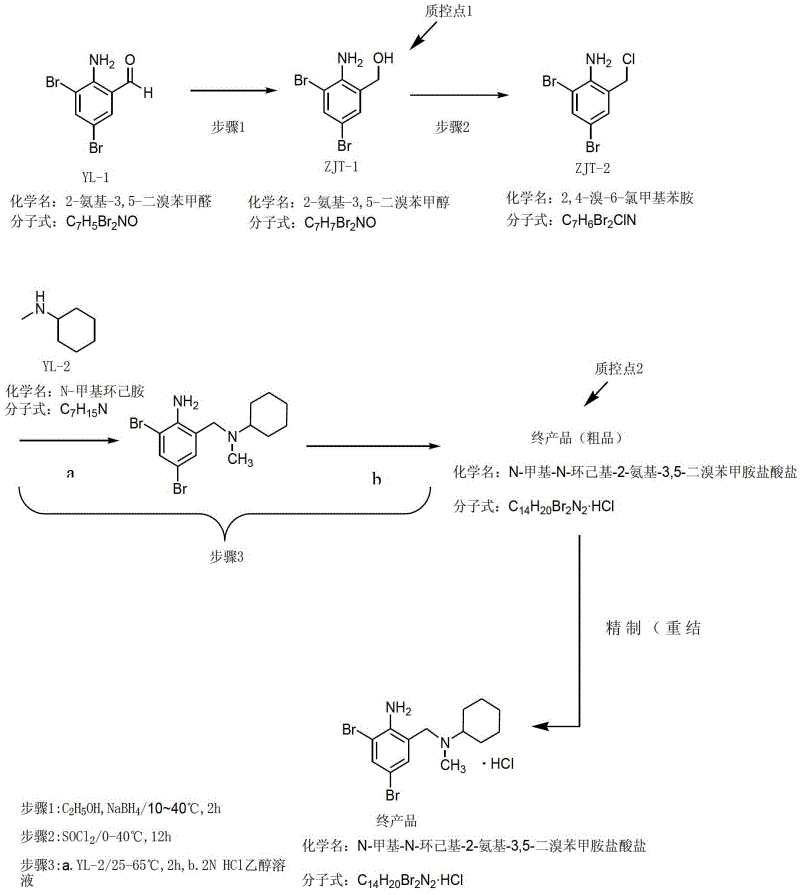
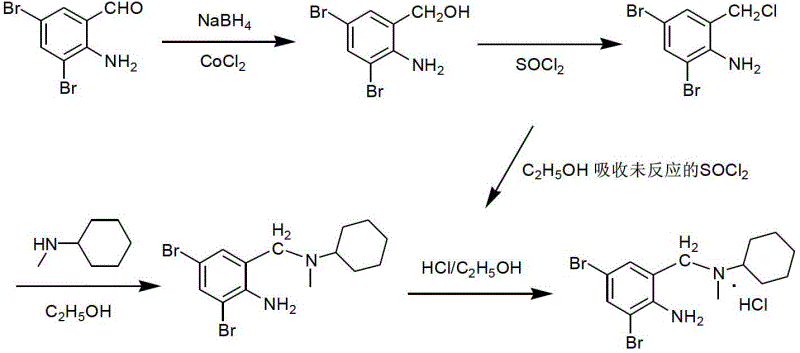
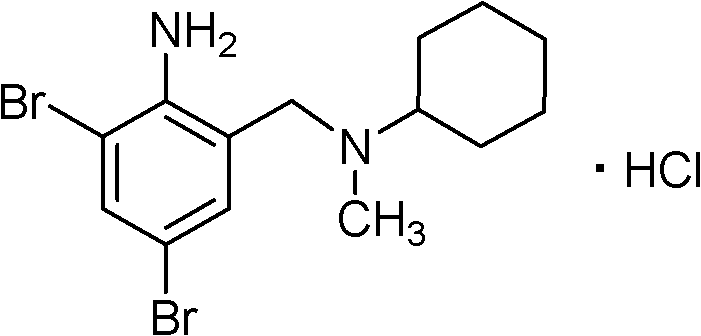
Method for preparing bromhexine hydrochloride
The invention provides a method for preparing bromhexine hydrochloride, which comprises the steps as follows: (1) 2-amino-3,5-dibromo benzaldehyde and reducing agents are in a reduction reaction to generate 2-amino-3,5-dibromo benzyl alcohol; (2) 2-amino-3, 5-dibromo benzyl alcohol that is obtained in the step (1) reacts with chlorinating agents to generate 2, 4-bromine-6-chloride methylaniline; and (3) 2,4-bromine-6-chloride methylaniline obtained in the step (2) and N-methylcyclohexylamine are in an amination reaction, and then 2, 4-bromine-6-chloride methylaniline and HCl salification agents are in a salification reaction, so that bromhexine hydrochloride is obtained. The preparation method adopts multiple advanced technologies, is easy to get starting materials, and has the advantages of stable property of intermediates, extremely low environment pollution, high yield coefficient of products and high purity.
Owner:SHIJIAZHUANG DONGFANG PHARMA
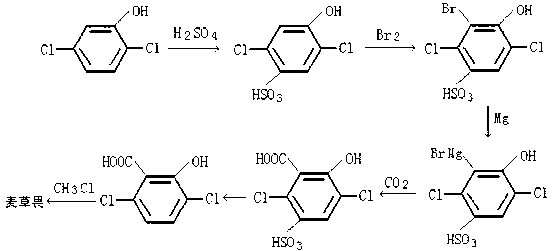
Synthesis method of dicamba
InactiveCN102838483AReaction raw materials are readily availableThe reaction process is green and environmentally friendlyOrganic compound preparationCarboxylic compound preparationElectrophilic additionSalicylic acid
The invention discloses a synthesis method of dicamba, which comprises the following steps of: using 2, 5-dichlorophenol as raw materials, conducting sulfonation and bromination reaction to obtain 4-hydroxy-2, 5-dichloro-3-bromobenzenesulfonic acid, treating the 4-hydroxy-2, 5-dichloro-3-bromobenzenesulfonic acid by using alkyl metal complexes of magnesium or lithium, conducting electrophilic addition reaction with CO2 to obtain 5-sulfo-3, 6-diclorsalicylic acid, removing the sulfo of the 5-sulfo-3, 6-diclorsalicylic acid and conducting O-methylation reaction with methyl chloride to obtain the dicamba. The synthesis method of dicamba has the advantages that since the 2, 5-dichlorophenol is used as the raw materials, the raw materials for reaction are easy to obtain, the reaction process is environmental-friendly, the quality of the obtained product is high, the yield is high and the application value is greater.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Brominated anionic styrenic polymers and their preparation
Concurrently fed into a reaction zone held at about 10° C. or less are brominating agent, aluminum halide catalyst, and a solution of anionic styrenic polymer having a GPC Mn about 2000-30,000. The components are in at least two separate feed streams. The feeds are proportioned to maintain (a) the amount of aluminum halide being fed at about 0.8 mole percent or less based on the amount of aromatic monomeric units in the polymer being fed, and (b) amounts of brominating agent and unbrominated polymer in the reaction zone that produce a final washed and dried polymer product containing about 60-71 wt % bromine. The catalyst is deactivated, bromide ions and catalyst residues are washed away from the reaction mixture, and the brominated anionic styrenic polymer is recovered and dried. The dried polymer has a volatile bromobenzene content of about 600 ppm (wt / wt) or less as well as other beneficial properties.
Owner:ALBEMARLE CORP
Organic micro-molecular electron transport material and preparation thereof, and n-doped electron transport layer and application thereof
ActiveCN108409730AImprove thermal stabilityGood film shape stabilityOrganic chemistrySolid-state devicesPhenylboronic acidBromine
The invention belongs to the technical field of electron transport materials and discloses an organic micro-molecular electron transport material and preparation thereof, and an n-doped electron transport layer and application thereof. The organic micro-molecular electron transport material has a structure shown as the formula I. The preparation method of the organic micro-molecular electron transport material comprises (1) subjecting 2-chloro-4, 6-diphenyl-1, 3, 5-triazine and 3-bromo-phenylboronic acid to coupling reaction, and then performing subsequent processing to obtain a bromine-containing intermediate; (2) subjecting the bromine-containing intermediate and bis-(pinacolato)-diboron to Suzuki reaction and then performing subsequent processing to obtain a borate intermediate; (3) subjecting the borate intermediate and 3-bromo-1, 10-phenanthroline to coupling reaction and performing subsequent processing to obtain the organic micro-molecular electron transport material. The organic micro-molecular electron transport material is simple in structure and high in thermostability and topographic stability; when n-doped, the organic micro-molecular electron transport material can beprepared into the n-doped electron transport layer, which achieves high luminous efficiency and stability when applied to organic electroluminescence devices.
Owner:SOUTH CHINA UNIV OF TECH
Soluble electro-green light organic molecule glass material and preparation method and use thereof
InactiveCN101392174AGood holeFacilitates electron injectionOrganic chemistrySolid-state devicesSolubilityCuprous salt
The invention discloses a soluble electroluminescent green light organic molecule glass material, a preparation method and applications thereof. The material comprises two types of a symmetric substituent benzothiadiazole derivative and a dissymmetric substituent benzothiadiazole derivative. The preparation method of the material comprises: carbazol and fluorine or anthracene are taken as raw materials, a bromide that contains Ar1 is obtained through palladium-catalyzed coupling reaction or cuprous salt catalyzed coupling reaction, and corresponding boric acid ester is generated through a next reaction; the boric acid ester is reacted with 4, 7-dibromo benzothiadiazole or a bromide of benzothiadiazole substituted by soluble resin Ar2 and a tiny molecule luminescent material which is symmetric or dissymmetric is obtained. The luminescent material prepared has good solubility in a solvent with high boiling point and weak polarity and can be purified by the solution method; simultaneously, the luminescent material has good thermal stability and morphologic stability, particularly the luminescent material with the dissymmetric structure has advantages in both synthesis and purification, thus having important application prospect in electroluminescence display, illumination and laser.
Owner:SOUTH CHINA UNIV OF TECH
Brominated anionic styrenic polymers and their preparation
Concurrently fed into a reaction zone held at about 10° C. or less are brominating agent, aluminum halide catalyst, and a solution of anionic styrenic polymer having a GPC Mn about 2000-30,000. The components are in at least two separate feed streams. The feeds are proportioned to maintain (a) the amount of aluminum halide being fed at about 0.8 mole percent or less based on the amount of aromatic monomeric units in the polymer being fed, and (b) amounts of brominating agent and unbrominated polymer in the reaction zone that produce a final washed and dried polymer product containing about 60-71 wt % bromine. The catalyst is deactivated, bromide ions and catalyst residues are washed away from the reaction mixture, and the brominated anionic styrenic polymer is recovered and dried. The dried polymer has a volatile bromobenzene content of about 600 ppm (wt / wt) or less as well as other beneficial properties.
Owner:ALBEMARLE CORP
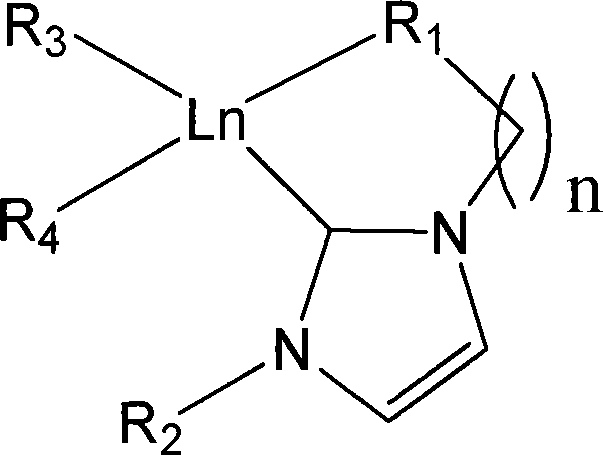
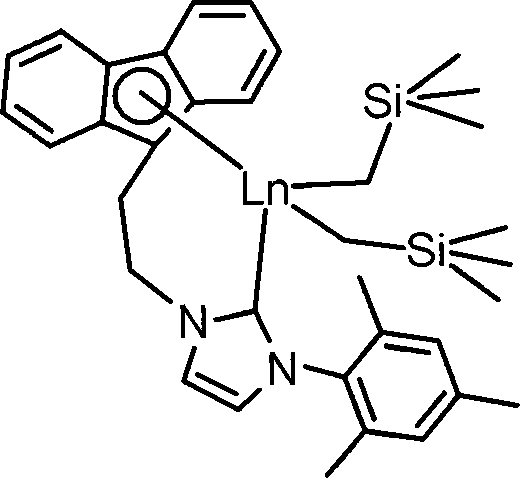
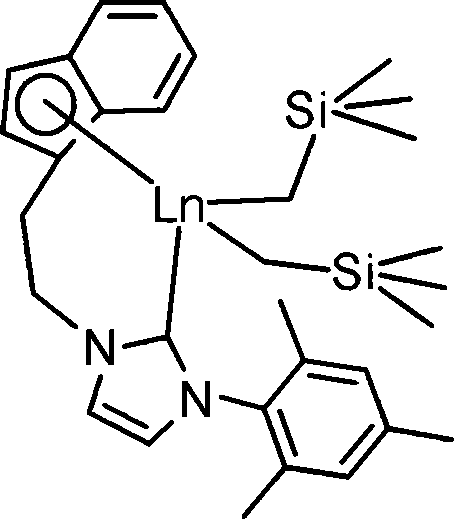
Aza cyclic carbine rear earth catalyst for crystallinity 3,4-polyisoprene
ActiveCN101157737AHigh molecular weightNarrow molecular weight distributionChlorobenzeneReaction temperature
The present invention relates to a nitrogen heterocyclic carbene rare earth complex, a catalyst system which consists of the present invention, aluminum alkyl and an organoboron salt can catalyse the isoprene to carry out the solution polymerization, so as to prepare the polyisoprene with the crystallization property, high 3, 4-structure and high glass transition temperature (Tg). The molar ratio of aluminum alkyl and the rare earth complex is within the scope of 1 to 100, the molar ratio of the organoboron salt and the rare earth complex is within the scope of 0.5 to 2.0; the solvent which is used for polymerization can be toluene, bromobenzene, n-hexane, dichloromethane or chlorobenzene; the polymerization temperature range is -20 DEG C to 80 DEG C, the reaction time of polymerization at minus 20 DEG C is 36 hours, the reaction time of polymerization at 80 DEG C is 1 hour, and the monomer conversion rate can be up to 100 percent. The reaction is characterized by active polymerization, the molecular weight of the product can be controlled by the molar ratio of the monomer and the catalyst, the molecular weight of the polyisoprene can achieve 360,000 at most, and the glass transition temperature Tg is equal to 5 DEG C to 50 DEG C. The content of the 3, 4 structure of the polyisoprene is affected by the spatial effect and the electronic effect of the rare earth complex, the solvent type for polymerization reaction and the polymerization reaction temperature and so on, which is changed between 76 percent and 99 percent.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
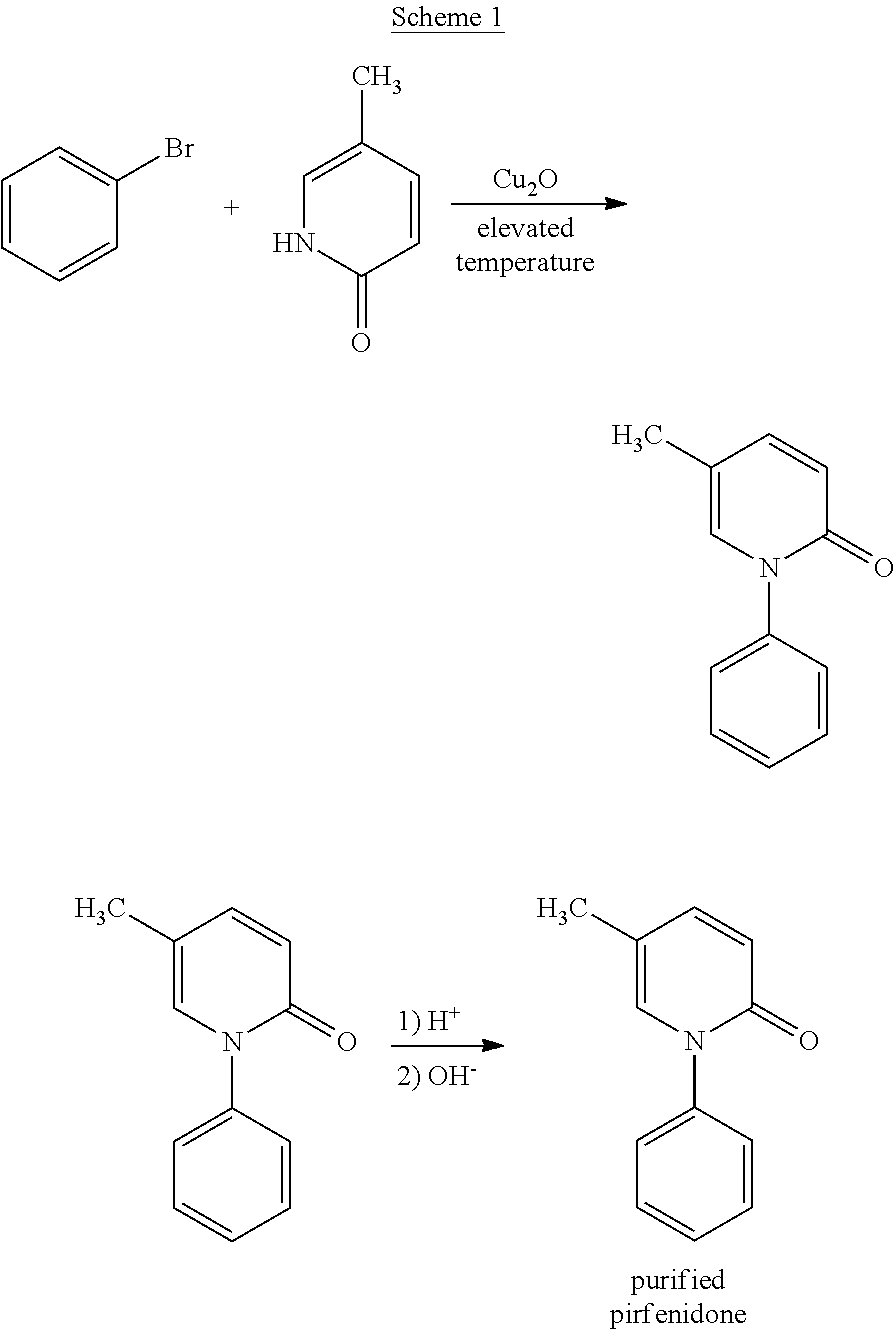
Method for Synthesizing Pirfenidone
ActiveUS20120016133A1Avoid insufficient purityEfficient and economical processOrganic active ingredientsBiocideAcetic acidRetention time
A process for synthesizing pirfenidone from bromobenzene having less than about 0.15% by weight dibromobenze is disclosed. Also disclosed are processes of synthesizing pirfenidone without using ethyl acetate or n-butanol, and pirfenidone having controlled levels of ethyl acetate, n-butanol, di(5-methyl-2-pyridinone)benzenes, and other impurities having specified retention times. Also disclosed are formulated dosage forms including the disclosed pirfenidone.
Owner:ZACH SYST SPA +2
Method for preparing fluorine-containing benzoxazole liquid crystal compound
The invention discloses a method for preparing a fluorine-containing benzoxazole liquid crystal compound. The compound has a structural formula shown in the description, wherein R refers to C2-16 straight-chain paraffin; X, Y and Z refer to respectively independent fluorine or hydrogen, X, Y and Z refer to hydrogen alternatively, and M refers to hydrogen, chlorine or nitro. The preparation method comprises the following steps: by taking 4-hydroxyl fluorinated bromobenzene, 1-bromoalkane and 4-formylphenylboronic acid as reaction raw materials, preparing 4'-alkoxyfluorobiphenylcarboxaldehyde, and reacting 4'-alkoxyfluorobiphenylcarboxaldehyde with aminophenol to prepare the fluorine-containing benzoxazole liquid crystal compound under the action of an oxidant. Compared with the classical method and the improved method, the method has the advantages of fewer steps, simple operation, low cost and high yield and can be used for preparing fluorine-containing benzoxazole liquid crystal.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
Method for synthesizing doxylamine succinate
The invention discloses a method for synthesizing doxylamine succinate. 2-acetylpyridine is taken as an initiative material, and the method comprises the following steps of: reacting the 2-acetylpyridine with a Grignard reagent prepared from bromobenzene and magnesium to obtain 2-pyridyl phenyl methyl carbinol; reacting the 2-pyridyl phenyl methyl carbinol with sodium amide and 2-dimethylaminoethyl chloride in turn to obtain doxylamine; and performing salt-forming reaction on the doxylamine and succinic acid to obtain the doxylamine succinate. The synthesis route is orientation reaction without byproduct. The doxylamine succinate is an ethanol antihistamine, and has antihistaminic and cholinolytic effects and obvious tranquillizing effect.
Owner:HEFEI UNIV OF TECH +1
Preparation method of umeclidinium bromide
InactiveCN105461710AMild reaction conditionsReduce cooling costsOrganic chemistryBenzeneChlorobenzene
The invention discloses a preparation method of umeclidinium bromide. The method is suitable for industrial routine reactions, and industrial production schemes exist at present. A technical scheme adopted in the invention is characterized in that a Grignard reagent is adopted to substitute a lithium reagent, bromobenzene, chlorobenzene and other benzene halides are adopted as raw materials, and ether and tetrahydrofuran can be used as a solvent. Compared with the prior art, the method disclosed in the invention has the following advantages: 1, the lithium reagent with high price is substituted by magneson; 2, reaction conditions are mild, too low temperature is not needed, and the reaction temperature is about 0-5DEG C, so the refrigeration cost is reduced; and 3, a routine device can be used, and the Grignard reaction is a classic reaction and industrially exists for a hundred years or above, so people can be simply trained, and the device is mature and has high operating stability.
Owner:ANHUI DEXINJIA BIOPHARM
Synthetic method for 2-methyl-3-trifluoromethyl phenylamine
ActiveCN102491906AWide variety of sourcesReduce manufacturing costOrganic compound preparationAmino compound preparationMethylanilinePtru catalyst
The invention discloses a synthetic method for 2-methyl-3-trifluoromethyl phenylamine. The method comprises the following steps: 1) preparing 3-nitro-4-methyl benzenesulfonic acid; 2) preparing 3-nitro-4-methyl-5-bromobenzenesulfonic acid; 3) preparing 2-nitro-6-bromotoluene; 4) preparing 2-nitro-6-trichloromethyl toluene; 5) preparing 2-nitro-6-trifluoromethyl toluene; 6) preparing 2-methyl-3-trifluoromethyl phenylamine, that is, adding 5% palladium-charcoal and methanol in 2-nitro-6-trifluoromethyl toluene obtained in step 5), carrying out hydrogenation reduction at a temperature of 35 DEG C and removing a catalyst so as to obtain 2-methyl-3-trifluoromethyl phenylamine. According to the invention, p-toluenesulfonic acid is used as a starting material, and therefore, disadvantages of theprior art are overcome and yield of a target product is improved.
Owner:QILU ANIMAL HEALTH PROD +1
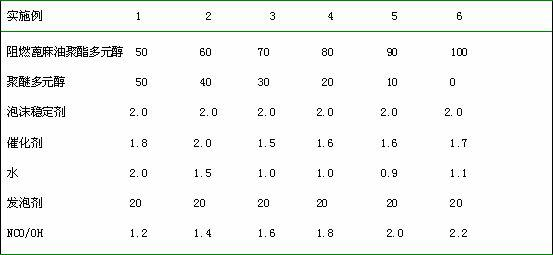
Method for preparing structure type flame-retardant castor-oil-based polyester polyol and applications thereof
InactiveCN102432850AImproved flame retardant performance of rigid foamImprove flame retardant performancePolyesterPolymer science
The invention relates to a method for preparing structure type flame-retardant castor-oil-based polyester polyol and applications thereof. The method comprises the following steps: castor oil and small-molecular alcohol generate alcoholysis reaction under the existence of an alkali catalyst, and then generate esterification reaction with tetrabromophthalic anhydride under the existence of an esterification catalyst, and the flame-retardant castor-oil-based polyester polyol is obtained, wherein the hydroxyl value is 100-450mg / gKOH, the acid value is less than or equal to 1.5mg / gKOH and the content of bromine is 5-30wt%. In the method, the middle process does not need to be separated, the cost is low and the process is simple, and the flame retardance of the prepared polyurethane foam is obviously improved. The prepared polyurethane foam has the advantages of no liquid drop of a product in burning, shape maintenance, less smoke density and the like and can be used for buildings, insulation and certain special fields.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY +1
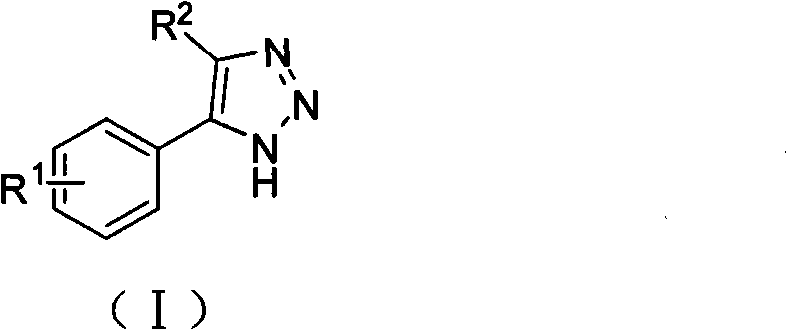
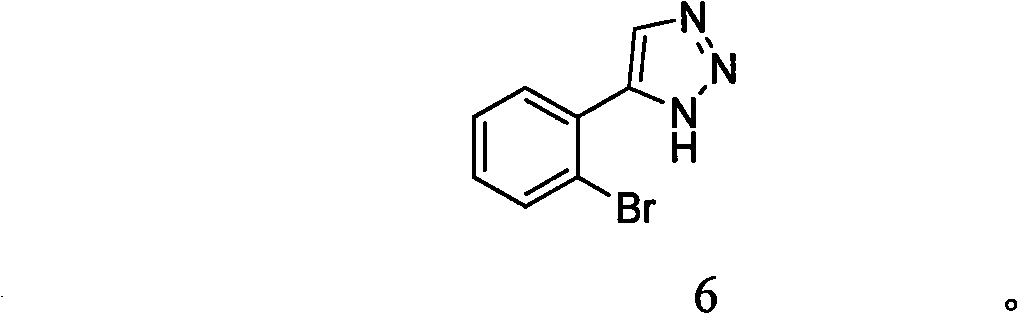
1, 2, 3-triazole compound and application thereof in preparing indoleamine 2, 3-dioxygenase inhibitor
InactiveCN101786993APromote decompositionLow toxicitySenses disorderNervous disorderDiseaseTryptophan
The invention belongs to the field of pharmaceutical chemistry and relates to a 1, 2, 3-triazole compound, comprising 4-(2-bromophenyl)-1H-1, 2, 3-triazole and 4-(2-chlorphenyl)-1H-1, 2, 3-triazole. The result of an inhibitory activity test of indoleamine 2, 3-dioxygenase indicates that the disclosed 1, 2, 3-triazole compound used as the indoleamine 2, 3-dioxygenase inhibitor has broad prospect of application and can be applied to the treatment of diseases with indoleamine 2, 3-dioxygenase-mediated tryptophan metabolic pathway pathological characteristics, including cancer, AIDS (Acquired Immure Deficiency Syndrome), Alzheimer disease, tristimania, cataract and other critical diseases.
Owner:FUDAN UNIV
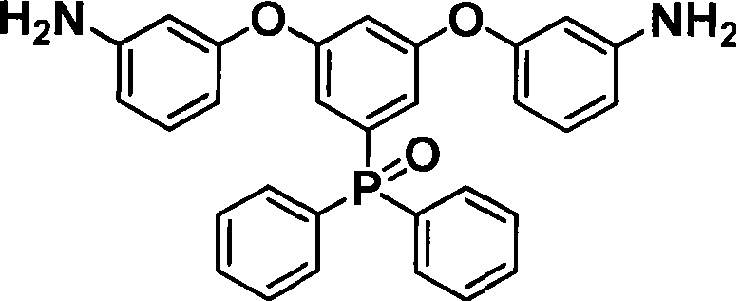
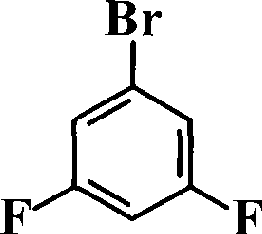
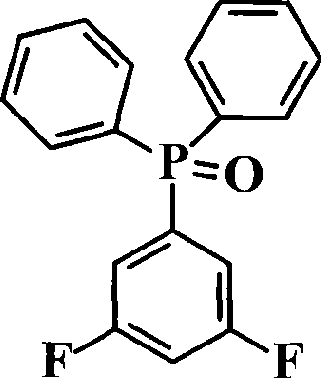
Phosphine-containing aromatic diamine compound, preparation method and application thereof
ActiveCN101531678AImprove heat resistanceHigh glass transition temperatureGroup 5/15 element organic compoundsGrignard reactionPhosphine oxide
The invention discloses an aromatic diamine compound containing aryl phosphine groups, a prepration method and an application thereof. The structural general formula of the compound is shown in a formula I. The compound takes 3,5-difluoro-bromobenzene and diphenylphosphinic chloride as raw materials, firstly prepares 3,5-difluorophenyl diphenyl phosphine oxide by Grignard reaction and then continuously reacts with 3-aminophenol for preparation. The compound has smaller steric effect and has great value for improving the heat resistance performance of atomic oxygen resistant polyimide thin film materials and improving the visible light transparency. In addition, polyimide phosphine-containing polymer materials prepared by utilizing the phosphine-containing aromatic diamine compound have good atomic oxygen resistance performance, higher glass transition temperature and better visible light transparency, thereby being capable of being applied in the manufacturing of satellites and other aircraft parts in the LEO environment.
Owner:INST OF CHEM CHINESE ACAD OF SCI
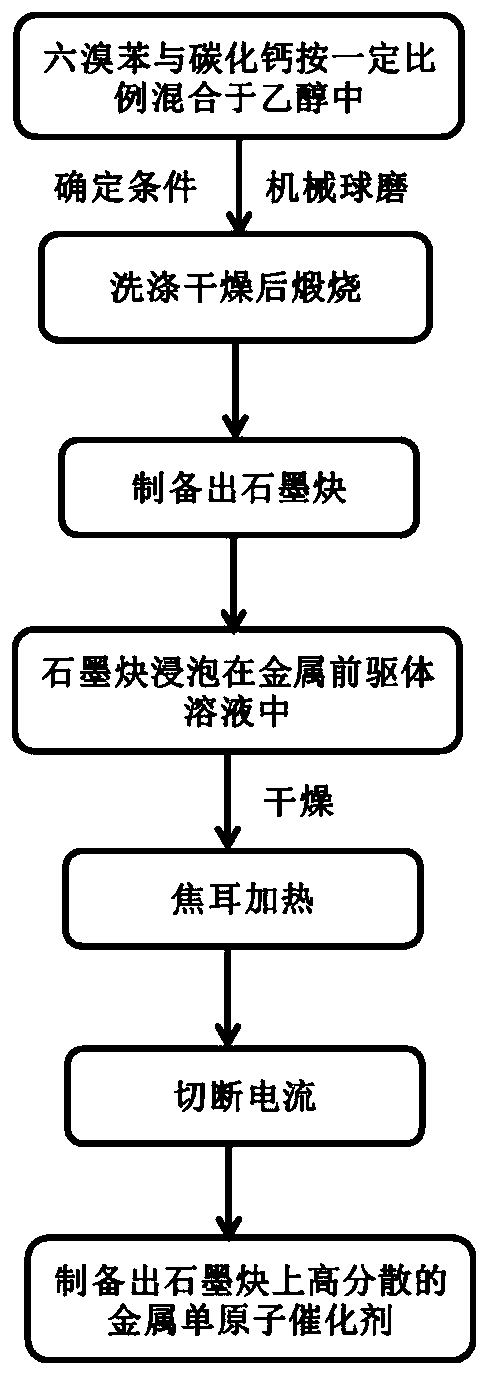
Preparation method of graphdiyne-loaded monatomic catalyst
PendingCN111490258AAvoid reunionDestabilizingCell electrodesSolid electrolyte fuel cellsPtru catalystPhysical chemistry
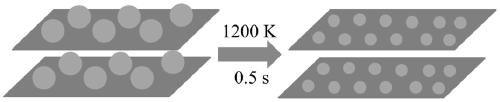
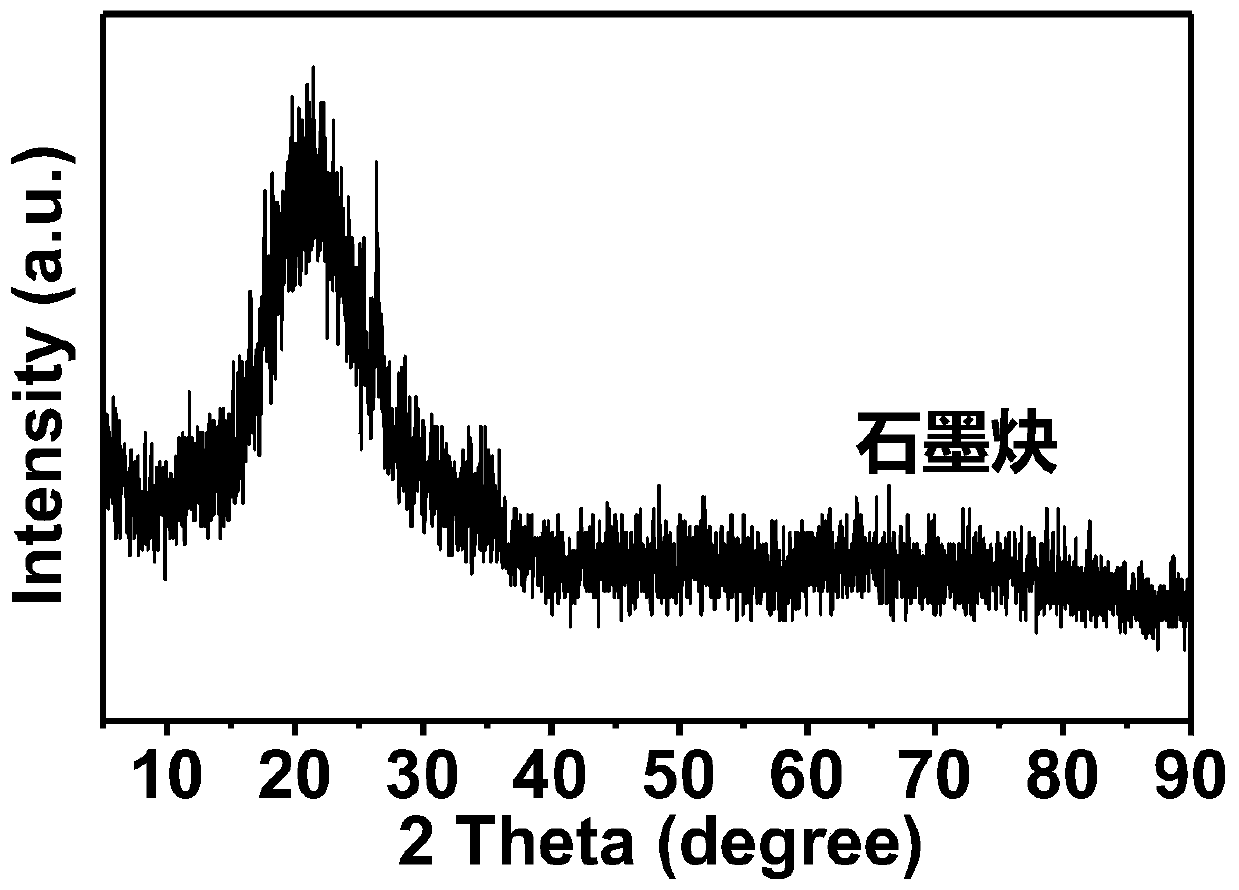
The invention relates to a preparation method of a graphdiyne-loaded monatomic catalyst, which belongs to the technical field of preparation of monatomic catalysts. The technical problems of atom aggregation and carrier structure damage easily caused by instability of existing metal monatomic and carrier materials at continuous high temperature is solved. The method comprises the steps of 1 mixinghexabromobenzene and calcium carbide, dissolving in ethanol, and grinding; 2 centrifuging, washing and calcining the generated reaction product; 3 carrying out acid washing treatment on the calcinedproduct to obtain graphdiyne; 4 soaking graphdiyne in a metal precursor solution to obtain graphdiyne loaded with a metal precursor; and 5 carrying out joule heating treatment on the graphdiyne loadedwith the metal precursor to a certain temperature to obtain the graphdiyne-loaded metal monoatomic catalyst. The monoatomic catalyst prepared by the method can prevent continuous high temperature from damaging the stability of the catalyst carrier, and atom aggregation is avoided.
Owner:CHAOWEI POWER CO LTD +1
Microporous polymer-nano-metal particle catalyst and its preparation method and use
InactiveCN106607091AReduce dosageQuick responseOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsPolymer scienceBromine
The invention discloses a microporous polymer-nano-metal particle catalyst and especially relates to a microporous polymer-nano-palladium catalyst and a preparation method thereof. The preparation method comprises that prepared bromine-substituted triarylimidazole group-containing monomer 2, 4, 5-tris(4-bromophenyl)-1-alkylimidazole (TAI) undergoes a reaction to produce a microporous polymer, the microporous polymer is dissolved in DMF, and an appropriate amount of a H2PdCl4 aqueous solution is added into the DMF solution and undergoes a reaction, and excess NaBH4 is added into the reaction product so that the microporous polymer-nano-metal particle catalyst is obtained. The microporous polymer has high nitrogen content, has a multi-pore structure and helps to increase the loading amount of the transition metal. The catalyst has strong catalytic activity, good selectivity, mild reaction conditions, good reusability and a good market application value.
Owner:XIANGTAN UNIV
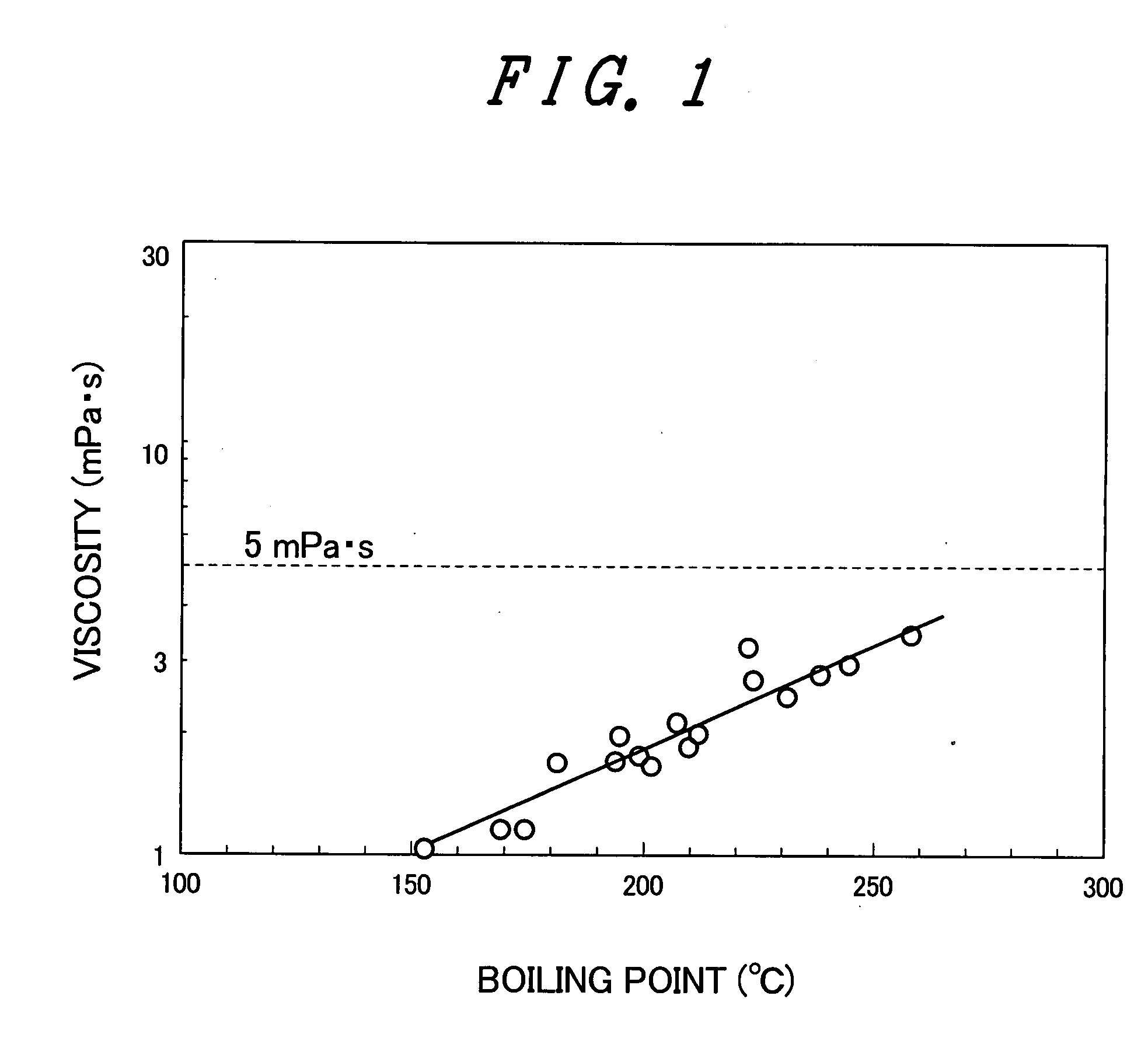
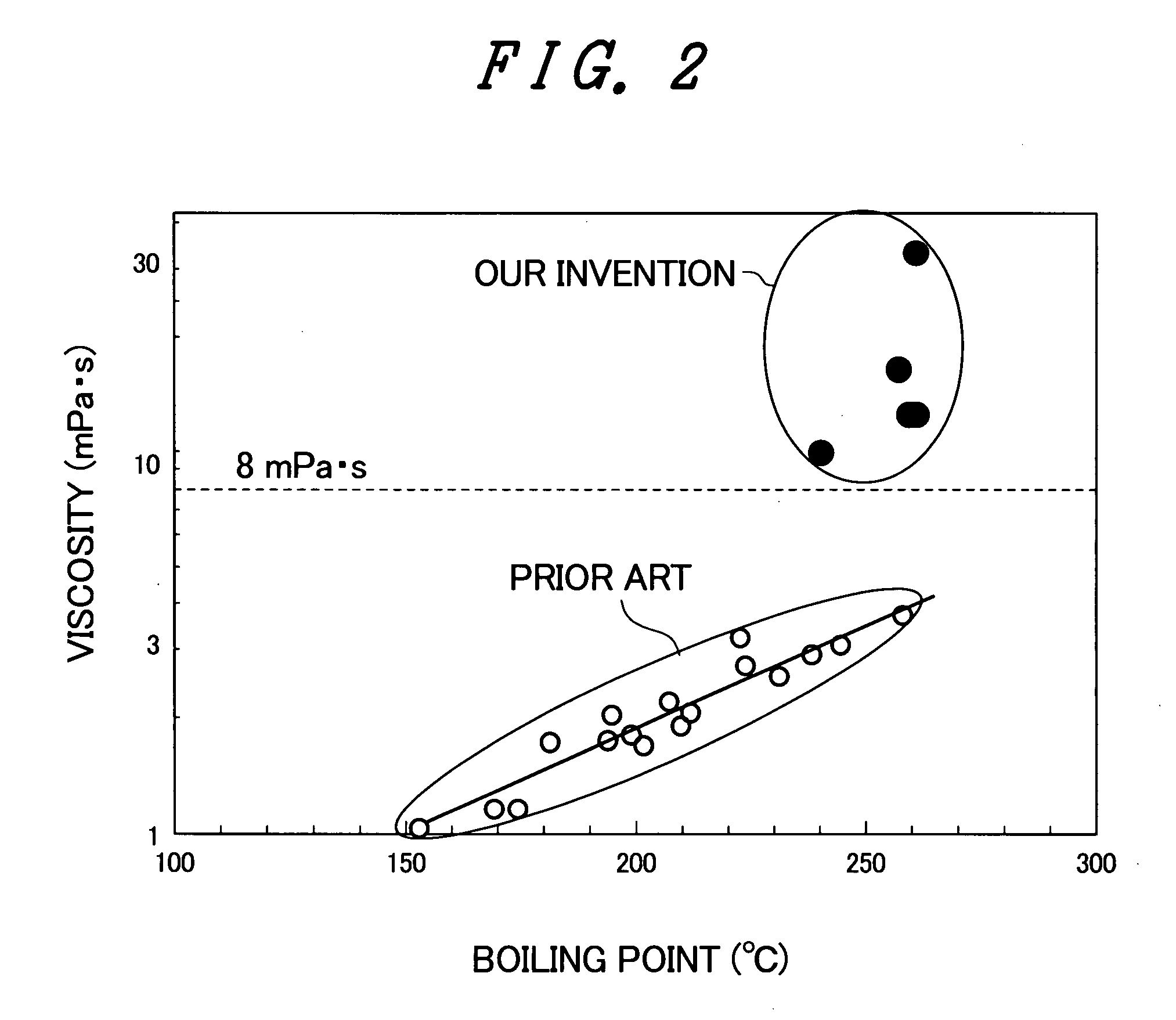
Organic EL display device and organic thin film device
InactiveUS20070254185A1High molecular weightViscosityDischarge tube luminescnet screensLamp detailsBenzeneOrganic film
An object of the invention is to provide a manufacturing method of an organic EL display device by fabricating a low molecular weight light emitting material on a lower electrode of an organic EL display device by an inkjet method. In order to achieve this object, in an organic EL display device including an organic material layer, the organic material layer is configured of a mixture of a low molecular weight material and a specified organic material. Concretely, at least one compound selected from 1,2,3-trimethoxytoleune, 1,2,3, 5-tetramethoxybenzene, 1,2-dimethoxy-5-bromobenzene, 3,4-dimethoxy-6-bromotoluene, 1,2,3-trimethoxy-5-bromo-benzene, and 3-bromo-1,2-benzodioxane is contained.
Owner:HITACHI DISPLAYS
Method for preparing 4 - bromine 2,6 - difluoro benzoic acid
ActiveCN101050176AEasy to industrializeSimple processOrganic compound preparationCarboxylic compound preparationLithiumBenzoic acid
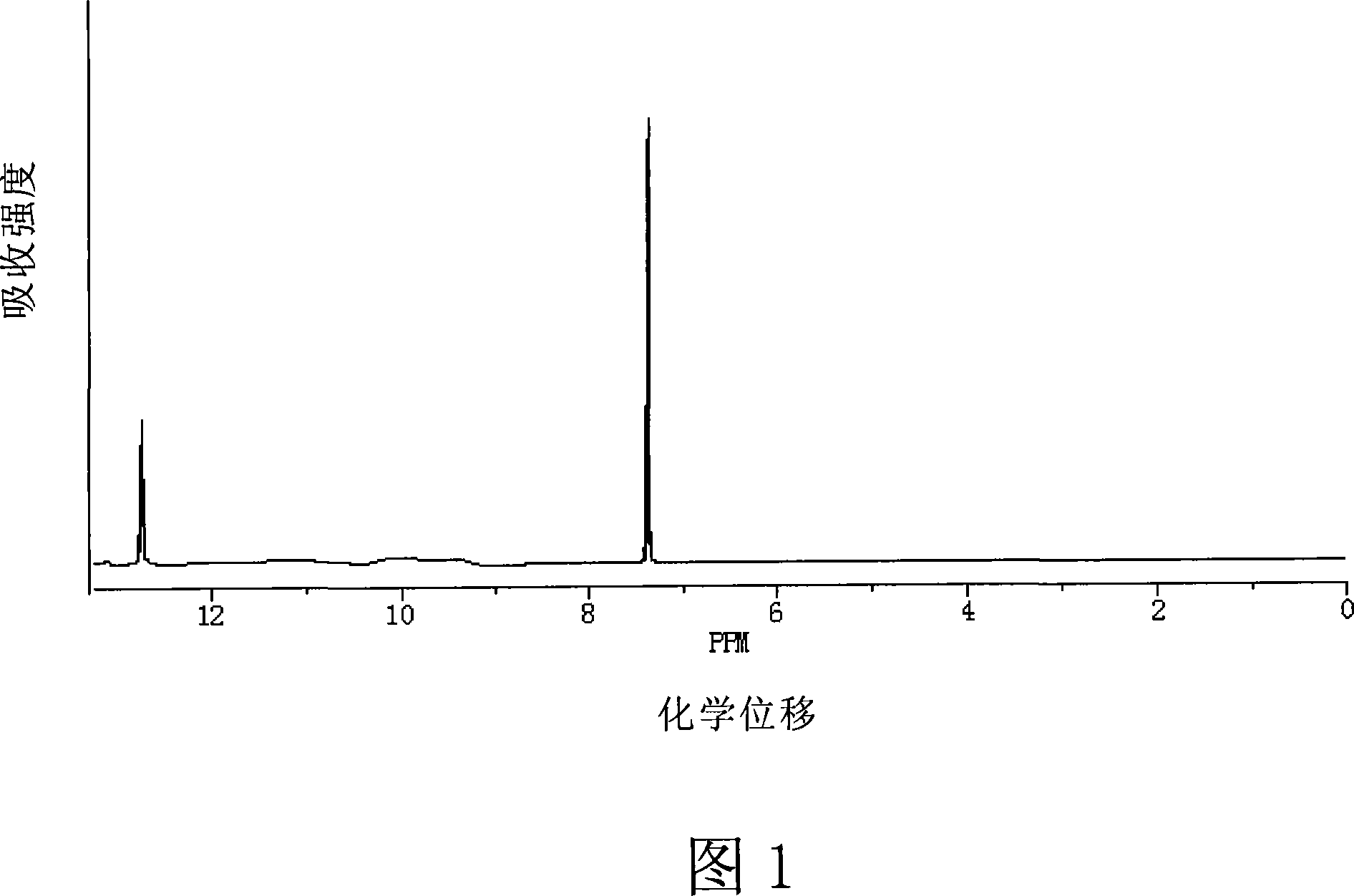
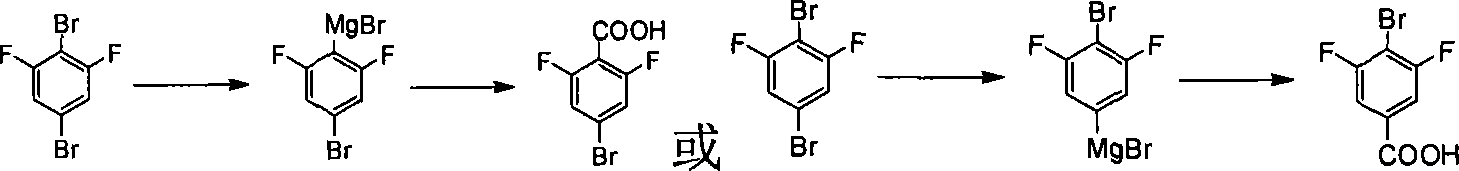
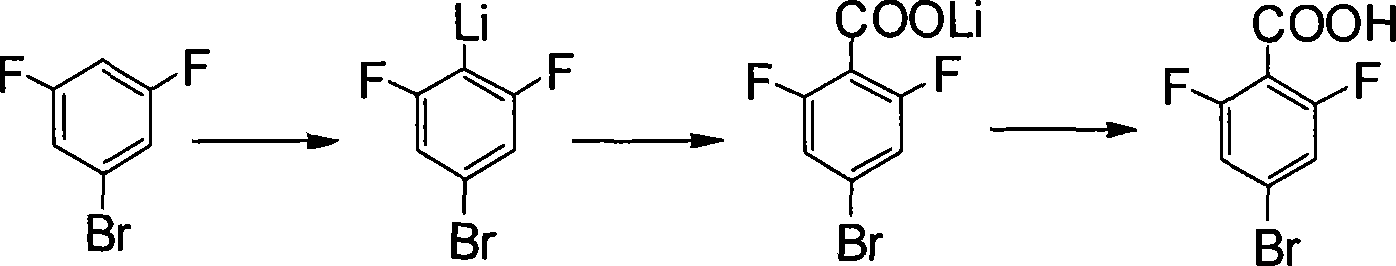
This invention provides a method for preparing 4-bromo-2, 6-difluorobenzoic acid. The method comprises: reacting 3, 5-difluoro bromobenzene and organic lithium reagent in organic solvent to obtain 4-substituted lithium salt of 3, 5-difluoro bromobenzene, and hydrolyzing to obtain 4-bromo-2, 6-difluorobenzoic acid. The method has such advantages as abundant raw materials, simple process, easy post treatment, mild reaction conditions, little pollution, and high yield.
Owner:SHANGHAI CHEMSPEC CORP
Elastic hydrophobic organic conjugated polymer, synthesizing method thereof and application thereof to removing of organic matters from water
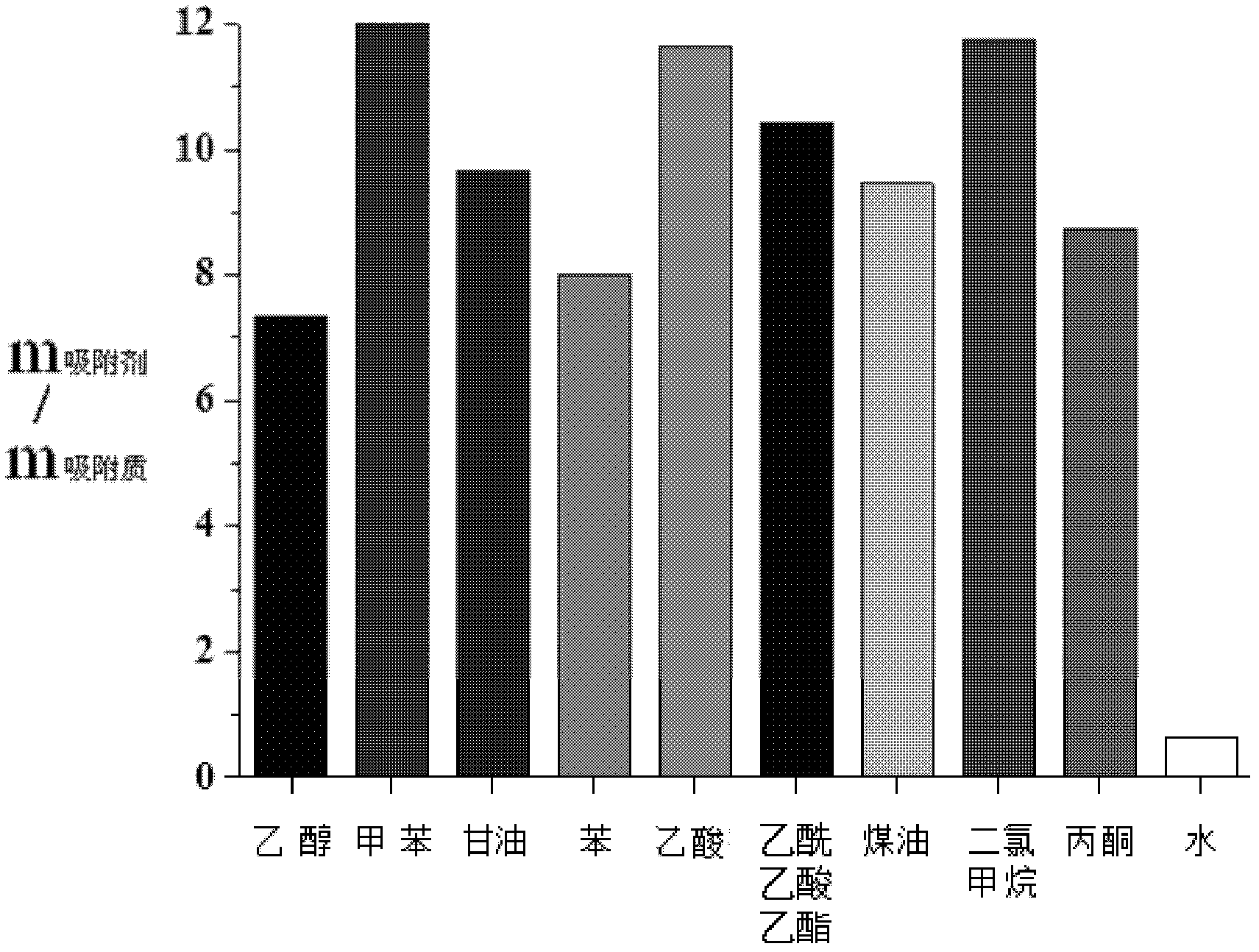
InactiveCN102504207ALarge particlesSmall particlesOther chemical processesWater/sewage treatment by sorptionSolventCopolymer
The invention discloses an elastic hydrophobic organic conjugated polymer, a synthesizing method thereof and an application thereof to removing of organic matters from water. The synthesizing method comprises the following steps of: connecting a benzene ring with an acetylene bond; particularly adding a catalyst, a nonpolar solvent and the like into 1.4-diacetylene-benzene and a 1,3,5-tribromobenzene derivative or a 1,3,5-triiodo benzene derivative serving as substrates in an inert atmosphere; undergoing a polymerization reaction; and filtering, washing, drying and the like to obtain a product. The product is centimeter grade or millimeter grade elastic particles containing a plenty of mesopores and gaps, so that material density is very low; and meanwhile, the copolymer has high elasticity and high hydrophobic property. When adsorption of liquid organic matters is saturated in the polymer, the polymer is taken out of an aqueous solution, organic matters are removed through mechanicalextrusion, and polymer particles can be used repeatedly, so that the cost on removal of the organic matters from water is lowered. The elastic hydrophobic organic conjugated polymer is suitable for batch production and environmental protection.
Owner:DALIAN UNIV OF TECH
Poly(bromoaryl)alkane additives and methods for their preparation and use
InactiveUS6743825B1Reduce quantityHalogenated hydrocarbon active ingredientsBiocideAlkaneFire retardant
An additive mixture useful as a flame retardant is described. The mixture is comprised of (i) a poly(bromophenyl)alkane having in the molecule in the range of 13 to 60 carbon atoms and in the range of two to four aryl groups and (ii) a poly(bromophenyl)bromoalkane having in the molecule in the range of 13 to 60 carbon atoms and in the range of two to four aryl groups, said poly(bromophenyl)bromoalkane being in an amount which is greater than 25 wt %, based on the total weight of the additive mixture. A facile process for making the poly(bromophenyl)bromoalkane is also described.
Owner:ALBEMARLE CORP
Thiophene polycyclic organic semiconductor material synthesis based on pyrene
InactiveCN105111216AImprove stabilityPlanar structureOrganic chemistrySemiconductor materialsSynthesis methods
The invention discloses thiophene polycyclic organic semiconductor material synthesis based on pyrene and belongs to the field of photoelectric conversion materials. The molecular formulas of compounds are C<76>H<86>N<4>S<4> and C<62>H<80>N<2>S<2>, and the structural formula can be seen in the Application of the invention. A synthesis method comprises the steps of making the pyrene react with liquid bromine, and obtaining 1,3,6,8-tetrabromo pyrene; making the 1,3,6,8-tetrabromo pyrene react with 1-octyne, obtaining a product, and then using palladium / carbon for conducting catalytic reaction,and obtaining 1,3,6,8-quadri-octane pyrene; using ruthenium trichloride for catalyzing the 1,3,6,8-quadri-octane pyrene, adjusting the using amount of sodium periodate, and obtaining tetrone and diketone; then making o-phenylenediamine and sulfoxide chloride react with liquid bromine, and obtaining 4,7-dibromo-2,1,3-diazosulfide; making thiophene react with thiadiazole, and obtaining diazosulfide; then making o-phenylenediamine and paratoluensulfonyl chloride react with liquid bromine, subsequently, making the mixture react with concentrated sulfuric acid, then making the mixture react with thionyl chloride, and obtaining 5,6-dibromo-2,1,3-diazosulfide; conducting operation same with the reaction process, and obtaining another type of diazosulfide; making a thiadiazole product to be subjected to ring-opening reaction, and obtaining two types of o-phenylenediamine products; making the tetrone and the diketone react with the two types of o-phenylenediamine respectively, and obtaining the compounds.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
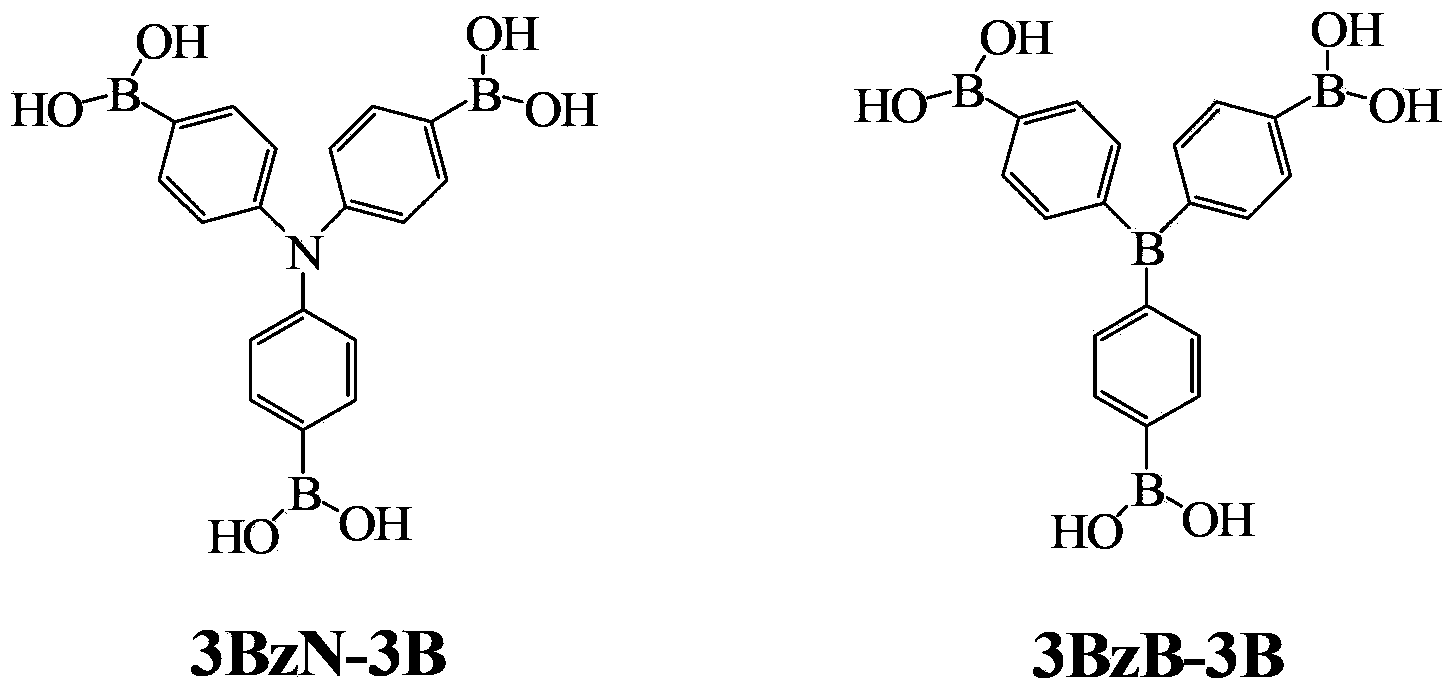
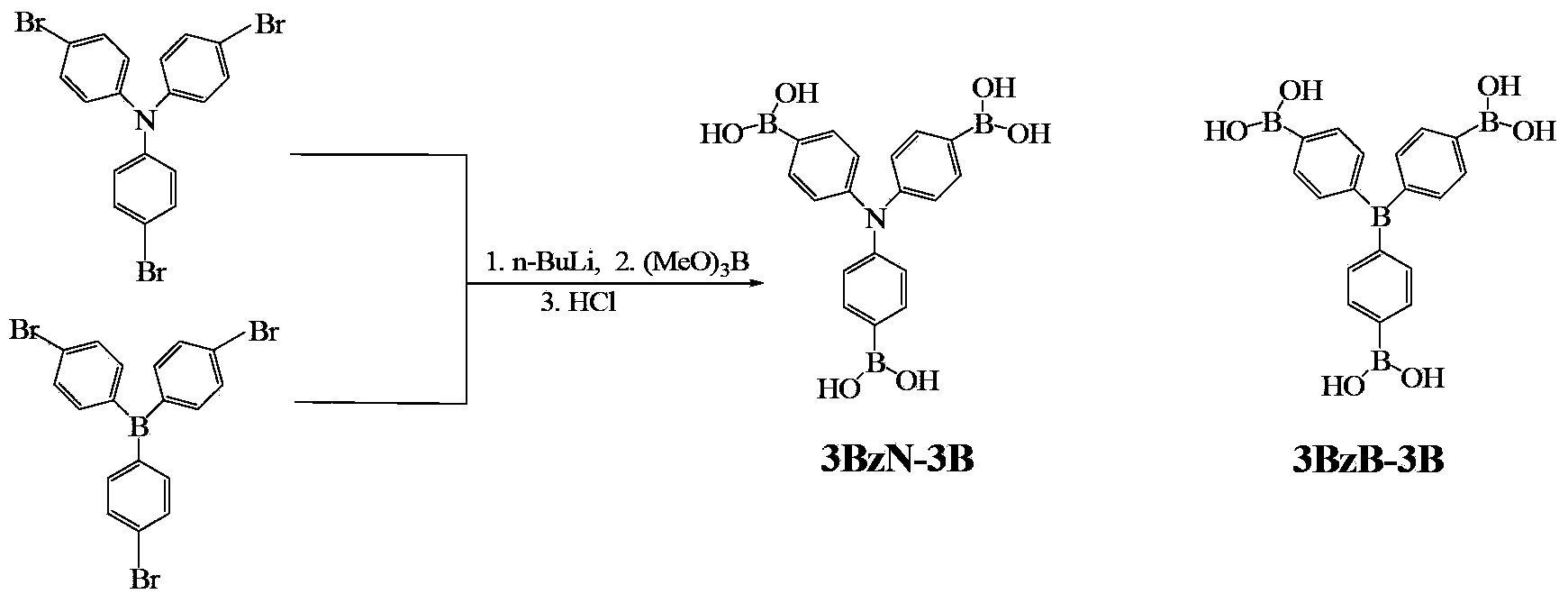
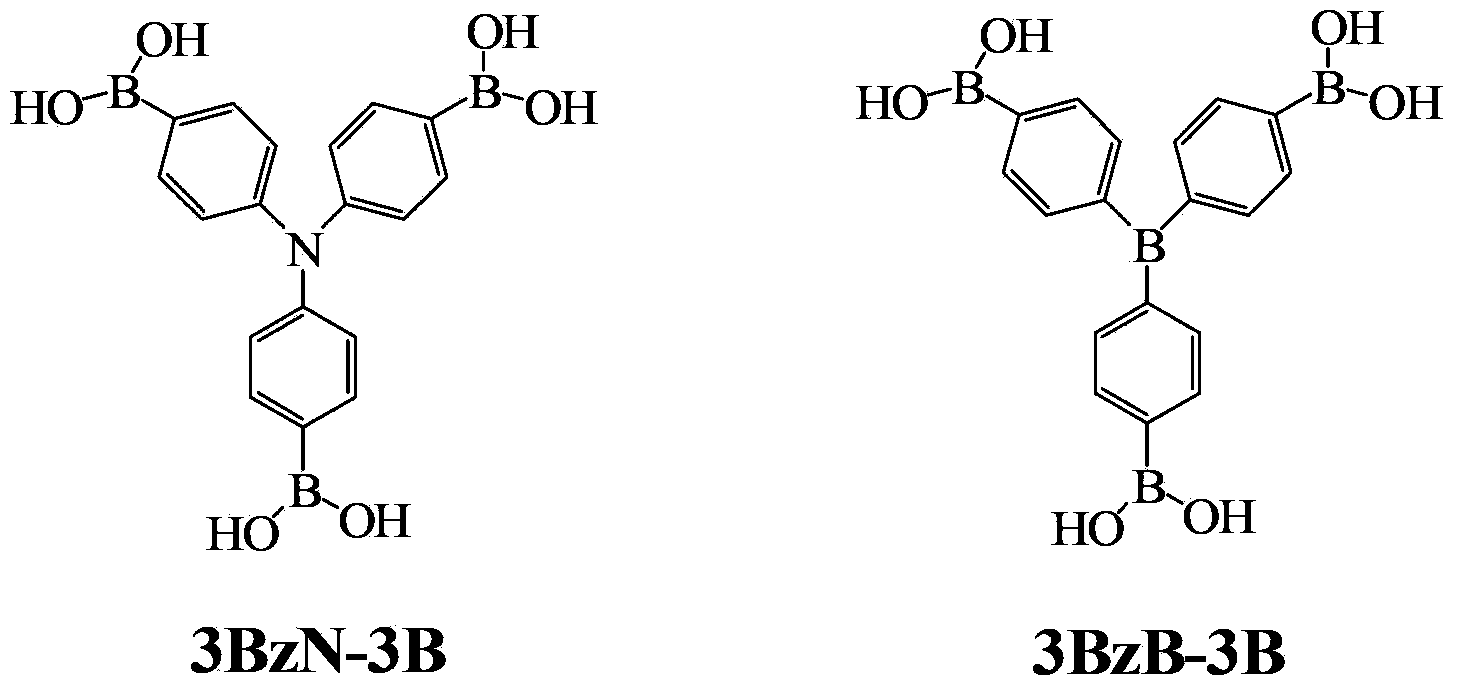
Arylboronic acid derivatives and preparation method thereof
ActiveCN103374024AStop burningTo solve the toxicGroup 3/13 element organic compoundsArylN-Butyllithium
The invention provides arylboronic acid derivatives and a preparation method thereof. The two arylboronic acid derivatives are tri(4,4',4'-triboric acid-phenyl)amino(3BzN-3B) and tri(4,4',4'-triboric acid-phenyl)boron(3BzB-3B), and the molecular structures are as shown in the specification. 3BzN-3B and 3BzB-3B are prepared by firstly, respectively reacting tri(4,4',4'-triboric acid-phenyl)amino and tri(4,4',4'-triboric acid-phenyl)boron with n-butyllithium, secondly, respectively carrying out nucleophilic substitution reaction with boric acid ester, and finally carrying out acidolysis by using diluted hydrochloric acid. The products of the arylboronic acid derivatives, namely, the 3BzN-3B and the 3BzB-3B are expected to be excellent environment-friendly organic fire retardants.
Owner:SOUTH CHINA UNIV OF TECH
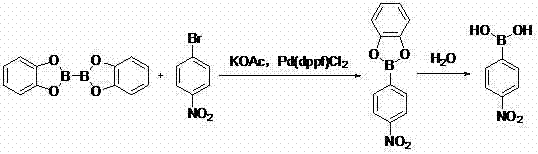
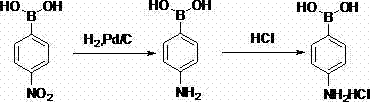
Method for preparing 4-amino benzene boric acid hydrochloride
InactiveCN103044471AEasy to handleAvoid cryogenicsGroup 3/13 element organic compoundsNitrobenzeneReaction temperature
The invention discloses a method for preparing 4-amino benzene boric acid hydrochloride. The method comprises the steps that 1), catechol and 4-nitrobromobenzene are subjected to a reaction for 2-3h in a polar solvent under the action of potassium acetate and Pd (dppf) C12, the temperature is lowered to a room temperature, filtering is performed, ethyl acetate is extracted, and a crude product is pulped and filtered through nonpolar varsol after an organic layer is concentrated, so that 4-nitrophenylboronic acid is obtained; and 2), the 4-nitrophenylboronic acid is subjected to palladium carbon catalytic hydrogenation in the ethyl acetate, hydrogenation is performed for 6-8h at the temperature ranging between 60 DEG C and 80 DEG C and under 0.8-1.0 MPa, filtering is performed at the temperature of 15-25 DEGC after the reaction, concentrated hydrochloric acid is added into the filtering liquid at the temperature of 0-10 DEG C, filtering is performed, and the crude product is obtained, then pulped with acetone, filtered and dried, so that the 4-amino benzene boric acid hydrochloride is obtained. According to the method, raw materials are low in toxicity and can be processed easily, copious cooling and low temperature are avoided simultaneously, the reaction temperature can be reached easily, the conditions are mild, the yield is high, the process is safe and reliable, and method is suitable for industrial production.
Owner:DALIAN NETCHEM CHIRAL TECH
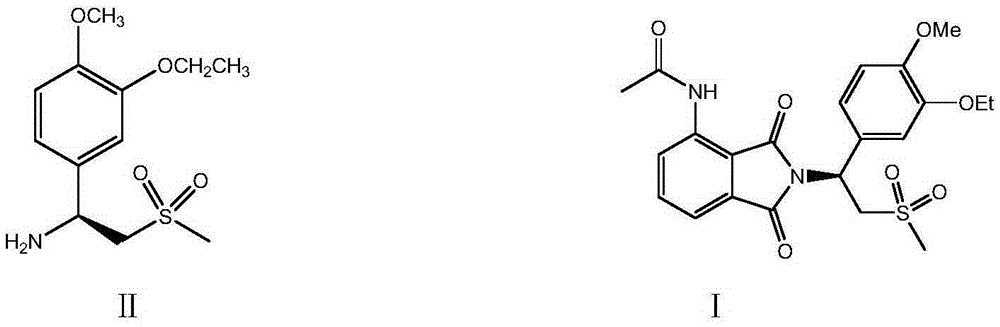
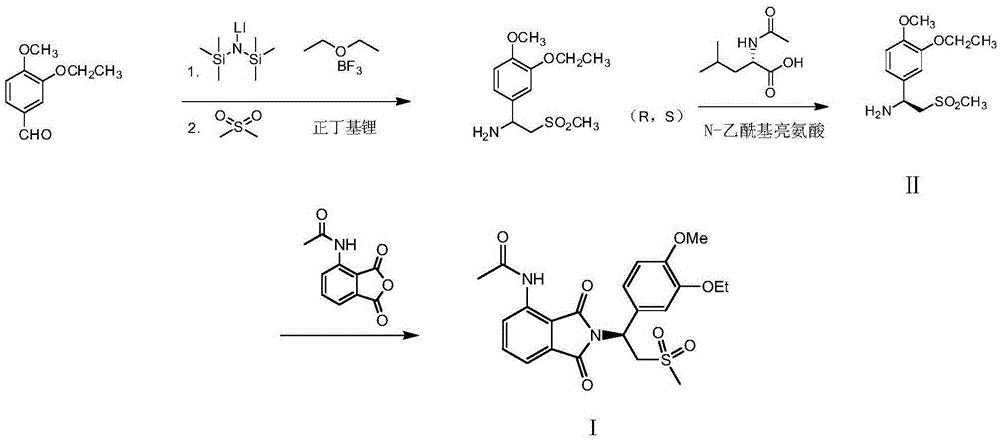
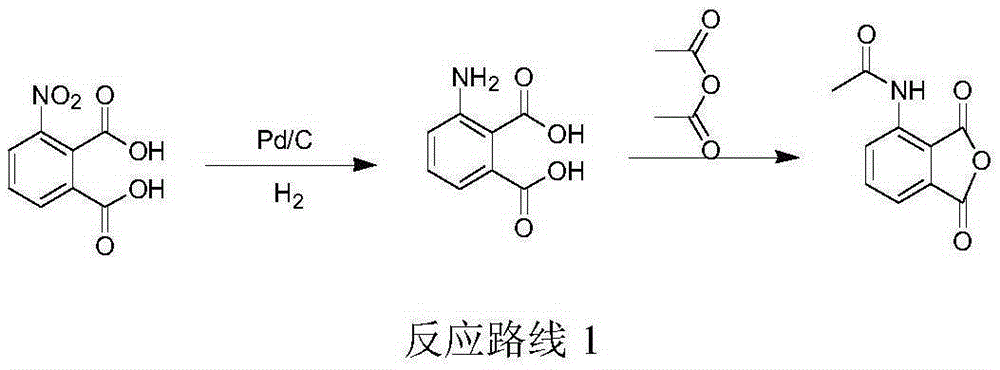
Preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine and preparation method of apremilast
ActiveCN105348172AHigh yieldEmission reductionOrganic chemistryOrganic compound preparationPhenylmagnesium bromideGrignard reaction
The invention relates to preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine and a preparation method of apremilast. 4-methoxy-3-ethoxy phenylmagnesium bromide is prepared from 4-methoxy-3-ethoxy bromobenzene through Grignard reaction; 4-methoxy-3-ethoxy phenylmagnesium bromide is in addition reaction with methylsulfonyl acetonitrile, and then hydrolysis and reduction are carried out, so that (R,S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine (III) is obtained; separation and filtering are carried out, so that (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine N-acetyl-L-leucine salt (IV) is obtained; through neutralization, (S)-1-(4-methoxy-3-ethoxy)phenyl-2-methylsulfonyl ethylamine (II) is obtained; the compound (II) is subjected to imidization, so that apremilast (1) is obtained. The mother liquor obtained after separation is recycled and converted into a compound IV, so that discharge of waste liquid is reduced, environment friendliness is realized and the cost is reduced.
Owner:XINFA PHARMA
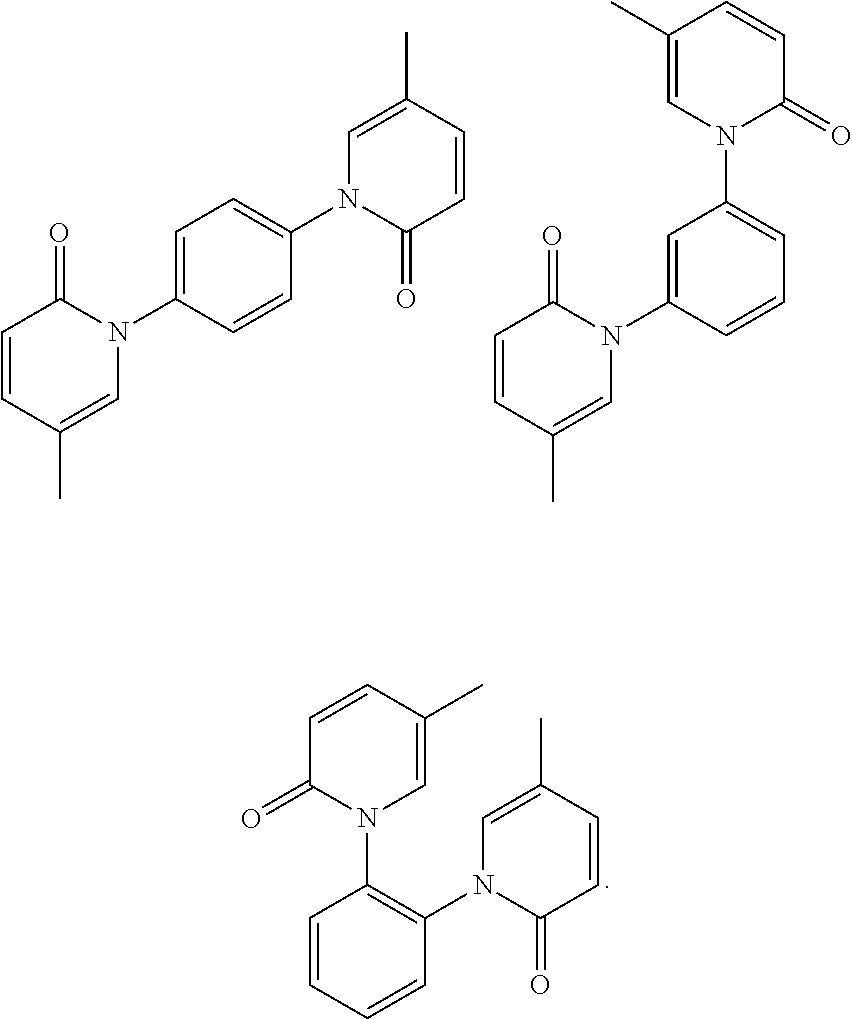
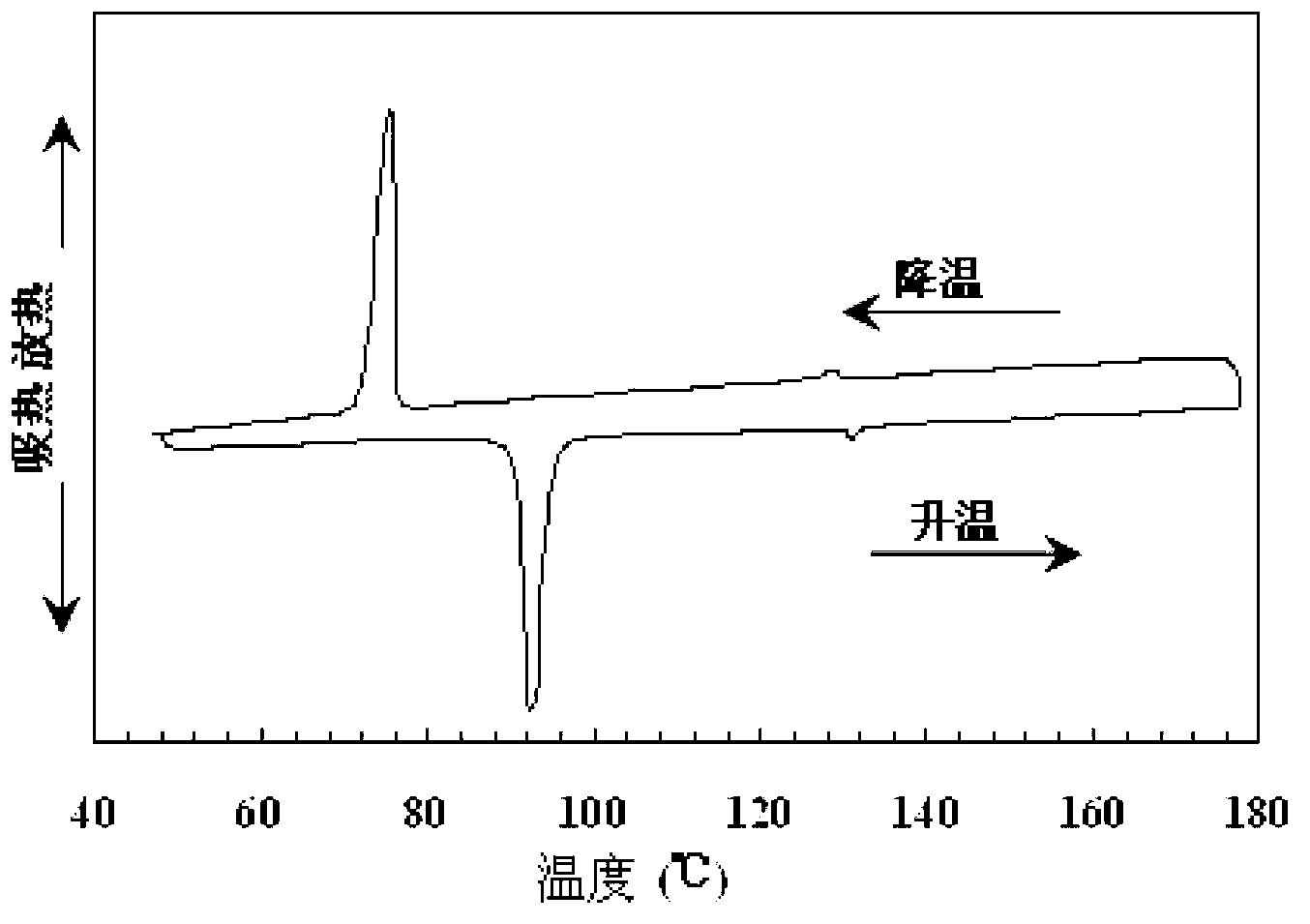
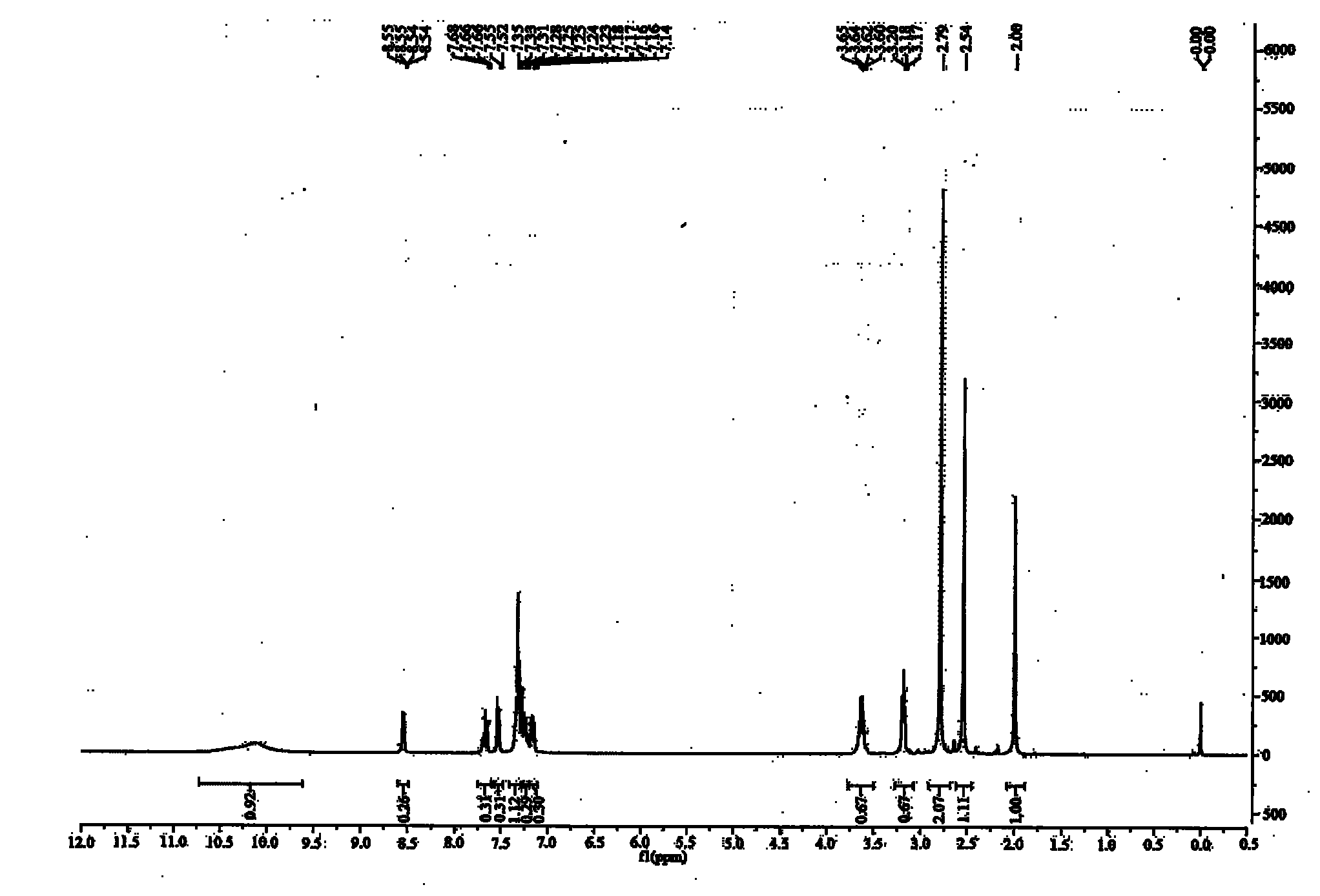
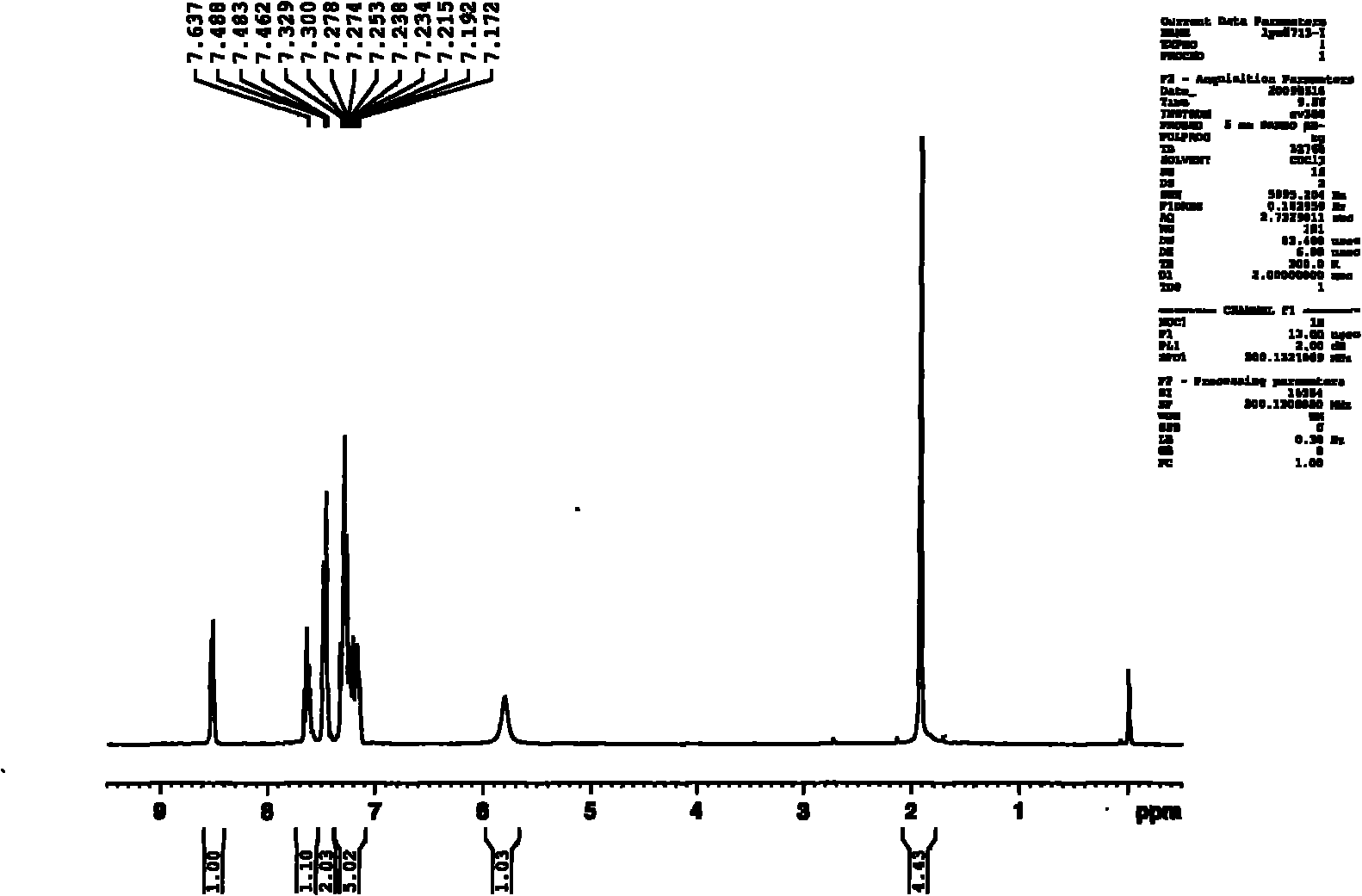
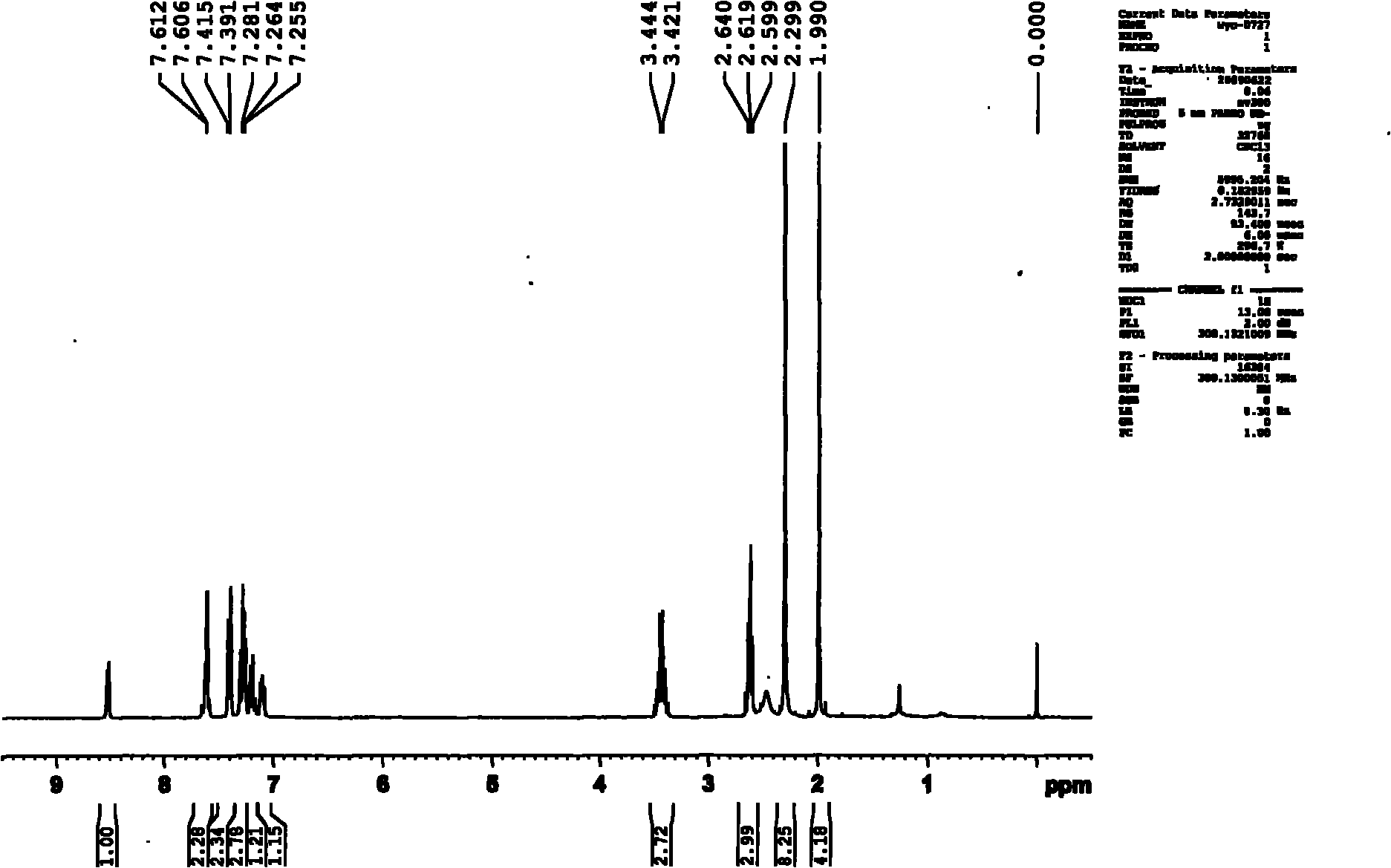
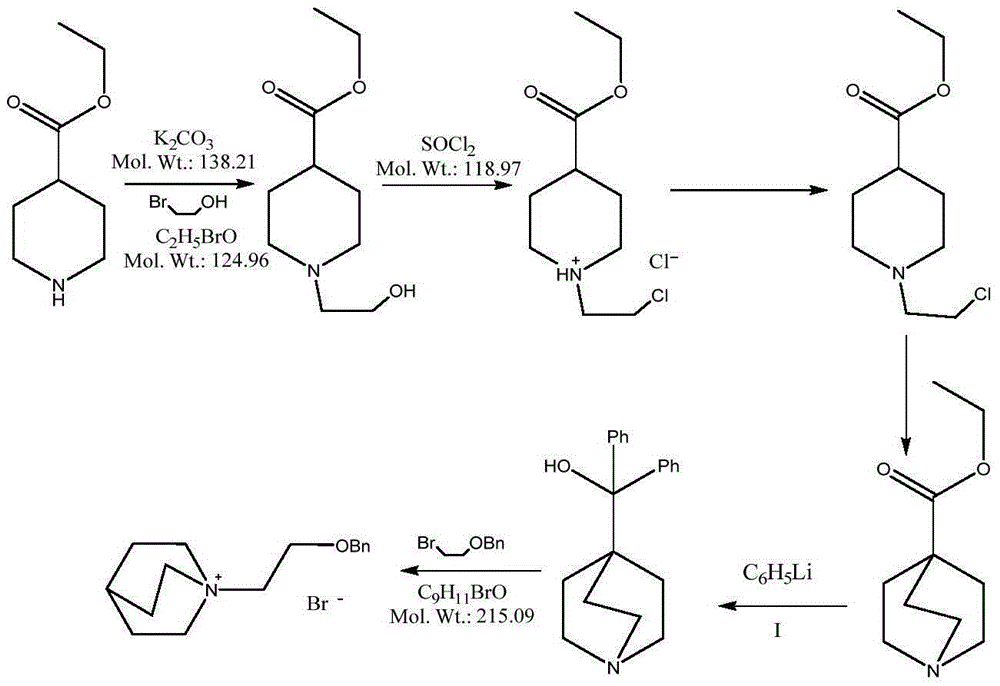
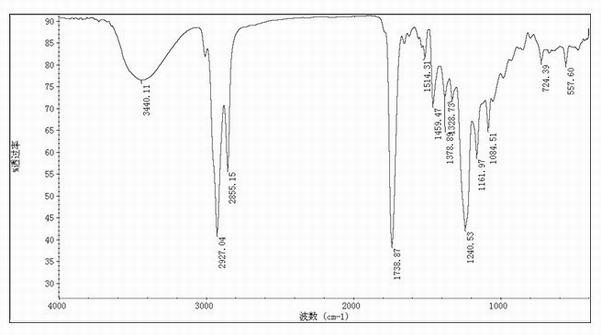
4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs
The invention belongs to the technical field of new drug synthesis, in particular to a class of 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-L-xylofuranose)pyrrolo [2,3-d]pyrimidine, its similar derivatives, and drugs for preparing anti-human liver cancer cells, human lung cancer cells, human breast cancer cells, human cervical cancer cells, and human gastric cancer cells. The compounds of the present invention are directed against 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-L-xylofuranose)pyrrolo[2,3 -d] The C6 position of pyrimidine is modified and synthesized by chemical methods. Experiments have shown that this type of compound can significantly inhibit the proliferation of various cancer cells, effectively induce apoptosis of cancer cells, including highly metastatic cancer cells, and can be used as drugs and drug components for treating cancer.
Owner:JILIN UNIV
Recoverable ligand-free mesoporous polymer palladium catalyst and synthetic method and application thereof
InactiveCN106334579AImprove stabilityHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsFiltrationPalladium catalyst
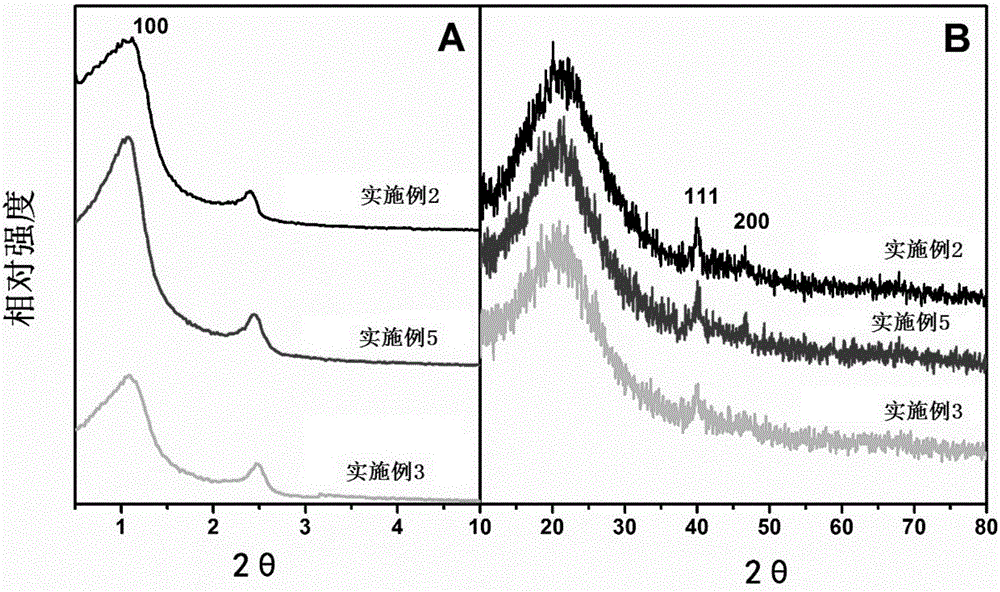
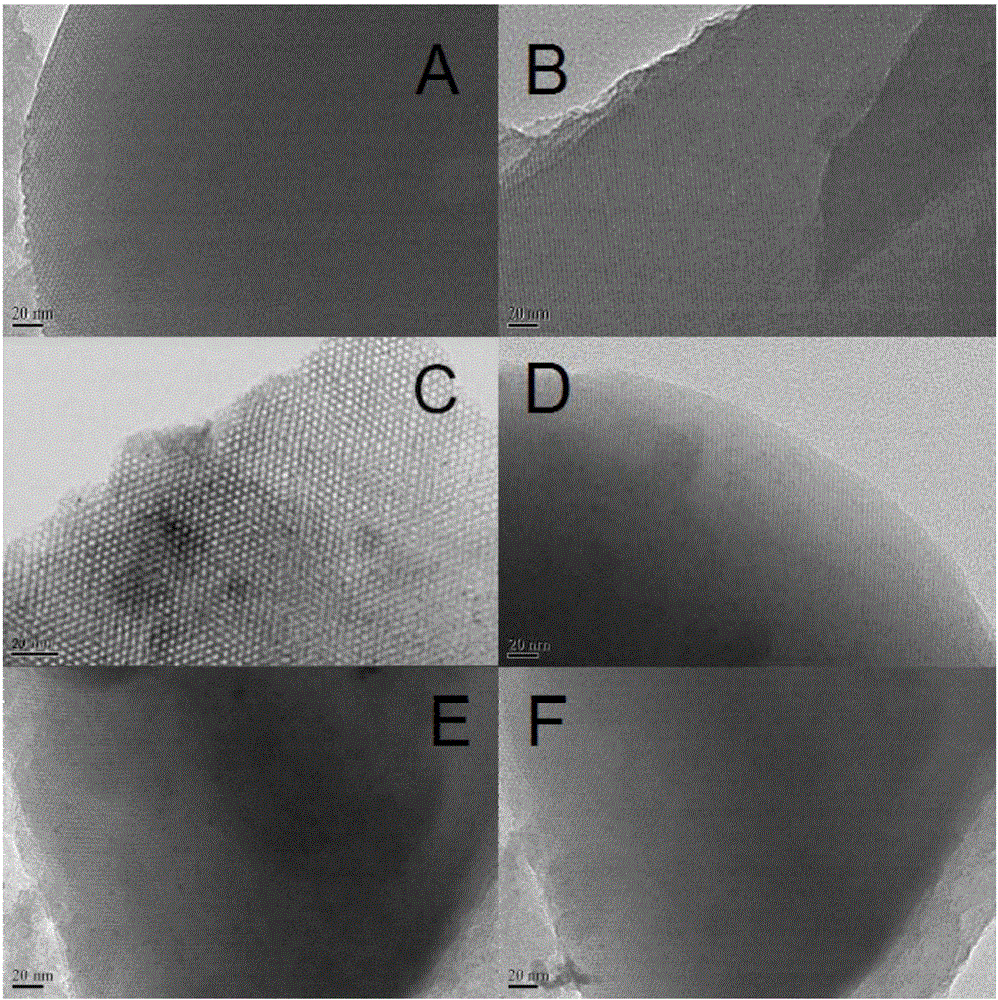
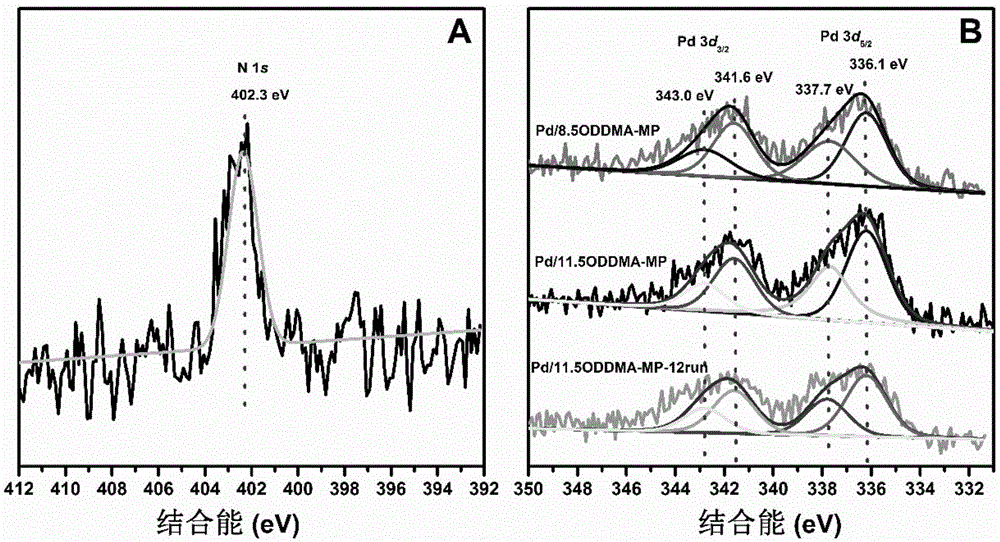
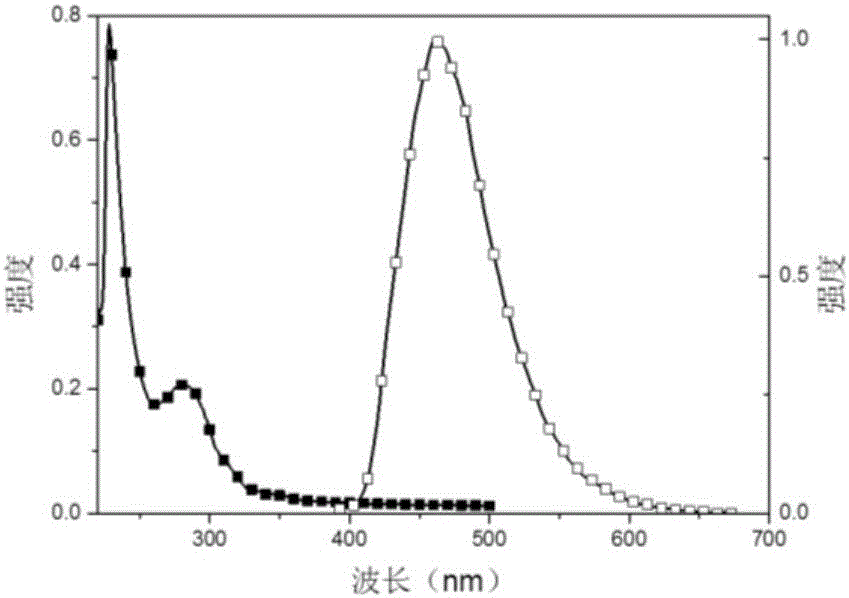
The invention relates to a recoverable ligand-free mesoporous polymer palladium catalyst and a synthetic method and application thereof. The catalyst has a two-dimensional hexagonal ordered mesostructure. specific surface area is 250-380 m<2> / g; pore diameter is 3.0-7.0 nm; mass content of a functional phase transfer agent is 8.5-13 wt%; mass content of metal palladium is 1-5 wt%; and particle size of palladium particles is 3-5 nm. The preparation comprises the following steps: adding a surfactant and hydrochloric acid into an organic solvent, stirring and reacting, successively adding a silicon source, quaternary ammonium salt and a carbon source, continuously stirring and reacting; carrying out a thermosetting reaction, and then carrying out high-temperature calcining to prepare a functional mesoporous polymer carrier; and mixing the functional mesoporous polymer carrier and a palladium source, stirring, carrying out suction filtration, washing, carrying out vacuum drying, and carrying out a reduction reaction. The catalyst is applied in aqueous medium catalysis of a Heck reaction between bromobenzene and styrene for generation of stilbene. In comparison with the prior art, the method of the invention is simple; and the catalyst is efficient and stable, can be recycled for many times and has a wide application prospect in the field of industrial catalysis.
Owner:SHANGHAI NORMAL UNIVERSITY
Thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, preparation method and application thereof
ActiveCN106831874AImprove efficiencyImproving Electron Injection Transport CapabilityGroup 5/15 element organic compoundsSolid-state devicesChlorodiphenylphosphinePhenyl group
The invention provides a thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, a preparation method and an application thereof and relates to the thermal excitation delayed fluorescence main material, the preparation method and the application thereof. The invention aims to solve the technical problem of low device efficiency caused by the easiness in quenching of the molecules of the present planar thermal excitation delayed fluorescence dye. The structural formula of the thermal excitation delayed fluorescence main material provided by the invention is as shown in the specification. The preparation method comprises the following steps: adding an o-dibromo-benzene derivative, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran and reacting; extracting and drying, and then directly purifying or vulcanizing / and oxidizing and then re-purifying, thereby acquiring the product; or adding o-dibromo-benzene derivative, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran and reacting and then reacting with chlorodiphenylphosphine, re-oxidizing and purifying, thereby acquiring the product. The thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives is applied to a thermal excitation delayed fluorescence electrofluorescence device.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka.patsnap.com/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000011.png)
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka.patsnap.com/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000012.png)
![4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs 4-amino-6-(3-(3-bromophenyl)phenyl)-5-cyano-7-(β-l-xylofuranose)pyrrolo[2, 3-d]pyrimidine, similar derivatives And for the preparation of antitumor drugs](https://images-eureka.patsnap.com/patent_img/03cb45ce-a15e-469a-98f4-94d24b90d681/HDA0000071034700000013.png)