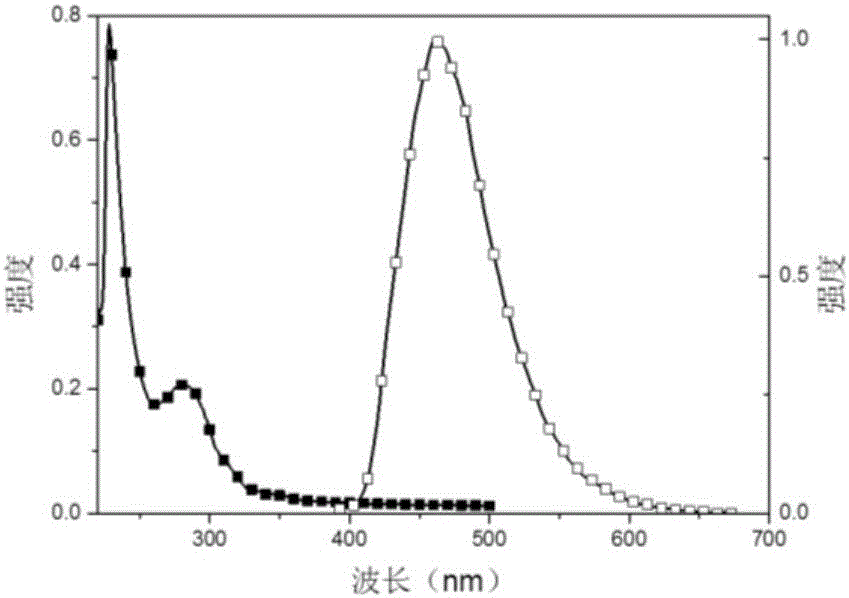
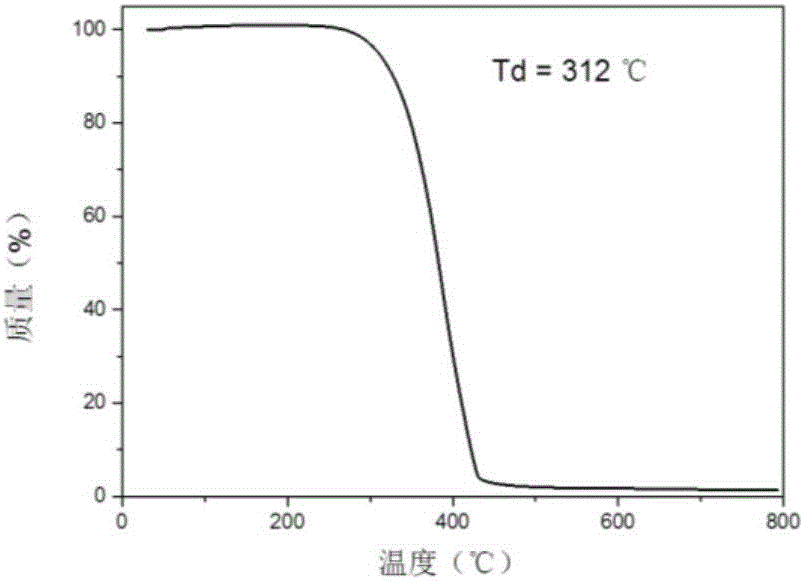
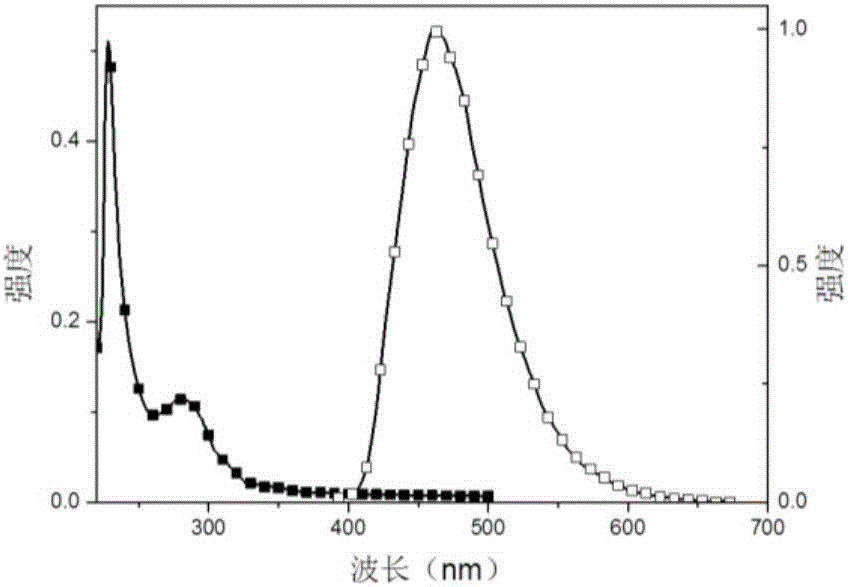
Thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, preparation method and application thereof
An aryl derivative and delayed fluorescence technology, which is applied in the fields of light-emitting materials, materials of organic semiconductor devices, chemical instruments and methods, etc., can solve the problems of easy quenching of molecules and low device efficiency, and achieve improved carrier injection. and transmission capacity, improve efficiency, and ensure the effect of effective transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0084] Embodiment 1: The structural formula of the thermally excited delayed fluorescence host material based on phosphine heteroaryl derivatives in this embodiment is Wherein R=Cz or t-Cz;
[0085] When R=Cz, it is recorded as 4CzDPDPA;
[0086] When R=t-Cz, it is recorded as 4tCzDPDPA;
[0087] or the structural formula is Wherein R=Cz or t-Cz;
[0088] When R=Cz, it is recorded as 4CzDPDPSA;
[0089] When R=t-Cz, it is recorded as 4tCzDPDPSA;
[0090] or the structural formula is Wherein R=Cz or t-Cz; X=S or O; Y=S or O;
[0091] When R=Cz, X=Y=S, it is recorded as 4CzDPDPS 2 A; When R=t-Cz, X=Y=S, it is recorded as 4tCzDPDPS 2 A;
[0092] When R=Cz, X=S, Y=O, it is recorded as 4CzDPDPSOA; when R=t-Cz, X=S, Y=O, it is recorded as 4tCzDPDPSOA;
[0093] When R=Cz, X=Y=O, it is recorded as 4CzDPDPO 2 A; When R=t-Cz, X=Y=O, it is recorded as 4tCzDPDPO 2 A;
[0094] The above Cz is a group t-Cz is a group
specific Embodiment approach 2
[0095] Specific embodiment two: The preparation method of the thermally excited delayed fluorescence host material based on phosphine heteroaryl derivatives described in specific embodiment one is as follows:
[0096] Add o-dibromobenzene derivatives, phenylphosphine dichloride, and n-butyllithium into tetrahydrofuran at a molar ratio of 1:(1~1.5):(2~4), under the protection of argon, React at a temperature of -60 to -85°C for 1 to 3 hours, then pour into water, extract with dichloromethane to obtain an organic layer, dry the liquid in the organic layer, and then directly purify or sulfide / oxidize and then purify , to obtain thermally excited delayed fluorescence host materials based on phosphine heteroaryl derivatives; wherein the derivatives of ortho-dibromobenzene are 1,2-dibromo-4,5-dicarbazolebenzene or 1,2-dibromo-4 ,5-Di-tert-butylcarbazolebenzene.
specific Embodiment approach 3
[0097] Specific embodiment three: the difference between this embodiment and specific embodiment two is that the vulcanization process is: adding sulfur powder to the organic layer liquid, stirring and reacting at a temperature of 15-30°C for 0.5-2 hours, Complete vulcanization. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com