Patents
Literature
61 results about "Chlorodiphenylphosphine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
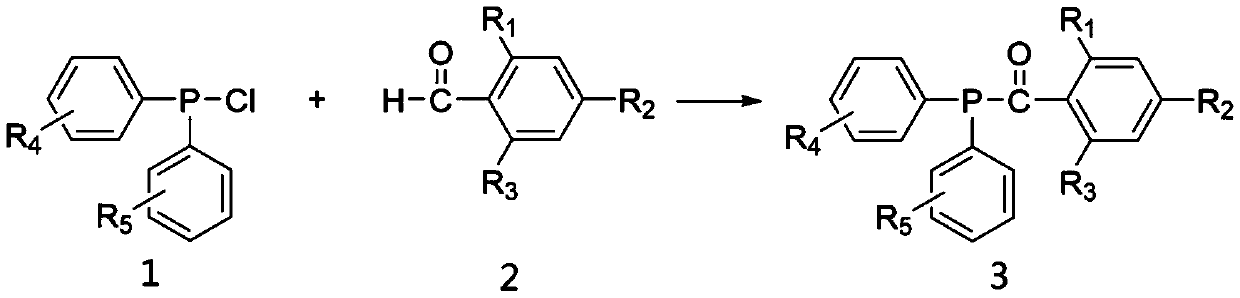
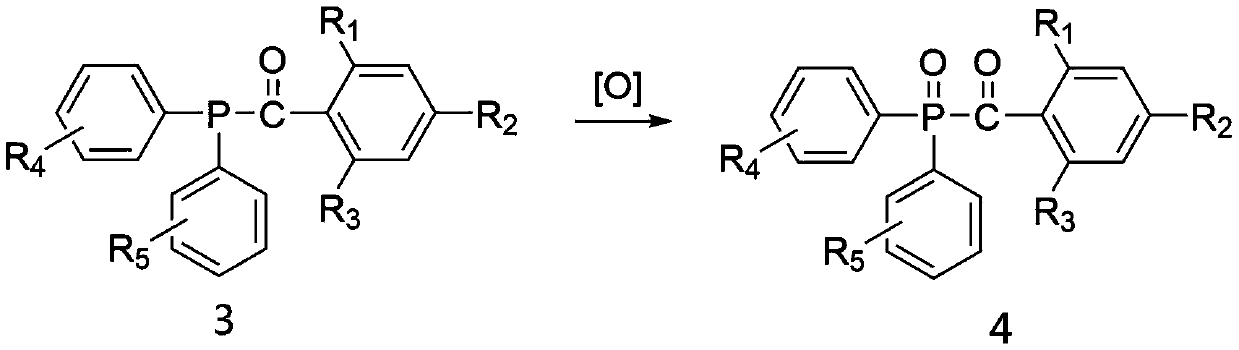
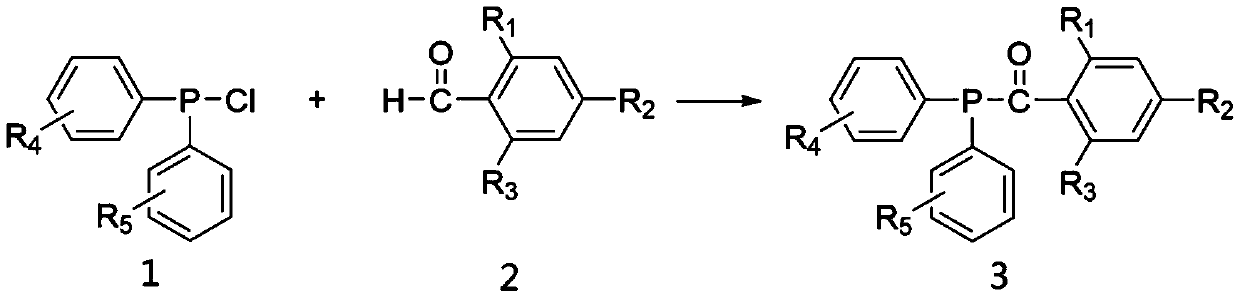
Chlorodiphenylphosphine is an organophosphorus compound with the formula (C₆H₅)₂PCl, abbreviated Ph₂PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph₂P group into molecules, which includes many ligands. Like other halophosphines, Ph₂PCl is reactive with many nucleophiles such as water and easily oxidized even by air.
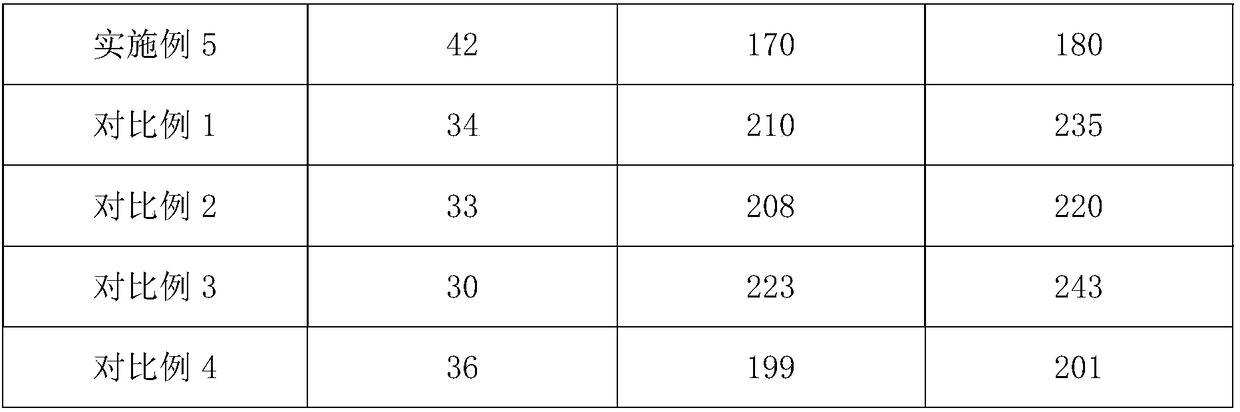
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
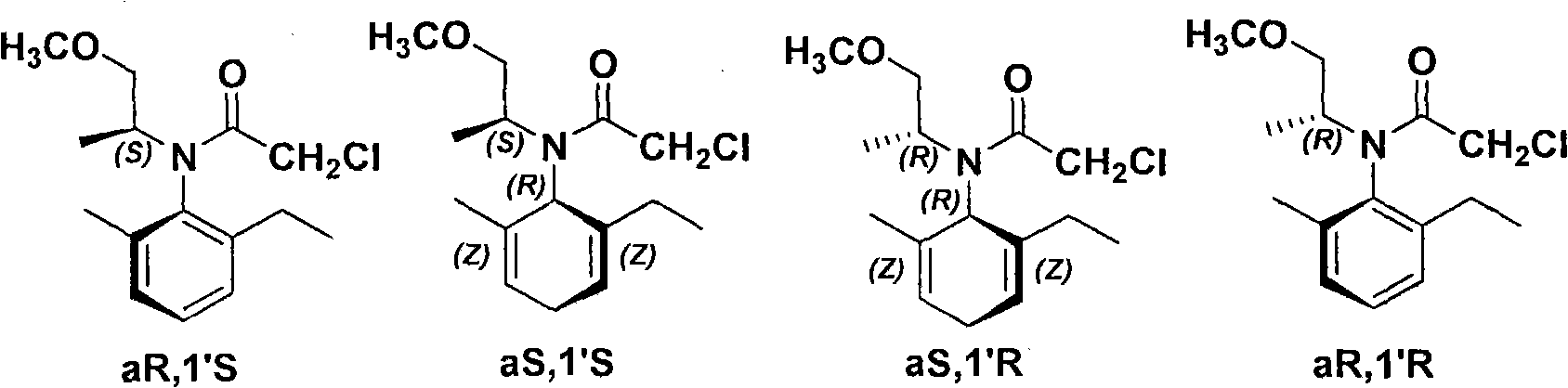
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
Ruthenium complex catalyzer for acetylene hydrochlorinate and preparation method and application thereof
ActiveCN108262072AImprove economyIncrease contentPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsChlorobenzeneRuthenium chloride
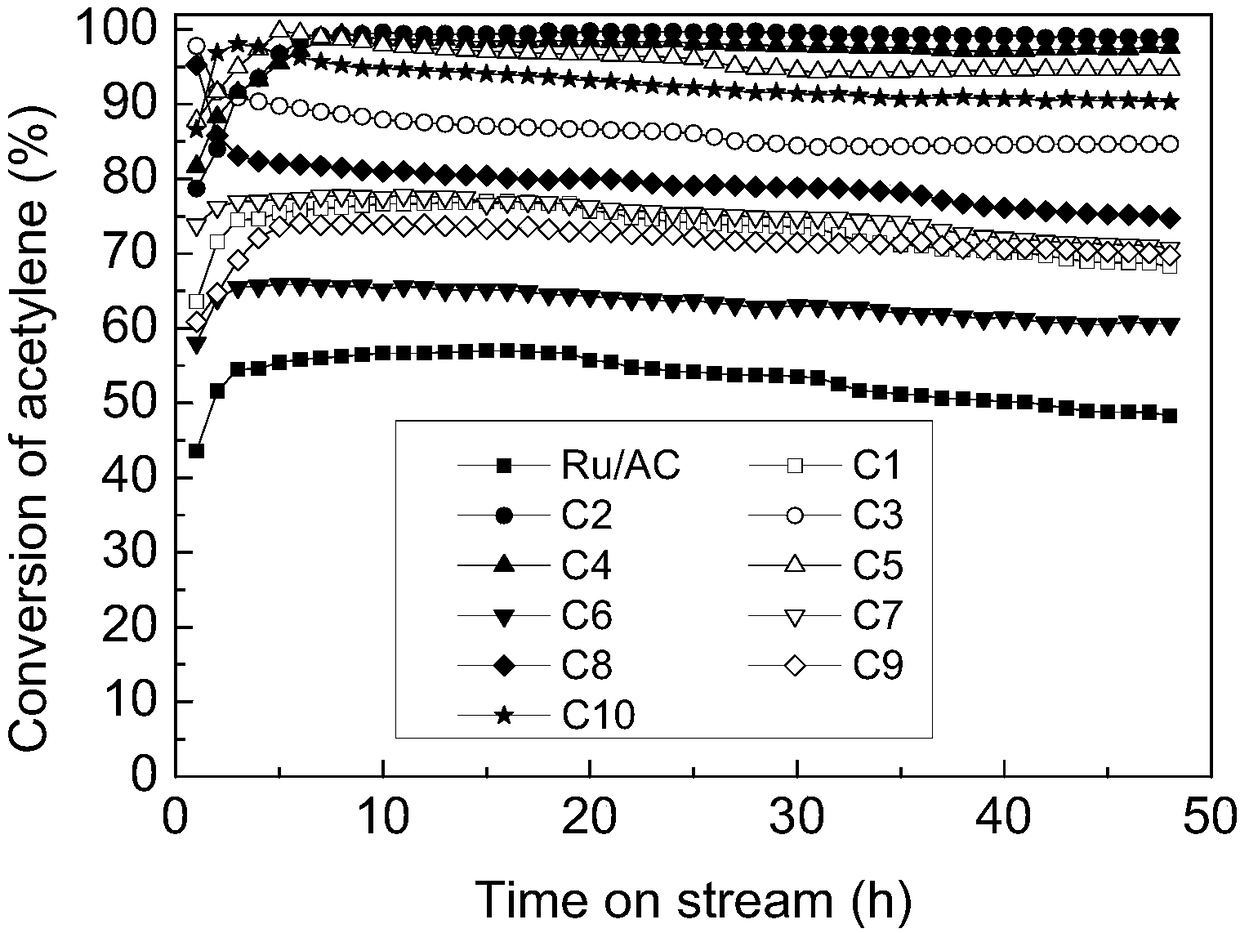
The invention discloses a ruthenium complex catalyzer for acetylene hydrochlorinate and a preparation method and application thereof. The catalyzer is characterized in that active carbon serves as catalyst support, and is loaded with ruthenium chloride and an organic ligand; the molar ratio of the ruthenium chloride and organic ligand is 1:1-6, and ruthenium accounts for 0.1-5.0% of the total weight of the catalyzer; the organic ligand is one or more of triphenylphosphine, pyridine, 2,2'-dipyridyl, acetylacetone, chlorodiphenylphosphine, chlorobenzene, cyclopentadiene, 4-methyl isopropyl benzene and 1,5-cyclooctadiene. The ruthenium complex catalyzer for the acetylene hydrochlorinate and the preparation method and application thereof have the advantages that the preparation process is simple, the catalytic activity and stability of the catalyzer are greatly improved, the catalyzer is more environmentally friendly, and the economy is high.
Owner:浙江天麟环境工程有限公司
Method for synthesizing diphenyl phosphonium chloride
InactiveCN102942591AReduce dosageDoes not destroy activityGroup 5/15 element organic compoundsChemical recyclingLiquid layerBenzene
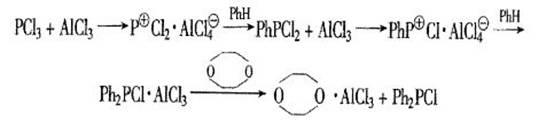
The invention discloses a method for synthesizing diphenyl phosphonium chloride. The method includes: performing reaction on raw materials of phosphorus trichloride and excessive benzene under catalyzing effect of Lewis acid ionic liquid, wherein after the reaction, the reaction liquid is divided into two layers, one layer is an ionic liquid layer, and the other layer is a mixed liquid layer. Through direct liquid separation, the ionic liquid layer is extracted, the extract liquor is mixed with the mixed liquid layer, and the mixture is respectively performed with normal pressure distillation and decompression distillation to obtain the target object diphenyl phosphonium chloride and byproduct phenyl phosphonium chloride. The ionic liquid is performed with normal pressure and decompression distillation so as to remove impurities and recover the diphenyl phosphonium chloride. Compared with the existing industrial process, the method has the advantages of being small in catalyst amount, requiring no decomplexing procedure, being easy to operate and control, not generating large amount of waste residue, being low in production cost, environment-friendly in production process and the like, and the catalyst is recoverable.
Owner:SHANDONG UNIV OF SCI & TECH
Environment-friendly synthesis method for diphenyl phosphine chloride
InactiveCN102399243AReduce pollutionIncrease incomeAluminium compoundsGroup 5/15 element organic compoundsNitrogen gasBottle
The invention discloses an environment-friendly synthesis method for diphenyl phosphine chloride and aims to solve the problem of environmental pollution in the conventional process. The method comprises the following steps of: adding phosphorus trichloride, benzene and aluminium trichloride into a three-mouth bottle respectively; introducing nitrogen for protection; stirring intensely; heating to 140 to 150 DEG C; maintaining reflux; discharging a large amount of white smoke until the white smoke disappears; cooling to room temperature; and performing aftertreatment operation comprising the steps of adding an organic solvent into reaction liquid in the step A, stirring for 0.5 hour, adding a decomplexing agent beta-chlorophosphate triethyl ester dropwise for 0.5 hour, stirring for 1.0 hour, standing for 1.0 hour, removing the lower layer of the decomlexing agent layer, distilling the organic solvent layer under reduced pressure to obtain a crude product diphenyl phosphine chloride, and distilling the crude product diphenyl phosphine chloride under high vacuum to obtain the pure product diphenyl phosphine chloride. By the method, the aluminium trichloride is recovered through an environment-friendly idea, so environmental pollution is reduced, a byproduct is increased, and yield is increased. The method is high in yield, low in cost and short in reaction time.
Owner:GANSU RES INSTION OF CHEM IND GRICI +1
Preparation method of photoinitiator TPO
InactiveCN103880882AReduce usageNo emissionsGroup 5/15 element organic compoundsBenzoyl chlorideAniline
The invention provides a preparation method of a photoinitiator TPO, comprises the following formula: 22.5% of diethyl aniline, 7.5% of absolute methanol, 34% of chlorodiphenylphosphine, and 36% of 2,4,6-trimethyl benzoyl chloride. The method comprises the following steps: 1, stirring and uniformly mixing diethyl aniline and absolute methanol; 2, warming to 20 DEG.C, slowly dropping chlorodiphenylphosphine, preserving heat for 2 hours; 3, pumping out the material, and filtering for later use; 4, uniformly mixing the 2,4,6-trimethyl benzoyl chloride; 5, warming to 80 DEG.C, slowly dropping the material for later use in the step 3, and then preserving for 1 hour; 6, carrying out suction filtering by using a suction filtering groove to obtain the photoinitiator TPO. The process is simple, the flammable and combustible solvent such as methylbenzene is unnecessary to use in the reaction process, the preparation method is safe, reliable and in favor of energy-saving and environment-protecting.
Owner:XIANGYANG KEMIN CHEM TECH CO LTD
Method for compounding 2,4,6-trimethylbenzoyl-diphenylphosphine oxide through 'one-pot process'
ActiveCN107304220AReduce manufacturing stepsReduce decreaseGroup 5/15 element organic compoundsDiphenylphosphine oxideBenzoyl chloride
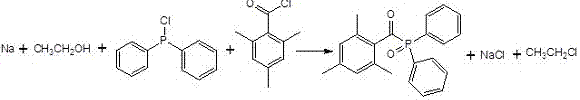
The invention aims to provide a method for compounding 2,4,6-trimethylbenzoyl-diphenylphosphine oxide through 'one-pot process'. According to the preparation method, a sodium, ethanol, chlorodiphenylphosphine and 2,4,6-trifluoromethyl benzoyl chloride 'one-pot process' is firstly adopted for replacing the original diphenyl oxethyl phosphine and acyl chloride process for preparing 2,4,6-trimethylbenzoyl-diphenylphosphine oxide, so that the method can greatly reduce the preparation steps and processes, has the characteristics of low cost, simplicity in operation and high yield and can more effectively meet the increasing market requirements at present.
Owner:INSIGHT HIGH TECH JIANGSU CO LTD
Thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, preparation method and application thereof
ActiveCN106831874AImprove efficiencyImproving Electron Injection Transport CapabilityGroup 5/15 element organic compoundsSolid-state devicesChlorodiphenylphosphinePhenyl group
The invention provides a thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives, a preparation method and an application thereof and relates to the thermal excitation delayed fluorescence main material, the preparation method and the application thereof. The invention aims to solve the technical problem of low device efficiency caused by the easiness in quenching of the molecules of the present planar thermal excitation delayed fluorescence dye. The structural formula of the thermal excitation delayed fluorescence main material provided by the invention is as shown in the specification. The preparation method comprises the following steps: adding an o-dibromo-benzene derivative, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran and reacting; extracting and drying, and then directly purifying or vulcanizing / and oxidizing and then re-purifying, thereby acquiring the product; or adding o-dibromo-benzene derivative, dichlorophenyl phosphine and n-butyllithium into tetrahydrofuran and reacting and then reacting with chlorodiphenylphosphine, re-oxidizing and purifying, thereby acquiring the product. The thermal excitation delayed fluorescence main material based on phosphonic aryl derivatives is applied to a thermal excitation delayed fluorescence electrofluorescence device.
Owner:HEILONGJIANG UNIV
Raw material components and production method of diphenyl phosphorus chloride
InactiveCN101481390AShort reaction timeLess side effectsGroup 5/15 element organic compoundsChemical productsSolvent
The invention relates to raw material components and a production method of chlorodiphenylphosphine, and belongs to the technical field of chemical product formulas and production methods. Based on mass part, the raw material components are as follows: 0.8-1.5 parts of an active metal, 1-4 parts of benzene halide, 1-3 parts of phosphorus trichloride, 1-8 parts of an organic solvent and 0.03-0.1 part of a catalyst. The production method comprises the following steps: 1. mixing and adding the organic solvent and the active metal to a dispersing kettle based on the mass part, heating and stirring to disperse the active metal, and adding the benzene halide for a first initiation; 2. cooling the materials after dispersion, adding the same amount of the benzene halide for a second initiation, cooling and adding the remaining benzene halide and the phosphorus trichloride; 3. allowing reaction, adding the catalyst, and heating for reaction; 4. filtering through a press filter cone when the materials are hot; and 5. putting the filter pressed materials in a rectifying still and stirring, distilling off the solvent at normal pressure, rectifying a finished chlorodiphenylphosphine product at negative pressure, checking and packaging. The raw material components and the production method have the advantages of 1. shortening the reaction time, reducing the side reaction and improving the yield; and 2. avoiding a hydrolysis procedure, eliminating sewage discharge and reducing the raw material consumption.
Owner:ZHAOYUAN SONGHE CHEM
Synthesis method of diphenylphosphine chloride
PendingCN110218226AHigh yieldHigh purityGroup 5/15 element organic compoundsChemical synthesisBenzene
The invention discloses a synthesis method of diphenylphosphine chloride, belongs to that technical field of chemical synthesis and solves the problem of low yield of the prior diphenylphosphoric chloride product. The synthesis method of diphenylphosphine chloride is characterized by comprising the following steps: under that protection of dry nitrogen, opening and stirring, adding benzene, phosphorus trichloride and catalyst to a reactor, and raising the temperature to 50 DEG C-80 DEG C and performing reflux reaction at 50 DEG C-80 DEG C for 2-3 h; after the reaction in step S01 is completed, raising the temperature to 85-200 DEG C and performing reflux reaction at 85-200 DEG C; at that end of step S02, after the reactor is cooled down, adding benzene into the reactor, adding a decomplexing agent, stirring until the decomplexing agent is dissolved and distilling to remove benzol, and then performing vacuum distillation to obtain the front fraction and the rear fraction. The inventionhas the advantages of high product yield.
Owner:ZHEJIANG YANGFAN NEW MATERIALS
Aryl phosphine chloride production waste residue treatment process
ActiveCN108147431ASimple operation processLow costTransportation and packagingSolid waste disposalAluminium chlorideSolubility
The invention discloses an aryl phosphine chloride production waste residue treatment process, and particularly a diphenyl phosphine chloride production waste residue treatment process. According to the process, aluminium chloride is separated from potassium chloride by utilizing different solubility of the aluminium chloride and the potassium chloride in n-butyl alcohol; the recycled potassium chloride can be re-utilized directly as a complexing agent; aluminum chloride crystals obtained after hydrolysis can also be sold out directly as a product; a small amount of organic distillation residue is outsourced for treating.
Owner:CHANGSHANG NEWSUN CHEM IND
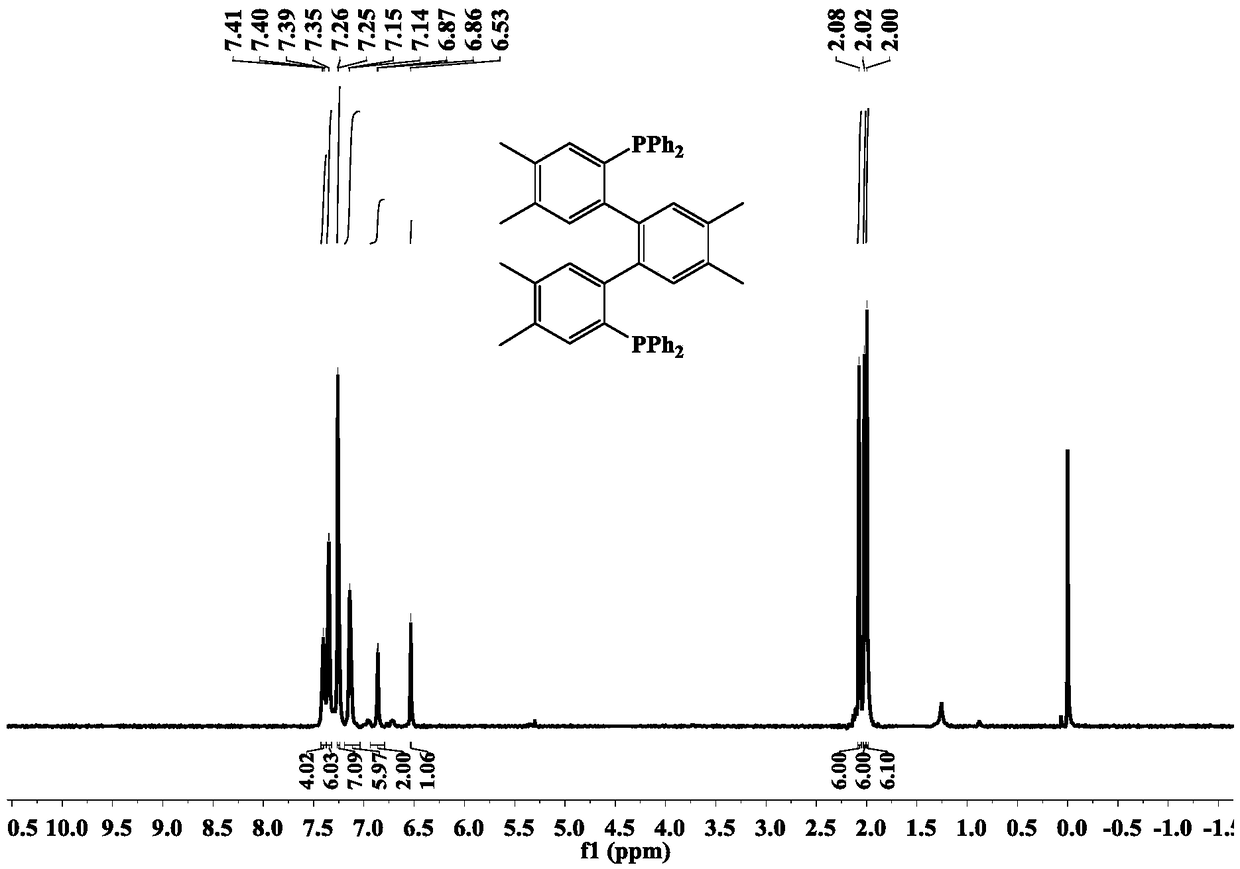
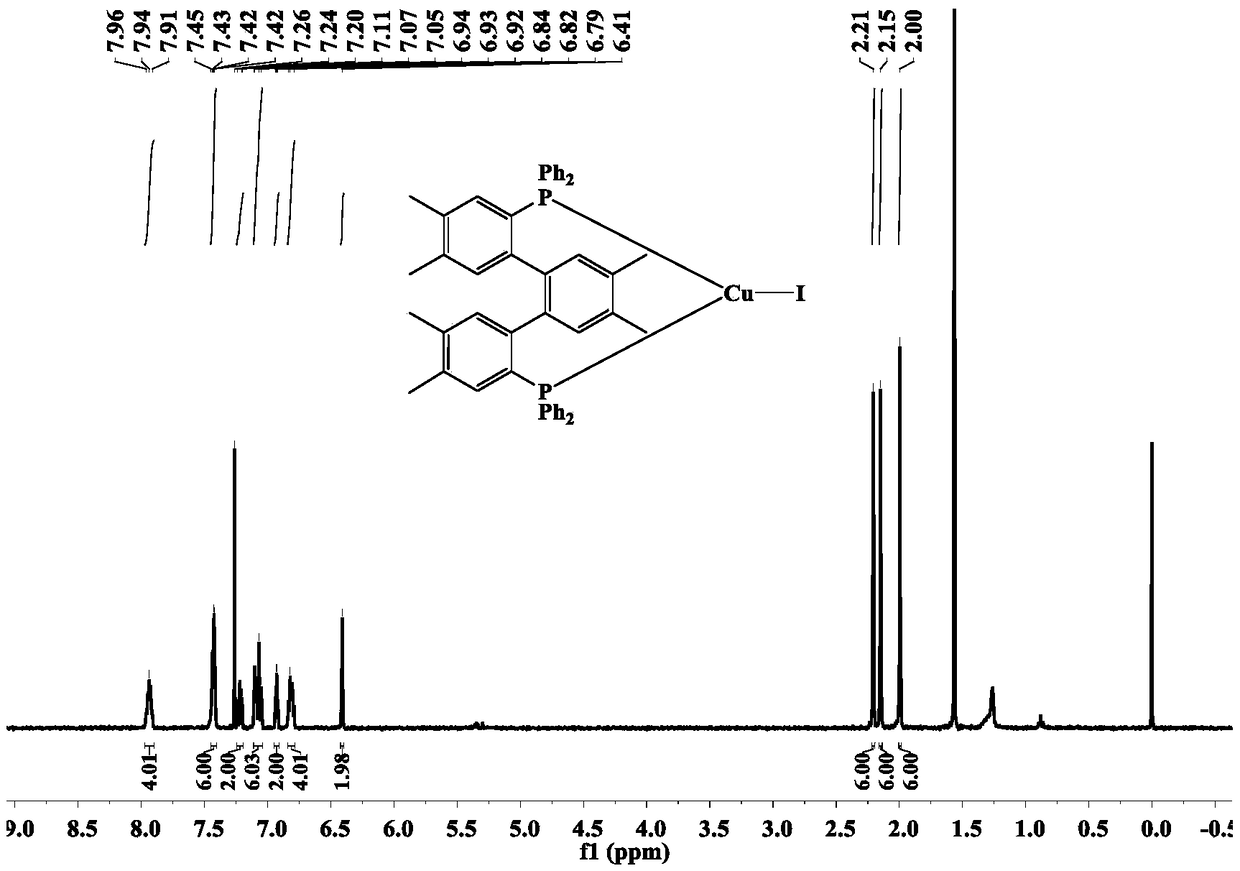
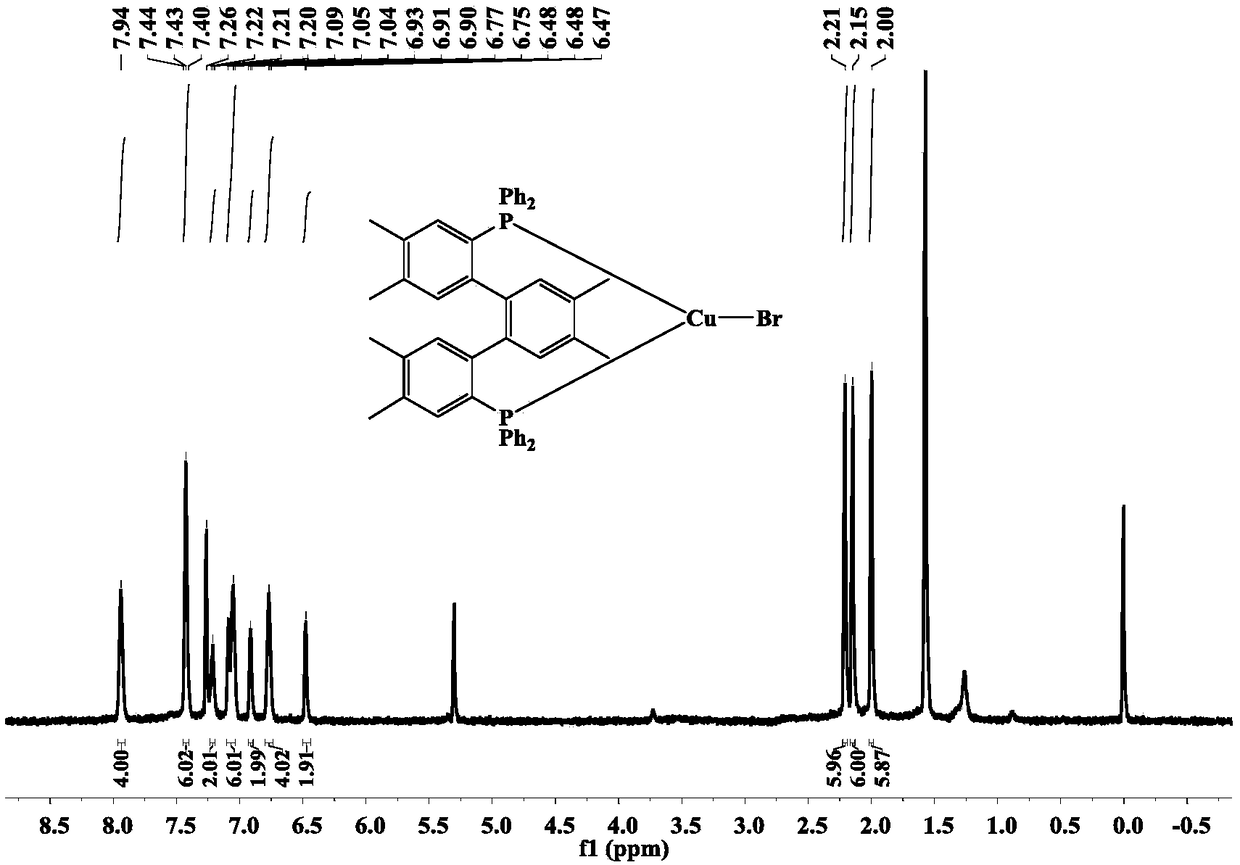
Terphenyl biphosphine three-coordination cuprous halide complex as well as synthesis method and application
ActiveCN108752382AHigh yieldThe synthesis method is simpleGroup 5/15 element organic compoundsOrganic chemistry methodsFluorescenceSynthesis methods
The invention relates to a terphenyl biphosphine three-coordination cuprous halide complex as well as a synthesis method and application and belongs to the technical field of light emitting materials.According to the complex, 1,2-dimethyl-4,5-dibromo-benzene is adopted as initial raw materials, a terphenyl bilithium reagent is synthesized through intermolecular coupling reactions, ligand dbdp issynthesized through a nucleophilic substitution reaction with diphenyl chlorphonium, dbdp is adopted as ligand to react with cuprous halide to synthesize a target product provided by the invention, and finally structures and light emission properties of the target product are represented. Results show that at a normal temperature, a solid terphenyl biphosphine three-coordination cuprous iodide complex emits green light, and a terphenyl biphosphine three-coordination cuprous bromide complex and a terphenyl biphosphine three-coordination cuprous chloride complex emit yellow and green light. Thesynthesis method is simple and easy to operate, in addition, the prepared target product has a microsecond-grade service life at the room temperature, and the result shows that the target product hasa light emission mechanism of thermal activity delayed fluorescence and has great potential of being used as an electroluminescent material applied to OLEDs (Organic Light Emitting Diodes).
Owner:HUBEI UNIV
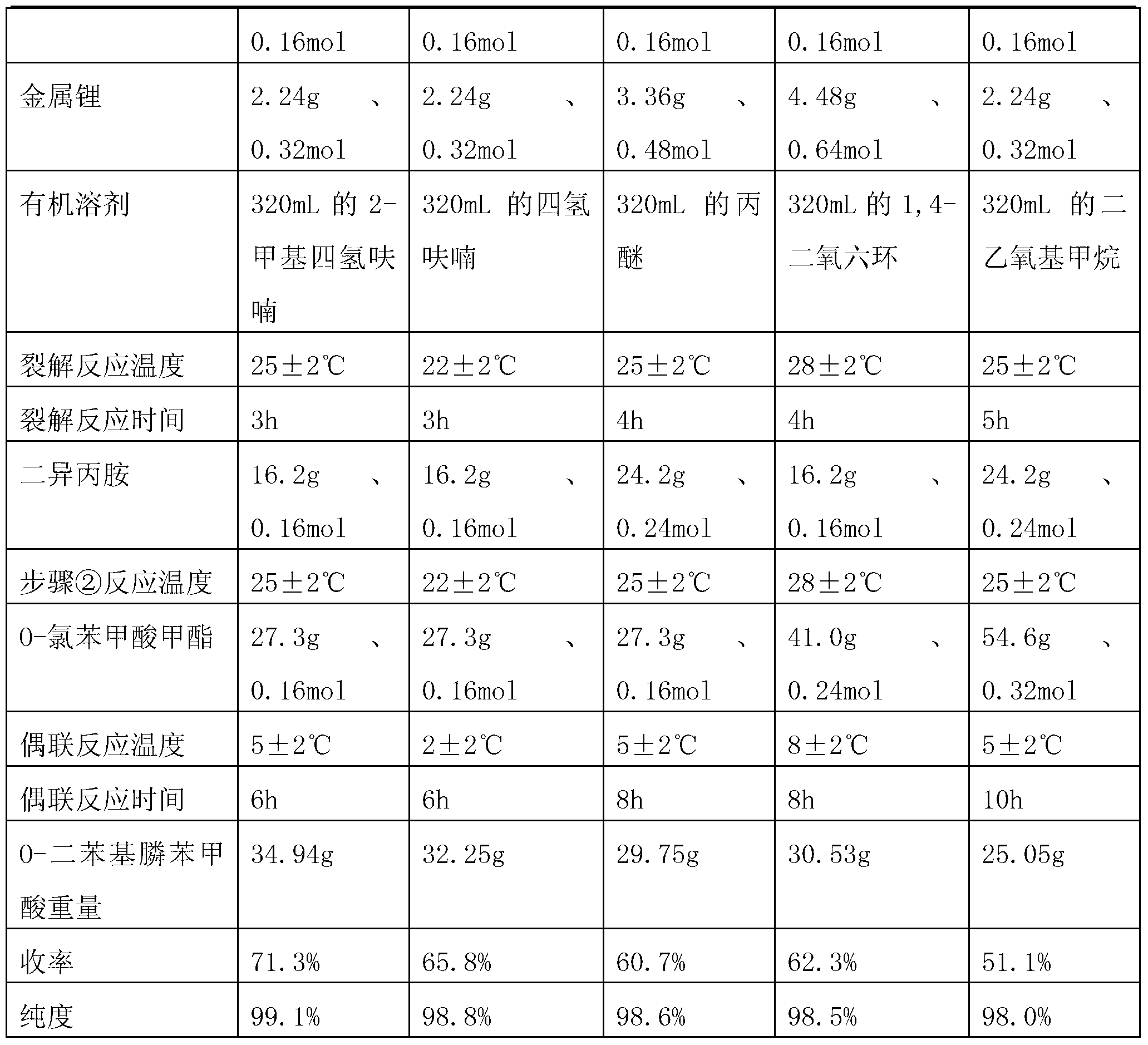
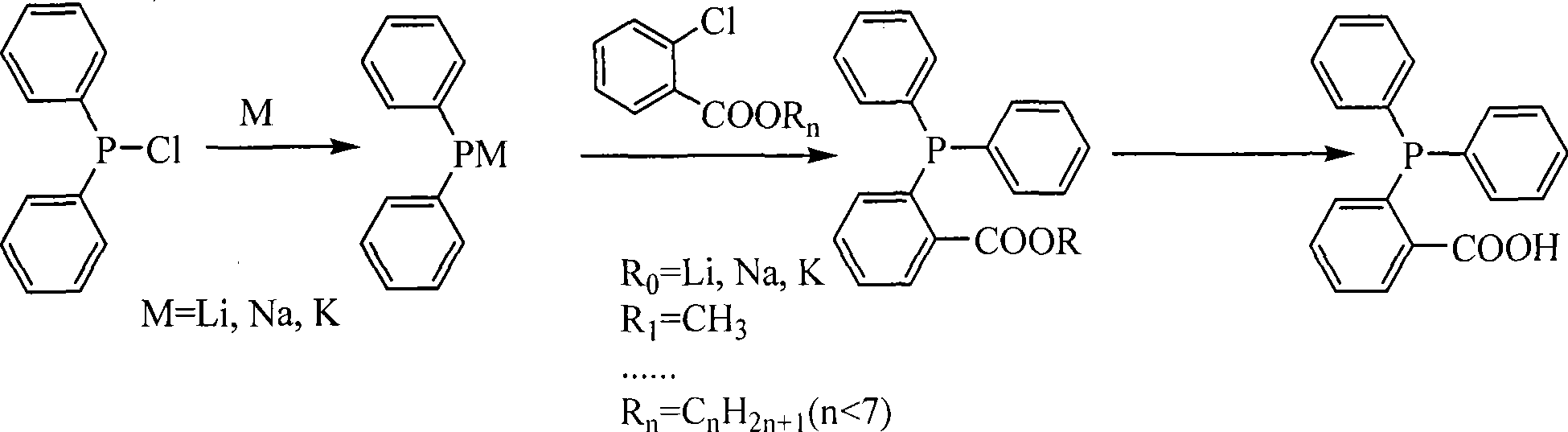
Preparation method of O-diphenylphosphine benzoic acid
InactiveCN103319530AInhibitionHigh reaction yieldGroup 5/15 element organic compoundsBenzoic acidFormate
The invention discloses a preparation method of O-diphenylphosphine benzoic acid. The preparation method comprises the following steps of: (1) carrying out cracking reaction on alkali metal and triphenylphosphine in an organic solvent to generate diphenylphosphine alkali metal salt and phenyl alkali metal salt; (2) slowly adding diisopropylamine, and enabling diisopropylamine to react with the phenyl alkali metal salt completely; (3) slowly adding O-chlorobenzol formate or O-bromobenzol formate, and enabling the diphenylphosphine alkali metal salt and the O-chlorobenzol formate or the O-bromobenzol formate to carry out coupling reaction and generate O-diphenylphosphino benzoate; (4) firstly hydrolyzing under the alkaline condition, obtaining O-diphenylphosphino sodium benzoate, acidizing and neutralizing under the acid condition, and obtaining the O-diphenylphosphine benzoic acid. The preparation method disclosed by the invention has the advantages that phenyllithium produced by cracking is completely reacted by adding the diisopropylamine, so that the generation of side reaction is effectively suppressed, and the reaction yield is greatly increased; since the triphenylphosphine is adopted as a reaction material, compared with the chlorodiphenylphosphine, the production cost is greatly reduced.
Owner:常州化工研究所有限公司
Process for synthesizing O-diphenylphosphinolbenzoic acid
ActiveCN101481388AGuaranteed supplyReduce manufacturing costGroup 5/15 element organic compoundsPotassiumHydrolysis
The invention relates to a synthetic method of o-diphenylphosphinolbenzoic acid. The synthetic method comprises the following steps: taking chlorodiphenylphosphine as a starting material, adding the chlorodiphenylphosphine and an alkali metal to a solvent for cracking to generate diphenylphosphine alkali salt, then adding ortho-chlorobenzoate or ortho-chlorobenzoic acid ether for reaction to generate diphenylphosphine benzoate or diphenylphosphine benzoic ether, and obtaining the o-diphenylphosphinolbenzoic acid after hydrolysis. The solvent is tetrahydrofuran, 2-methyltetrahydrofuran or an ether solvent such as n-butyl ether or 1,4-dioxane and the like, the alkali metal for cracking can be lithium, sodium or potassium, the ortho-chlorobenzoate can be lithium ortho-chlorobenzoate, sodium ortho-chlorobenzoate and lithium ortho-chlorobenzoate, and the ortho-chlorobenzoic acid ether can be ortho-chlorobenzoic methyl ester, ortho-chlorobenzoic acid ethyl ester, ortho-chlorobenzoic acid propyl ester, ortho-chlorobenzoic acid butyl ester, ortho-chlorobenzoic acid pentyl ester and the like. The synthetic method can prepare the o-diphenylphosphinolbenzoic acid above 0 DEG C at normal pressure with safe and stable operation, can save a large amount of energy sources and is suitable for large-scale production.
Owner:SINOMAX SOLUTIONS CO LTD
Continuous preparation method of trimethylbenzoyl-diphenylphosphine oxide compound
PendingCN110283206AImprove space utilizationImprove protectionGroup 5/15 element organic compoundsBenzaldehydeCatalytic oxidation
The invention discloses a continuous preparation method of trimethylbenzoyl-diphenylphosphine oxide (TPO) and its derivatives. The preparation method comprises: in a tubular reactor, benzaldehyde or its derivative reacts with chlorodiphenyl phosphine or its derivative; and then a catalytic oxidation reaction is directly carried out without separation, and the reaction solution is desolvated and re-crystallized to obtain a TPO product. The conversion rate of the product TPO is 98% and above, and the finished product content reaches 99.5% and above.
Owner:维思普新材料(苏州)有限公司
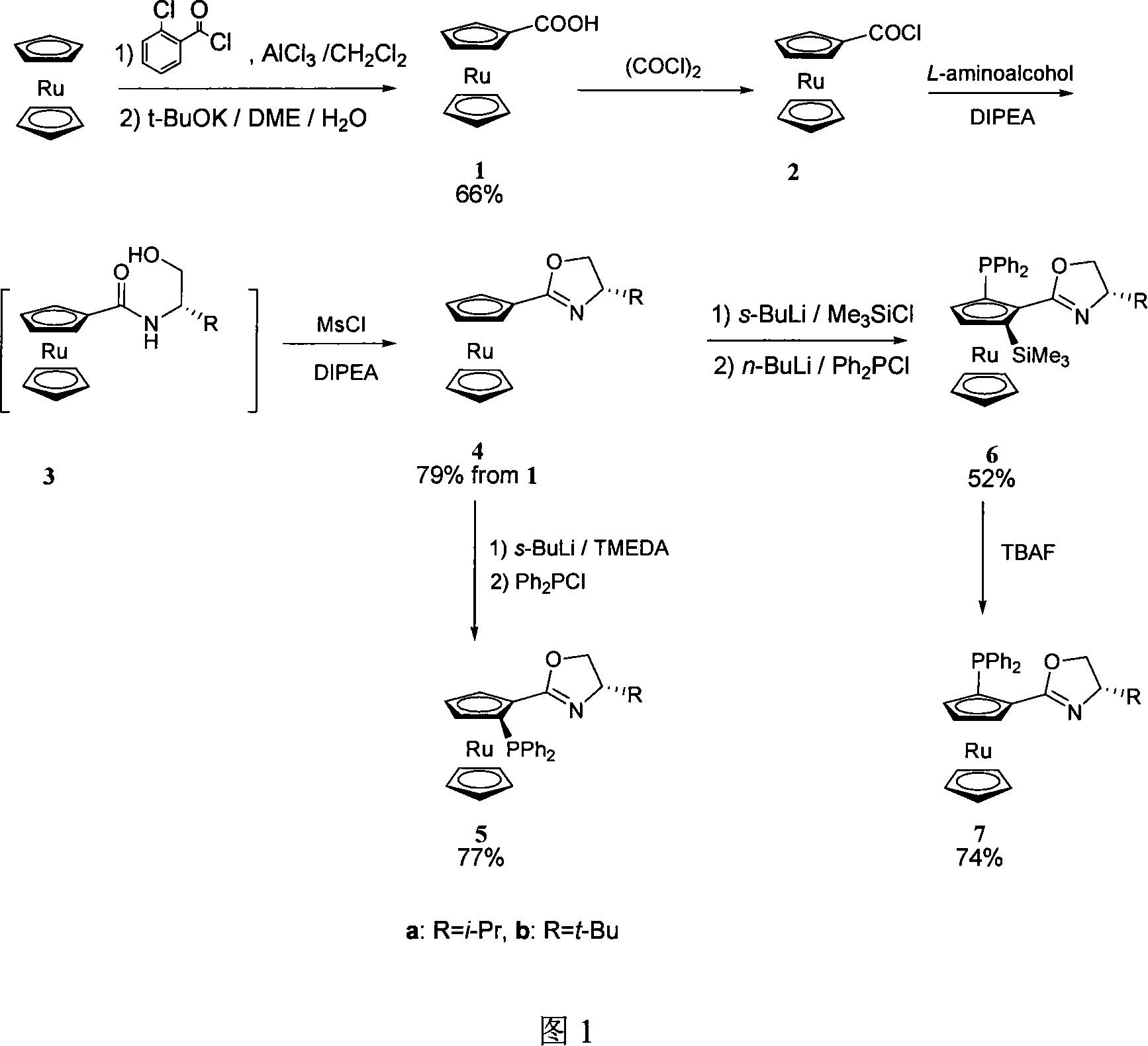
1,2-disubstituted bis ruthenium face chirality ligand and method for synthesizing same
InactiveCN101139367AHigh reactivityHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSulfonyl chloridePhosphate
The present invention relates to 1, 2- substitute cyclopentadienyl ruthenium surface chiral ligand in the chemical field and the correspondence synthetic method. The present invention comprises six reaction steps: the cyclopentadienyl ruthenium, after the Fuke acyl reaction, is hydrolyzed into 1-carboxyl cyclopentadienyl ruthenium; then 1-carboxyl cyclopentadienyl ruthenium reacts with oxalyl chloride to generate 1-carboxyl carbonyl cyclopentadienyl ruthenium; 1-oxazoline carboxyl carbonyl cyclopentadienyl ruthenium reacts with beta-amino alcohol to generate lactam compound, and then reacts with methane-sulfonyl chloride to generate 1-oxazoline cyclopentadienyl ruthenium; 1-oxazoline cyclopentadienyl ruthenium reacts with sec-butyllithium first, and then reacts with diphenyl chloride phosphate to generate (S, Sp)-1,2-substitute cyclopentadienyl ruthenium P, N- ligand; 1-oxazoline cyclopentadienyl ruthenium reacts with sec-butyllithium first, and then reacts with trimethylchlorosilane to generate (S,Sp)-1- oxazoline-2-trimethyl cyclopentadienyl ruthenium. Then the trimethyl is shucked off to generate (S,Rp)-1,2-substitute cyclopentadienyl ruthenium P,N- ligand. The structure is as follows: in the formula: R: a represents isopropyl, b represents tert-butyl.
Owner:SHANGHAI JIAO TONG UNIV +1
Novel synthesis method of clean and safe photoinitiator (2,4,6-trimethylbenzoyldiphenyl-diphenylphosphine oxide)
InactiveCN109776608AHigh yieldHigh recovery rateGroup 5/15 element organic compoundsHalogenated hydrocarbon separation/purificationSynthesis methodsDiphenylphosphine oxide
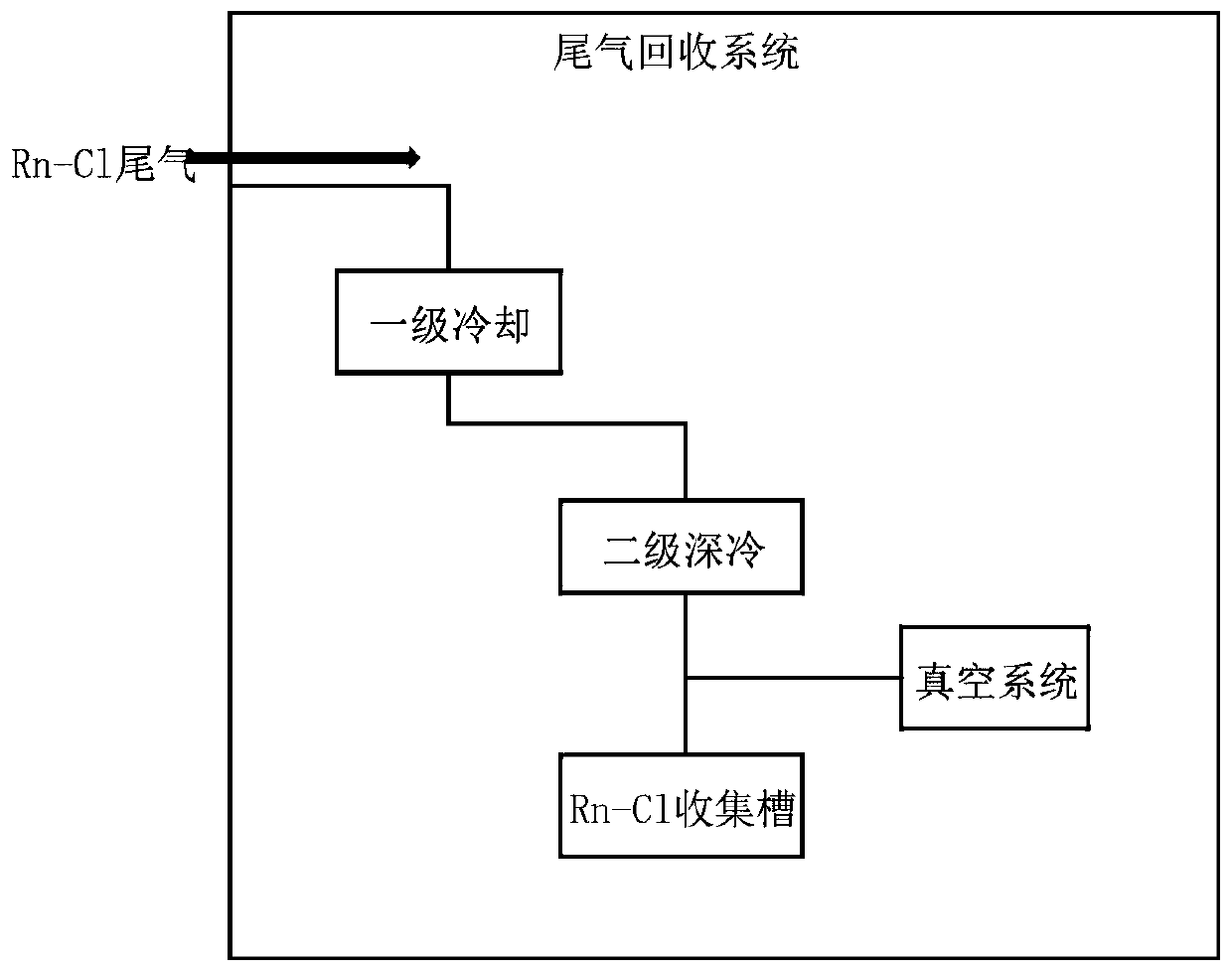
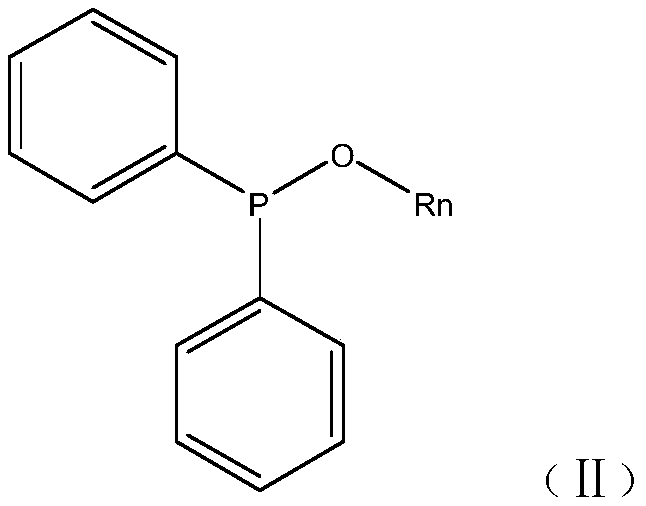
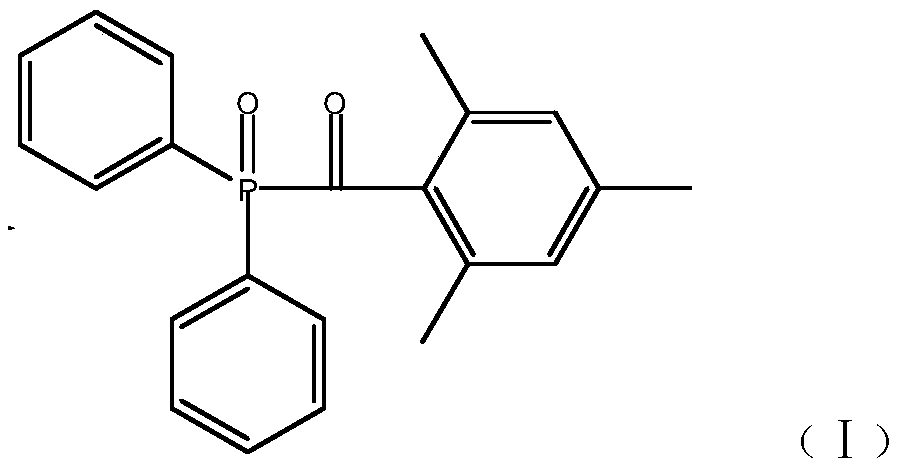
The invention relates to the technical field of synthesis of photoinitiators, in particular to a novel synthesis method of a clean and safe photoinitiator (2,4,6-trimethylbenzoyldiphenyl-diphenylphosphine oxide). The method includes the steps of evenly mixing anhydrous alcohol with N-methylimidazole at the room temperature, raising the temperature to 30-50%, controlling the temperature, dropwise adding diphenylphosphine oxide, raising the temperature to 50-100 DEG C, conducting the constant-temperature reaction for 2-3 h, conducting standing and layering to take supernatant liquid for decompressing and distilling to obtain diphenyl alkoxylphosphine, making the diphenyl alkoxylphosphine start to react with 2,4,6-trimethylbenzoyldiphenyl chloride at the controlled temperature of 50 DEG C under the vacuum condition of -0.098 Mpa, raising the temperature by 10 DEG C every 20 minutes until the temperature is raised to 90 DEG C, conducting the constant-temperature reaction for 4-8 h, and adding 50% ethyl alcohol crystals to obtain a target product. The produced byproduct is subjected to vacuum removal and then enters a tail gas recovery system to be recovered through two stages of condensation. The synthesis method is simple in step, safe, clean, low in running cost and high in byproduct recovery rate.
Owner:宁波易兮化工科技有限公司
Preparation method of diphenyl phosphine chloride
InactiveCN110615812ASolve Distillation DifficultySolve the pollution of the environmentGroup 5/15 element organic compoundsBenzeneChemical synthesis
The invention relates to a preparation method of diphenyl phosphine chloride, which belongs to the technical field of chemical synthesis, and comprises the following steps: adding benzene and aluminumtrichloride into a reaction kettle, adding phosphorus trichloride into the kettle while stirring, slowly heating the mixture to 120 DEG C, and carrying out a reaction for 12 hours; stopping heating,cooling the reaction product to 60 DEG C, adding a decomplexing agent in batches, heating the reaction product to 80 DEG C, and stirring the reaction product for 2 hours; desolventizing the reaction product, and rectifying the reaction product to separate out phenyl phosphine dichloride and diphenyl phosphine chloride; after the rectification, the product is properly cooled, then slowly adding residual liquid into a 20% hydrochloric acid solution, stirring the mixture for 2 hours, centrifuging the mixture to obtain a wet decomplexing agent product and an aluminum chloride solution, drying thewet decomplexing agent product, and recycling the dried wet decomplexing agent product, and using the aluminum chloride solution for preparing a flocculating agent. According to the method, a new decomplexing agent is used, so that the solid waste problem of diphenyl phosphine chloride production is solved, the production cost is reduced, and the product has market competitiveness.
Owner:江苏多森新材料科技有限公司
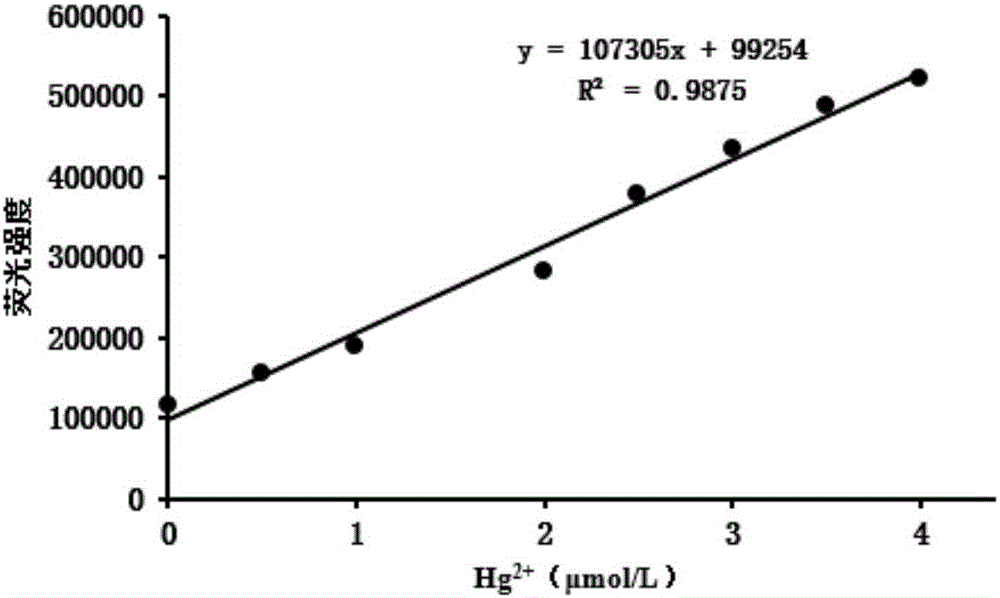
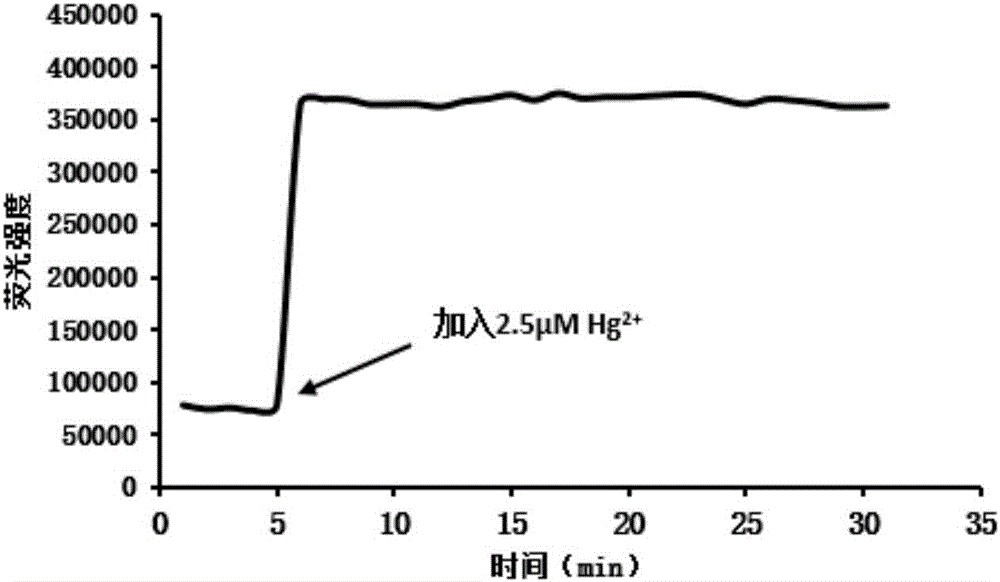
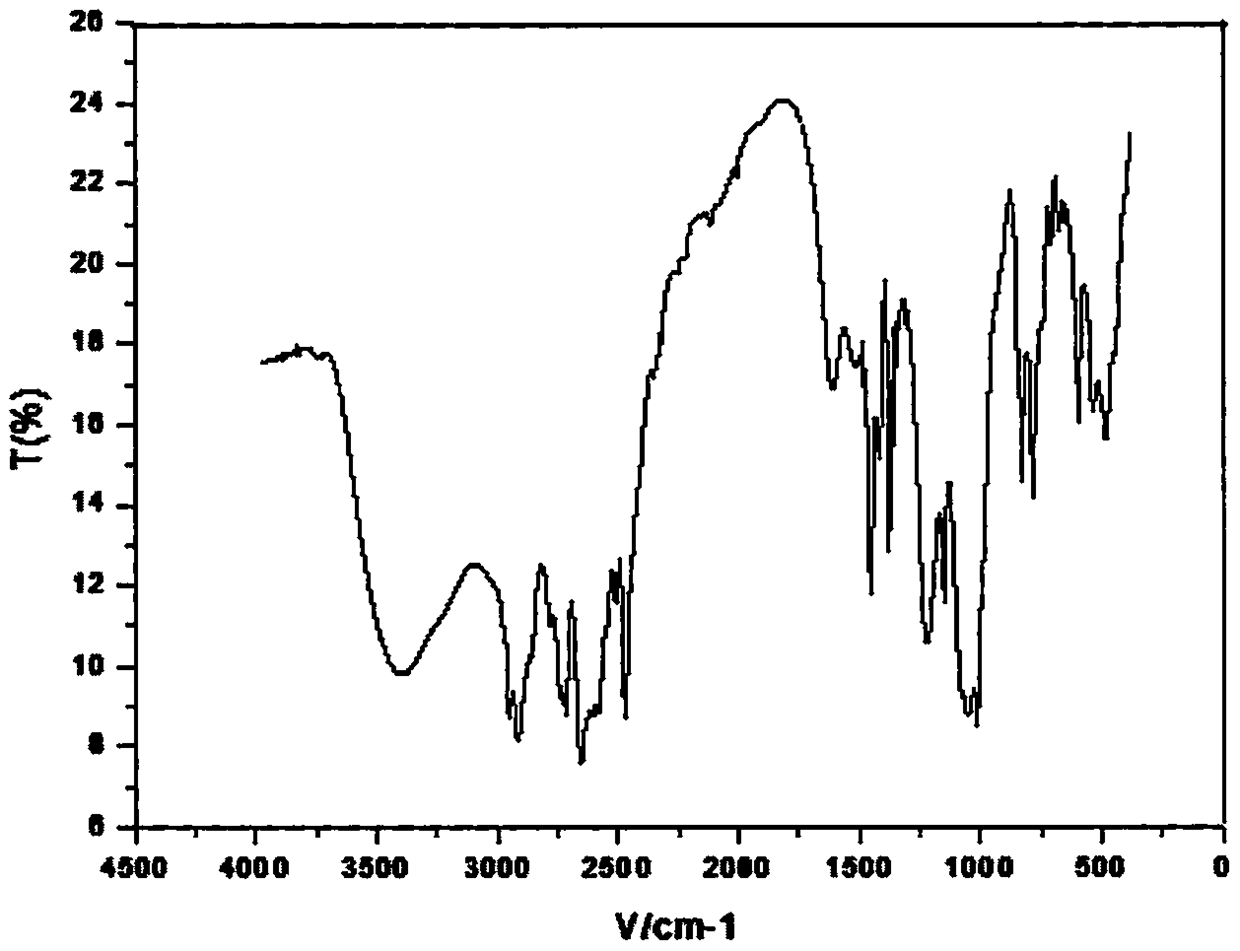
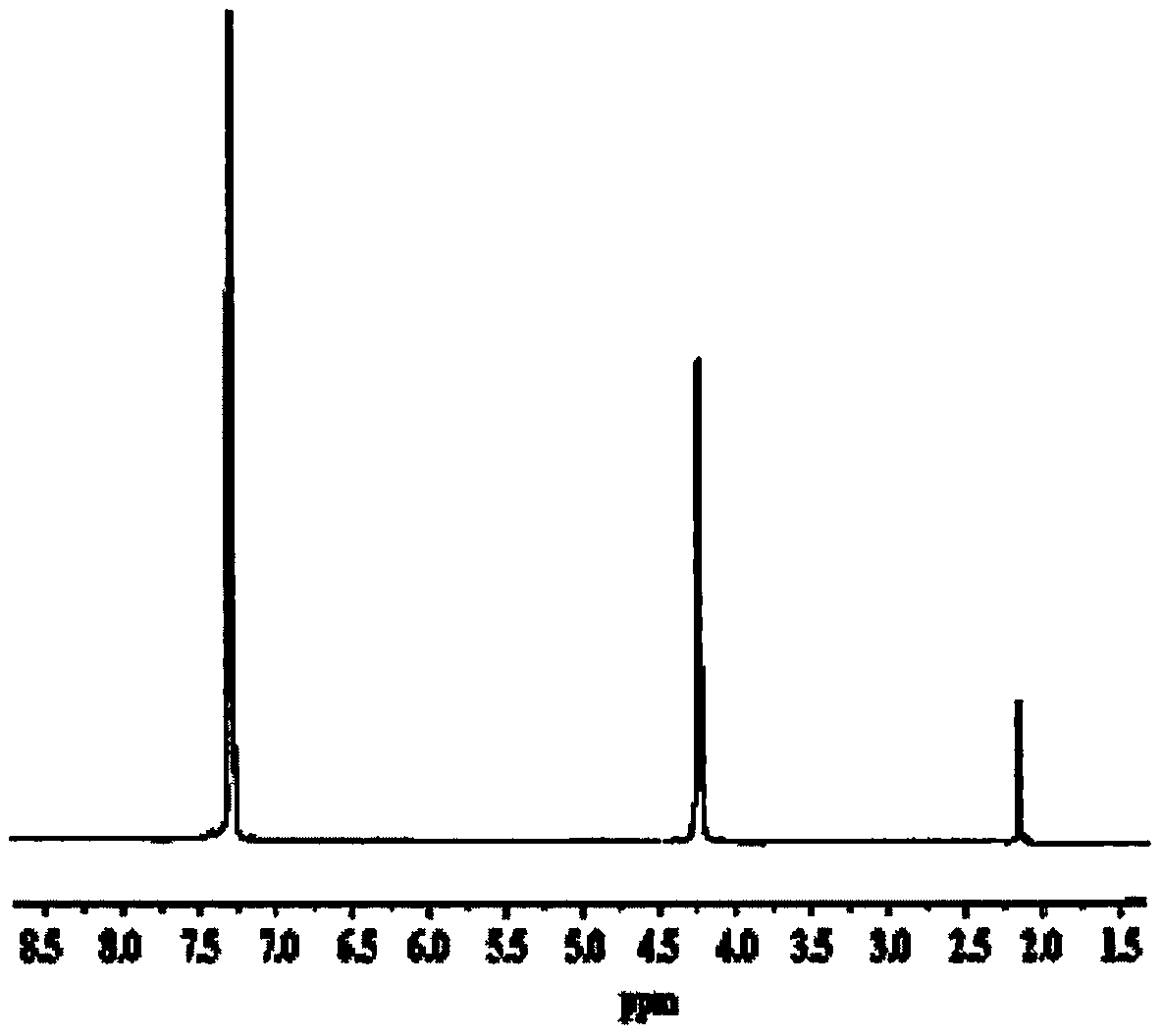
Method for preparing highly selective colorimetric probe for detecting mercury ions in sample
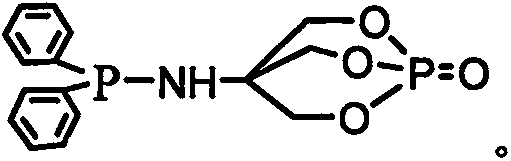
ActiveCN106588988AGroup 5/15 element organic compoundsFluorescence/phosphorescenceSolventChlorodiphenylphosphine
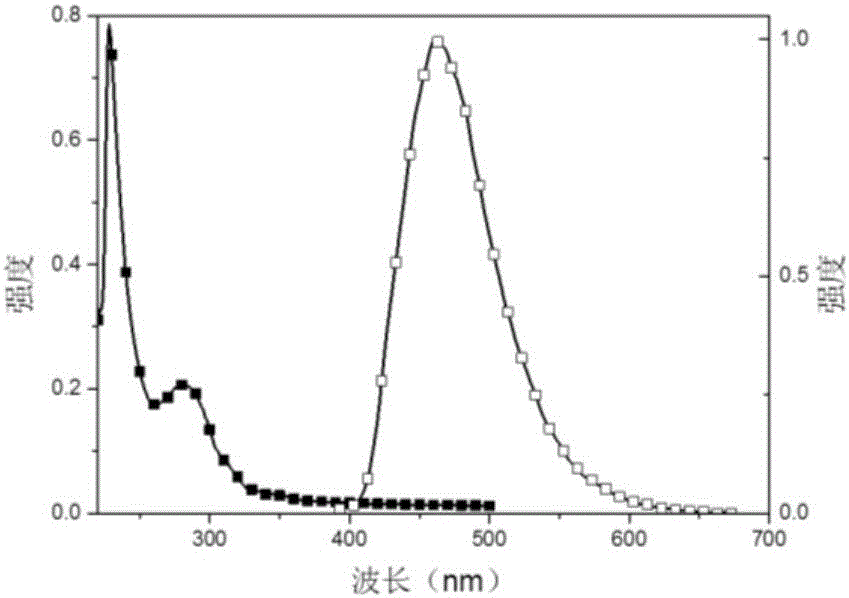
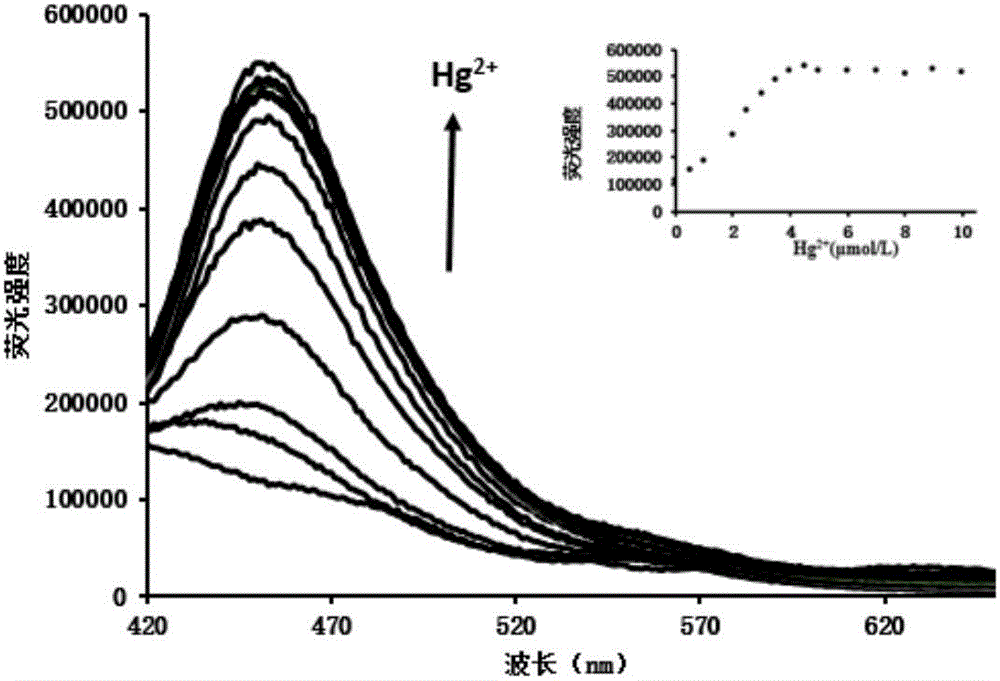
The invention relates to a method for preparing a highly selective colorimetric probe for detecting mercury ions in a sample. The method specifically comprises the following steps: dissolving 7-hydroxycoumarin in THF; dropwise adding a THF solution of selenium powder slowly under protection of nitrogen; stirring at room temperature; adding chlorodiphenylphosphine dissolved in a mixed solution of triethylamine and THF; stirring under protection of nitrogen for reaction; spin-drying the solvent; adding toluene and backwashing with a water separator; removing solids; spin-drying an organic phase; separating and purifying a target product through column chromatography. The probe synthesized in the invention specifically responds to mercury ions, is not affected by coexisting ions K<+>, Na<+>, Mg<2+>, Zn<2+>, Ni<2+>, Cr<3+>, Cd<2+>, Pb<2+> and Cu<+>, and has good selectivity to Hg<2+>.
Owner:LESHAN NORMAL UNIV
Organic phosphonate nitrogen fire retardant dibenzyl phosphinous-N-cage phosphate amine compound, and preparation method thereof
InactiveCN109180731AHigh in phosphorusImprove flame retardant performanceGroup 5/15 element organic compoundsPolyesterPolyolefin
The invention relates to an organic phosphonate nitrogen fire retardant dibenzyl phosphinous-N-cage phosphate amine compound, and a preparation method thereof. The structure of the organic phosphonatenitrogen fire retardant dibenzyl phosphinous-N-cage phosphate amine compound is disclosed in the invention. The preparation method comprises following steps: a cage phosphate amine and triethylamineare added into an organic solvent, the air in a reactor is replaced by nitrogen gas, at 20 DEG C, dibenzyl phosphonium chloride is added drop by drop in 1h, wherein the dibenzyl phosphonium chloride:cage phosphate amine :triethylamine molar ratio is controlled to be 1:1:1 to 1:1.2:1, and the dropwise adding temperature is controlled to be 50 DEG C or lower; after dropwise adding, the temperatureis increased to 80 to 110 DEG C for 4 to 6h of backflow reaction, then an obtained product is subjected to cooling, filtering, water washing, and vacuum drying so as to obtain the slightly yellow solid dibenzyl phosphinous-N-cage phosphate amine. The dibenzyl phosphinous-N-cage phosphate amine is an excellent phosphonate nitrogen cooperative fire retardant, and is suitable to be taken as fire retardants of polyamide, polyester, polyurethane, glass fiber reinforced plastic, and polyolefin materials; the preparation method is simple; equipment investment is low; and industrialized production isconvenient to realize.
Owner:SUZHOU UNIV OF SCI & TECH
Method for preparing bis(diphenylphosphino)
InactiveCN104558030AHigh yieldLess investmentGroup 5/15 element organic compoundsLithium metalAntioxidant
The invention discloses a method for preparing bis(diphenylphosphino), and belongs to the technical field of diphosphine ligands. According to the technical scheme, the method for preparing bis(diphenylphosphino) comprises the following steps: adding tetrahydrofuran and a lithium metal into a reaction kettle, dropwise adding chlorodiphenylphosphine at 20-60 DEG C, dropwise adding dichloralkane at 20-60 DEG C after the chlorodiphenylphosphine is completely reacted, adding an amino antioxidant or a phenol antioxidant after the reaction is completed, depressurizing, removing tetrahydrofuran, adding diluted hydrochloric acid, filtering, and recrystallizing the filter cakes, thereby obtaining bis(diphenylphosphino). The method disclosed by the invention is typical one-pot reaction, is small in equipment investment, simple in equipment and convenient to operate, and due to addition of the antioxidant, the operation steps are greatly simplified, and the yield of the product is increased.
Owner:HENAN NORMAL UNIV
Method for preparing triphenylphosphine in reducing manner
ActiveCN108409785AReduce dosageImprove stabilityGroup 5/15 element organic compoundsOrganic synthesisPhosphate
The invention provides a method for preparing triphenylphosphine in a reducing manner. Phenylphosphonicdichloride or chlorodiphenylphosphine is used as a reducing agent, and raw materials are low in price and easily obtained; under the effect of a catalyst, the phenylphosphonicdichloride or chlorodiphenylphosphine and triphenylphosphine oxide are subjected to reducing reaction, stabilities of phosphine oxygen double bonds in the triphenylphosphine oxide and oxidation products (phenylphosphonic dichloride or diphenylphosphinyl chloride) of a reducing agent are different, the stabilities of thedouble bonds of the phenylphosphonic dichloride and the diphenylphosphinyl chloride are good, the double bonds of the triphenylphosphine oxide are broken under the effect of the catalyst, the double bonds of the formed phenylphosphonic dichloride and the formed diphenylphosphinyl chloride are not easily damaged by the catalyst, and therefore, triphenylphosphine can be obtained under gentle conditions; the oxidation products (the phenylphosphonic dichloride or the diphenylphosphinyl chloride) of the reducing agent are important organic synthesis intermediate (such as synthesis of organic phosphate); and moreover, the amount of the catalyst required in the method is small, and the catalysis efficiency is high.
Owner:江苏富比亚化学品有限公司
Selenium atom-containing fluorescence probe, and preparation method and application thereof
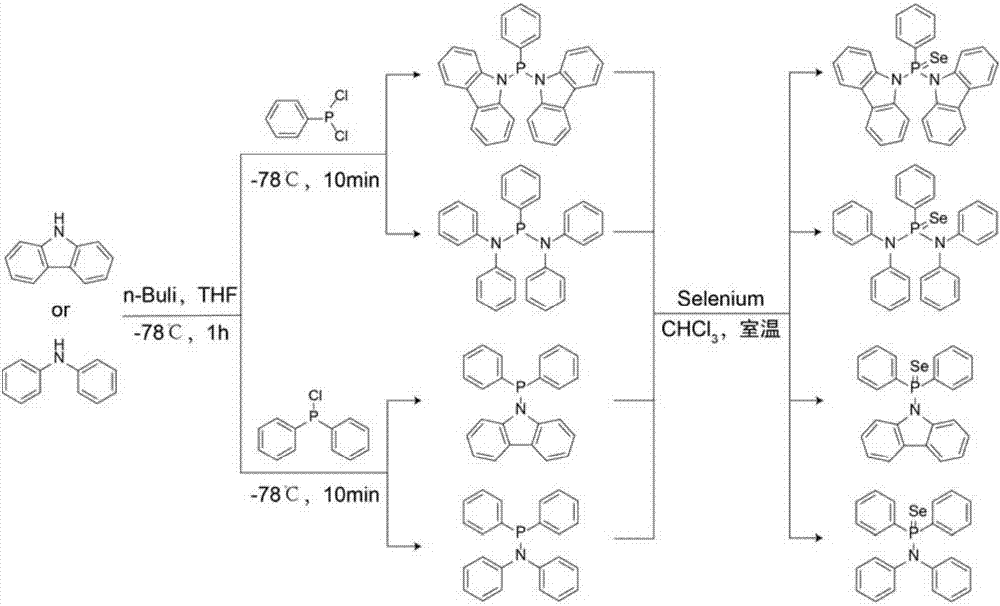
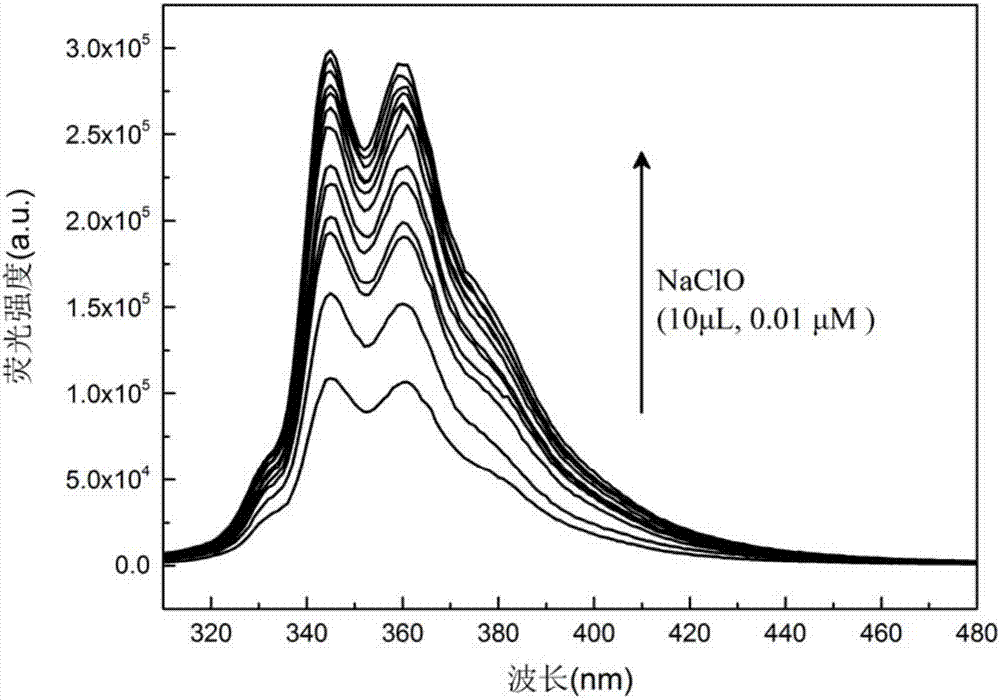
ActiveCN107129512ASimple preparation processMild conditionsGroup 5/15 element organic compoundsFluorescence/phosphorescenceDichlorophenylphosphineCarbazole
The invention discloses a selenium atom-containing fluorescence probe, and a preparation method and an application thereof, and belongs to the field of functional materials. The structural general formula of above material is represented by formula (I) shown in the description; and in the formula (I), Ar is a carbazolyl or diphenylamino group, and Ph is a phenyl group. The preparation method of the material concretely comprises the following steps: reacting carbazole / diphenylamine with dichlorophenylphosphine / diphenylchlorophosphine to form a derivative containing an N-P structure, and carrying out chemical modification to introduce selenium atoms in order to form the selenium atom-containing fluorescence probe. The method has the advantages of simple synthesis steps, mild conditions, low production cost and high thermal stability. Se=O double bonds are formed through the reaction of the selenium atoms and hypochlorite radicals in order to generate fluorescence change. The selenium atom-containing fluorescence probe has the advantages of high sensitivity and high selectivity.
Owner:NANJING UNIV OF POSTS & TELECOMM
Nine-site functional fluorenyl aromatic single-phosphine oxide electroluminescent material and preparation method thereof
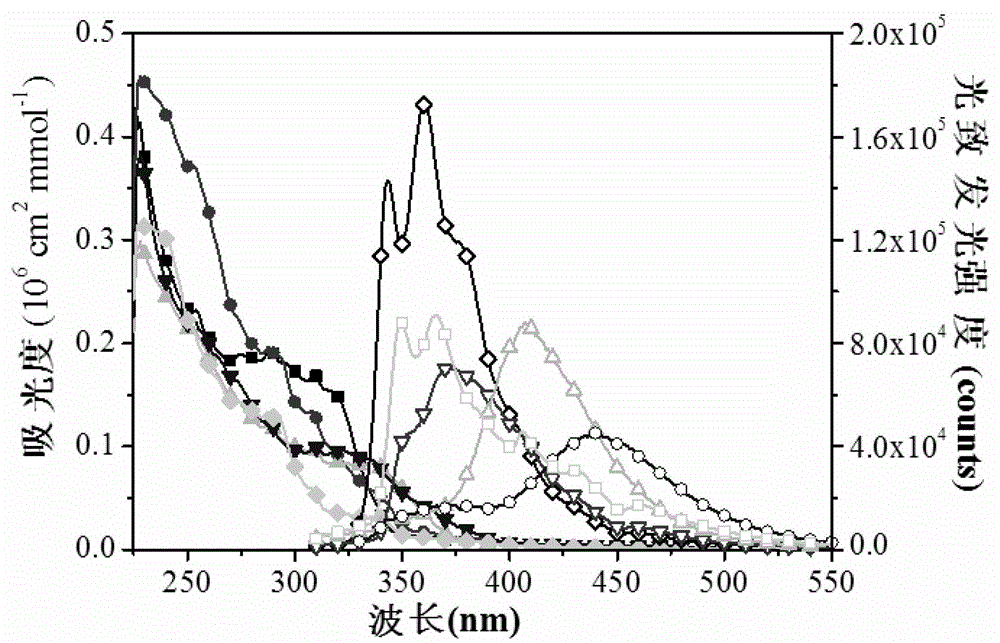
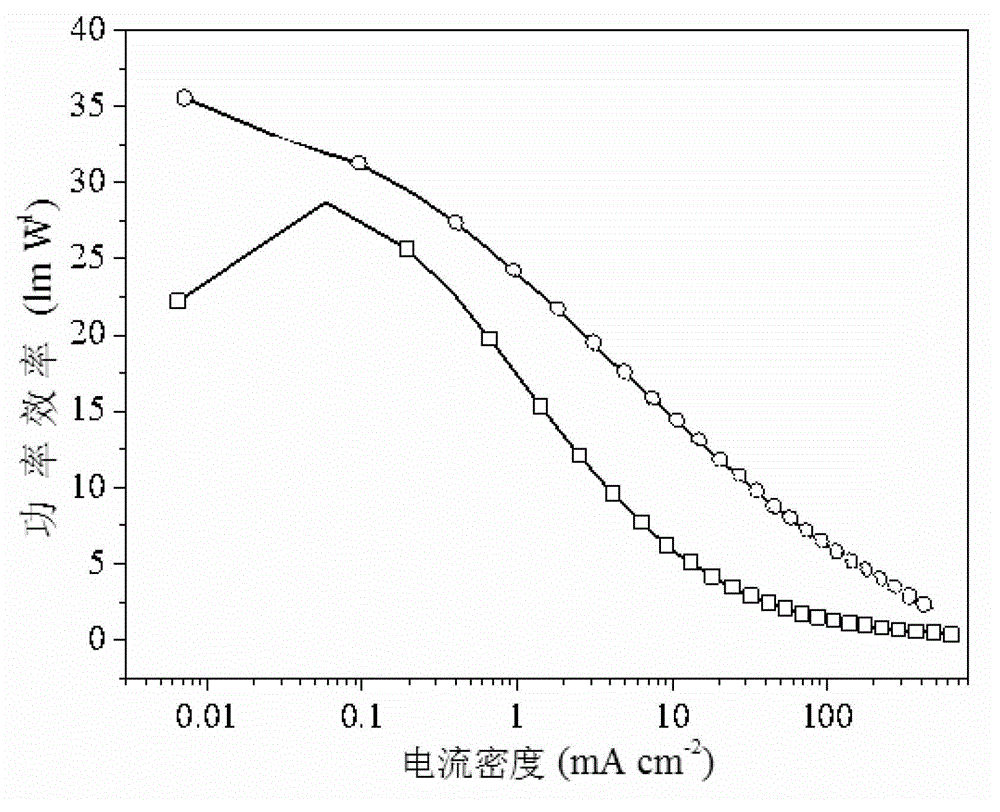
InactiveCN102911658AMaintain good performanceBalance injectionGroup 5/15 element organic compoundsSolid-state devicesMethylene DichlorideTriplet state
The invention provides a nine-site functional fluorenyl aromatic single-phosphine oxide electroluminescent material and a preparation method thereof, and relates to a luminescent material and a preparation method thereof, aiming at solving the technical problem that a blue light phosphorescent device is low in external quantum efficiency due to the existing electroluminescent material. The preparation method provided by the invention comprises the following steps of: 1, preparing a bromo-intermediate; and 2, dropwise adding n-butyllithium into the bromo-intermediate, cooling, dropwise adding chlorodiphenylphosphine for reaction, reacting under room temperature, extracting, drying, dissolving with methylene dichloride, oxidizing by adding hydrogen peroxide, extracting, drying, and carrying out column chromatography to obtain the electroluminescent material. The electroluminescent material prepared by the invention is taken as a phosphor material, so that the high-efficiency deep blue light emission can be successfully realized; and the electroluminescent material is taken as a blue light phosphorescent body material, so that the high triplet-state energy level (3.01eV) and the stable power efficiency (35.61m / W) can be realized.
Owner:HEILONGJIANG UNIV
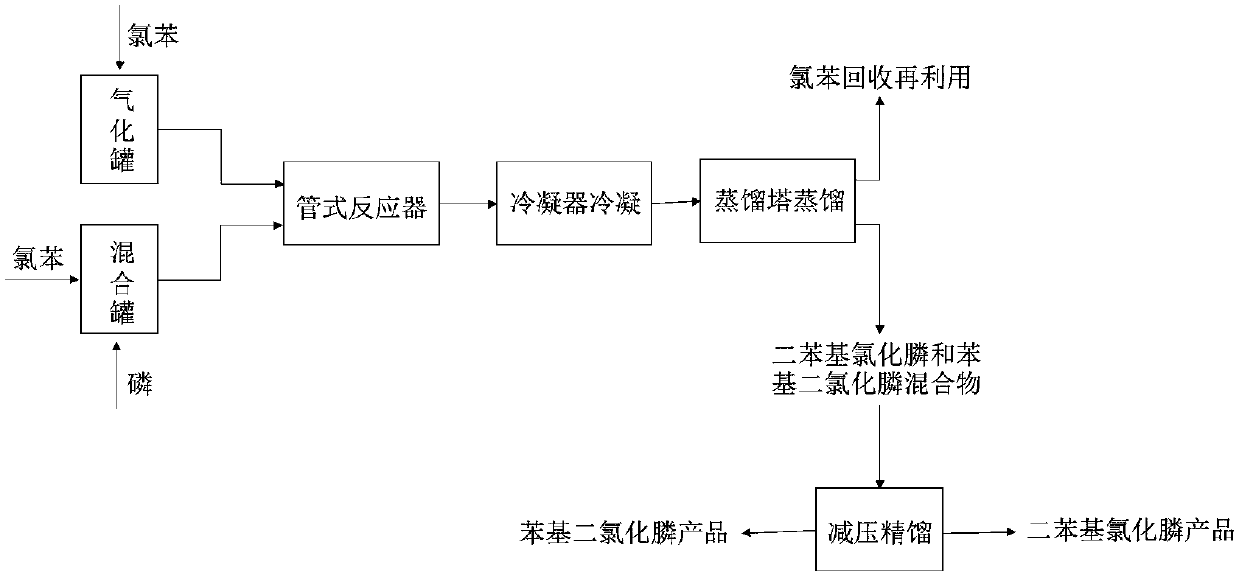
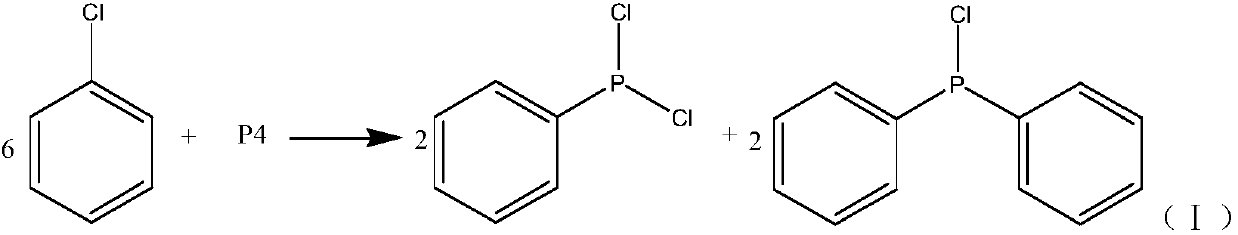
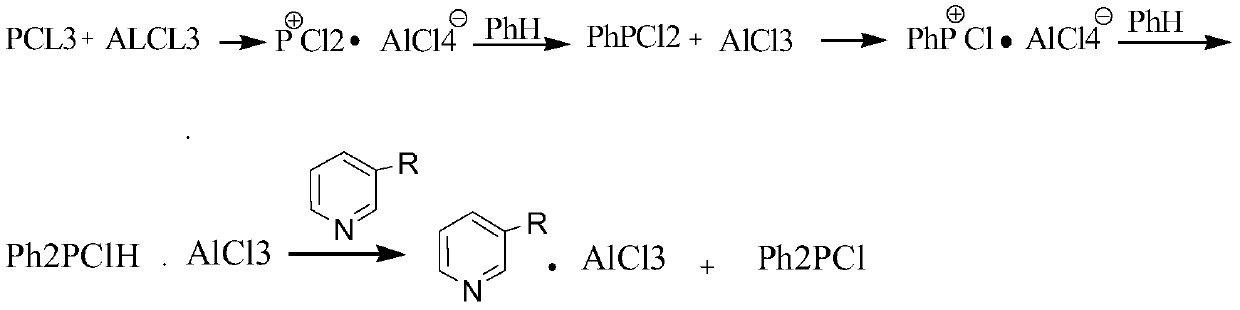
New synthesizing method of chlorodiphenylphosphine
InactiveCN109575072AIncrease profitHigh yieldGroup 5/15 element organic compoundsChlorobenzeneContact reactions
The invention discloses a new synthesizing method of chlorodiphenylphosphine and belongs to the field of synthesizing technology. The method comprises the following steps of filling chlorobenzene intoa gasification tank for gasification, then uniformly mixing chlorobenzene and phosphorous into a mixing tank to obtain a mixed solution; guiding the gasified chlorobenzene into a tubular reactor, pumping the mixed solution into the tubular reactor through a metering pump for normal-temperature contact reaction between the chlorobenzene and the phosphorous in the tubular reactor at 580-610 DEG C;condensing and crudely distilling the reacted materials to separate chlorobenzene, chlorophenylphosphine and chlorodiphenylphosphine; recycling the separated chlorobenzene, and performing reduced pressure rectification on the mixture of the chlorophenylphosphine and the chlorodiphenylphosphine to obtain the products of chlorodiphenylphosphine and the chlorophenylphosphine. The new synthesizing method of the chlorodiphenylphosphine achieves continuous reaction and simple operation with a raw material utilization rate up to 100%, saves solvent and catalyst and is high in yield and purity of products.
Owner:刘碧见
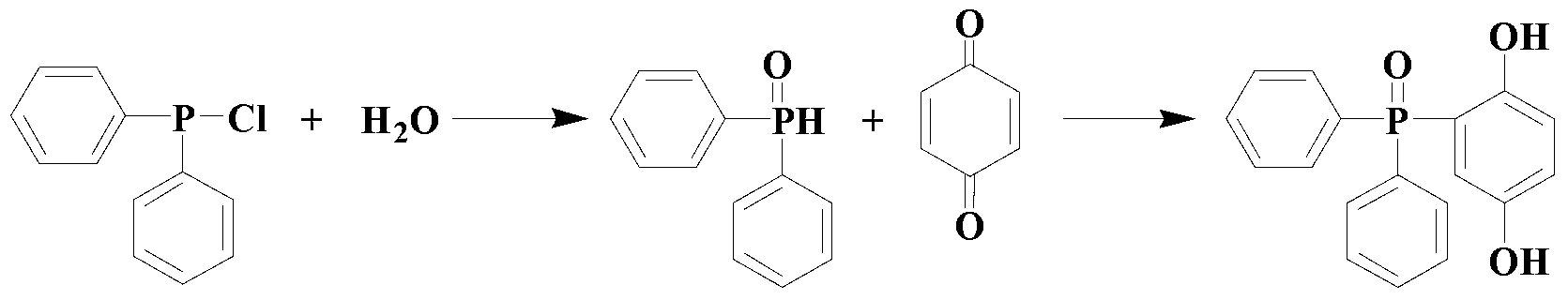
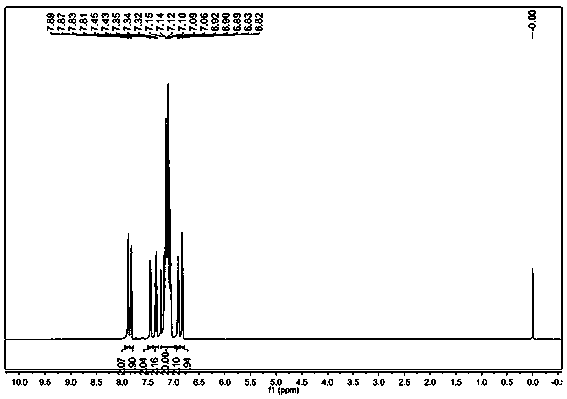
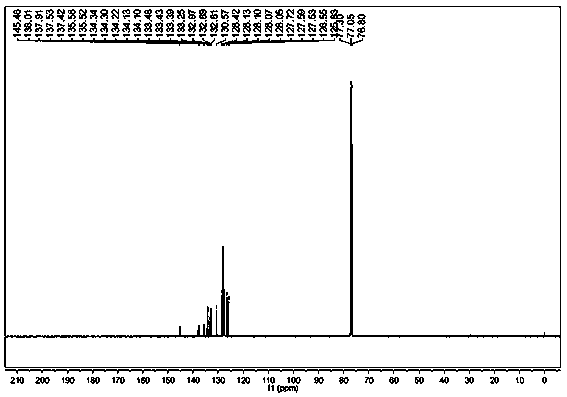
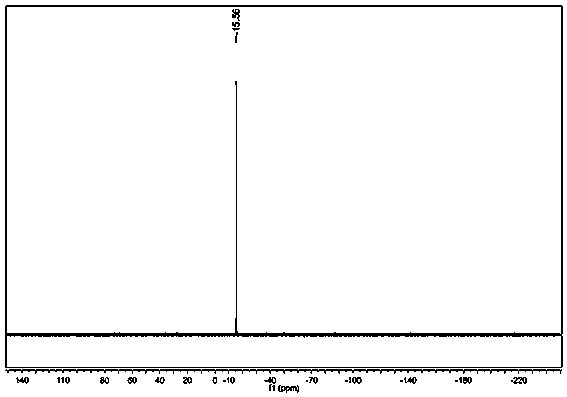
Environment-friendly preparation method of phosphorus flame retardant, namely 2-(diphenylphosphinoyl)-1, 4-benzenediol
InactiveCN103319533AMeet environmental protection requirementsHigh yieldGroup 5/15 element organic compoundsAlcoholSolvent
The invention discloses an environment-friendly preparation method of a phosphorus flame retardant, namely 2-(diphenylphosphinoyl)-1, 4-benzenediol. Chlorodiphenylphosphine is taken as a reaction raw material, a one-pot method is adopted for skipping the purification process of the chlorodiphenylphosphine and further directly synthesizing the 2-(diphenylphosphinoyl)-1, 4-benzenediol, and a solvent and a purification solvent, which are used by the environment-friendly preparation method are alcohols, so that the environment-friendly preparation method has the advantages of low toxicity, high yield of above 90%, capability of greatly reducing production cost and effect of meeting environment-friendly and high-efficient requirements.
Owner:QINGDAO FUSILIN CHEM SCI & TECH
A kind of synthetic method of 2,2'-bisdiphenylphosphino-1,1'-binaphthalene
ActiveCN108586528BHigh reaction yieldLow priceLithium organic compoundsGroup 5/15 element organic compoundsMetallic lithiumEthoxidine
The invention discloses a synthetic method of 2,2'-double diphenyl phosphine-1,1'-dinaphthalene. The method comprises the following steps: adding a lithium metal sheet, a ligand, 2,2'-diethoxy-1,1'-dinaphthalene and an ethers solvent are added in a reaction still, heating the materials to the temperature of 60-140 DEG C, reacting the materials for 6-12 hours, dropping chlorodiphenylphosphine at the temperature of 0 DEG C, heating the material to room temperature, and obtaining the product after the reaction is completed; equivalent water quenching is carried out, a product solution is concentrated and filtered, a filter cake is washed with methanol, and vacuum drying is carried out to obtain the product 2,2'-double diphenyl phosphine-1,1'-dinaphthalene (BINAP). The method has the advantages of high yield, low preparation cost, simple post-treatment, high product purity, and is suitable for process enlargement.
Owner:XINXIANG RUNYU NEW MATERIAL TECH CO LTD
1,2-bis(diphenylphosphino) benzene
InactiveCN103936787AHigh yieldSimple processGroup 5/15 element organic compoundsChlorodiphenylphosphinePressure reduction
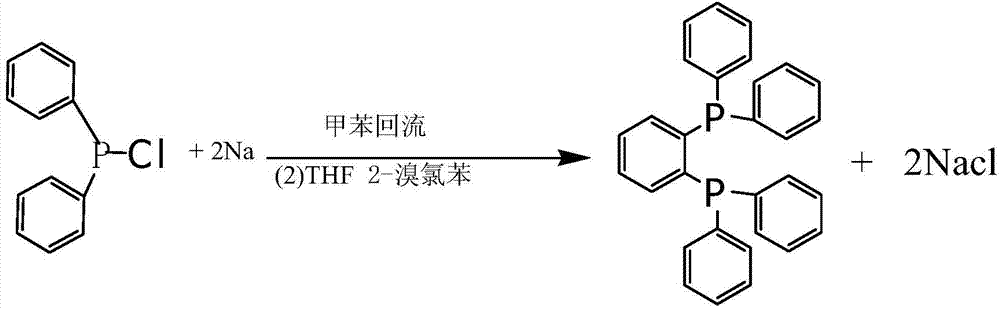
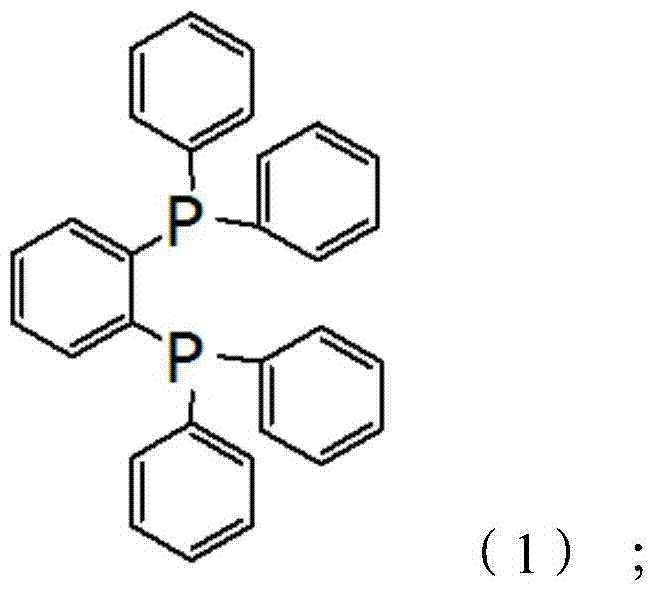
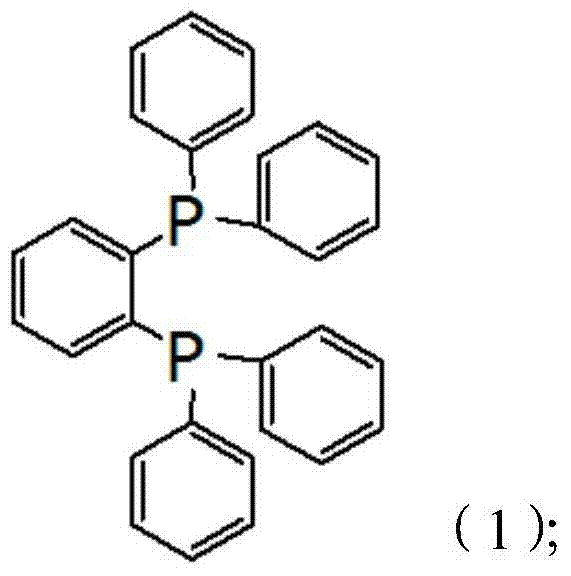
The invention discloses 1,2-bis(diphenylphosphino) benzene prepared by using a compound (chlorodiphenylphosphine) shown in the formula (2) as one of raw materials. The preparation method sequentially comprises the steps of (a) adding metallic sodium into a reaction container containing toluene, heating, reflowing and stirring; (b) adding mixed liquor of the compound shown in the formula (2) and toluene when metallic sodium is melted into sodium beads, and controlling the temperature to be 100-130 DEG C during the adding process; (c) continuously performing reaction for 11-12 hours at 100-130 DEG C; (d) slowly cooling a reaction system to the room temperature, then adding tetrahydrofuran, and stirring for 0.5-1.5 hours; (e) cooling to 18-20 DEG C, adding 2-bromochlorobenzene, and then stirring for 11-12 hours; and (f) performing pressure reduction and concentration on a reacted mixture, adding methanol, stirring and crystallizing to obtain 1,2-bis(diphenylphosphino) benzene. The preparation method is simple, efficient, and high in yield, and 1,2-bis(diphenylphosphino) benzene is high in purity.
Owner:BEIJING GUOFANG DINGSHENG TECH
Polycarboxylic acid water reducer and preparation method thereof
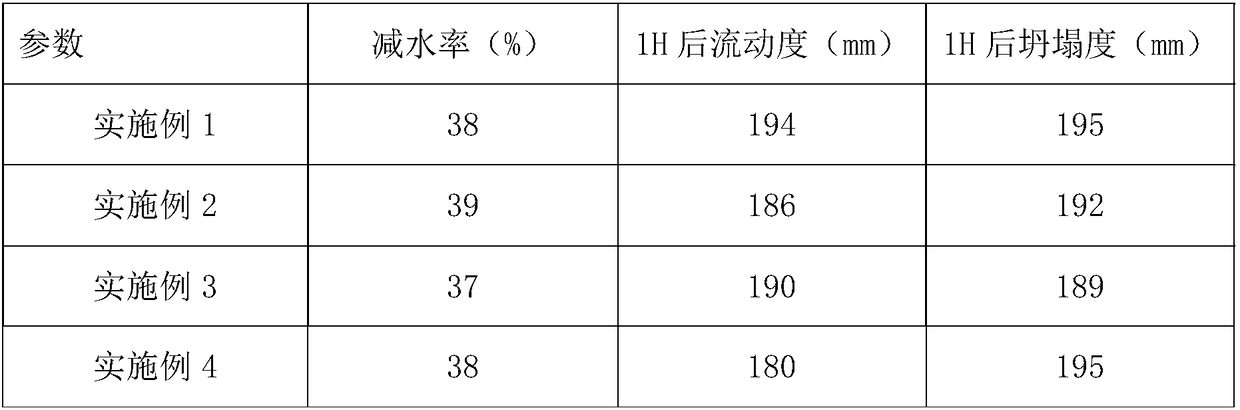
The invention discloses a polycarboxylic acid water reducer and a preparation method thereof. The polycarboxylic acid water reducer comprises the following raw materials in percentages by mass: 100-120 parts of polyoxyethylene-based allyl ester macromonomers, 1-5 parts of sodium methallyl sulfonate, 50-80 parts of acrylic acid, 5-10 parts of potassium hydroxide, 70-90 parts of tap water, 2-5 partsof chlorodiphenylphosphine and 0.5-1 part of copper chloride. Reaction conditions are simple, reaction steps are also simple, and the tap water can be directly used for carrying out charging reaction. The disclosed polycarboxylic acid water reducer has high water reducing ability and low slump, and can meet the requirement of processing of concrete well.
Owner:山西谷山建材有限公司
Novel method for synthesizing chlorodiphenylphosphine by one-step process
PendingCN109956971AAchieve recyclingReduce pollutionPhosphorus halides/oxyhalidesGroup 5/15 element organic compoundsBenzeneReflux
The invention relates to a novel method for synthesizing chlorodiphenylphosphine by a one-step process. The method comprises the following steps: respectively adding phosphorus trichloride, benzene and aluminum trichloride into a three-necked bottle, performing programmed heating to 140-150 DEG C while stirring under the protection of nitrogen, carrying out a heat insulation reaction at the temperature of 140-150 DEG C until no reflux state, and cooling the obtained reaction solution to room temperature; adding an organic solvent to the reaction solution, performing stirring for several hours,dropwise adding a decomplexing agent, stirring the obtained solution for several hours after the drop-by-drop addition of the decomplexing agent is finished, standing the solution to layer the solution, separating out the obtained decomplexing agent layer, carrying out reduced pressure distillation on the obtained organic solvent layer to obtain crude chlorodiphenylphosphine, and distilling the crude chlorodiphenylphosphine under a high vacuum to obtain pure chlorodiphenylphosphine. The method reduces the environmental pollution by recovering the aluminum trichloride, increases the yield of abyproduct, increases the income, and also has the advantages of high yield, low cost and short reaction time.
Owner:上海振品化工有限公司
Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor
ActiveCN108456235AShort reaction timeImprove reaction efficiencyMetallocenesDistillationContinuous flow
The invention discloses a method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by a continuous flow microchannel reactor. The method comprises the following steps that an organic solvent solution of N,N-dimethyl-(R)-1-[(S)-2-ferrocenyl]ethylamine and an n-hexane solution of n-butyllithium are respectively introduced into a microchannel reactor constant temperature module by a metering pump for constant temperature treatment; then, the solutions enter a first mixing module for reaction; after the reaction is completed, the mixed solution enters a second mixingmodule to react with chlorodiphenylphosphine; acidification, extraction, drying and distillation are performed to obtain a crude product of the product of N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine; the crude product is subjected to alcohol recrystallization to obtain the product of N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine. The method has the advantages that the operation is simple, convenient and safe; the yield is high.
Owner:XIAN MODERN CHEM RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

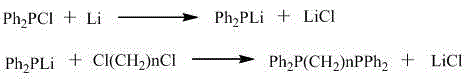
![Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor](https://images-eureka.patsnap.com/patent_img/6177b4dc-4293-432c-8f91-e738d6ef714d/HDA0001593729190000011.png)
![Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor](https://images-eureka.patsnap.com/patent_img/6177b4dc-4293-432c-8f91-e738d6ef714d/HDA0001593729190000012.png)
![Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor Method for preparing N,N-dimethyl-(R)-1-[(S)-2-(diphenylphosphine)ferrocenyl]ethylamine by microreactor](https://images-eureka.patsnap.com/patent_img/6177b4dc-4293-432c-8f91-e738d6ef714d/BDA0001593729180000011.png)