Method for preparing highly selective colorimetric probe for detecting mercury ions in sample
A high-selectivity, sample-detecting technology, applied in chemical instruments and methods, measuring devices, compounds of Group 5/15 elements of the periodic table, etc., can solve the problems of high cost and complicated operation of detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of fluorescent probe
[0023] The synthetic route and the method of mercury ion fluorescent probe of the present invention are as follows:
[0024]
[0025] Specifically, 7-hydroxycoumarin (0.49g, 3mmol) was dissolved in THF (2mL), and the solution of selenium powder (0.19g, 24mmol) in THF (5mL) was slowly added dropwise under nitrogen protection, and stirred at room temperature 10min, add diphenylphosphine chloride (0.53g, 24mmol) dissolved in a mixed solution of triethylamine (2mL) and THF (20mL), stir and react for 24h under nitrogen protection, spin to dry the solvent, add 25mL toluene with a water separator Backwash for 4 hours to remove the solid, spin the organic phase to dryness, and separate and purify the target product by column chromatography (dichloromethane: petroleum ether = 1:1) to obtain 1.01 g of red powder fluorescent probe with a yield of 52%. 1 H NMR (400MHz,D 2 O)δ7.77-7.68(m,6H),7.62(dd,J=10.9,4.0Hz,1H),7.55-7.43(m...
Embodiment 2
[0026] Embodiment 2: the preparation of reaction reagent
[0027] Probe stock solution and probe test solution: Accurately weigh 4.26 mg of the probe synthesized in Example 1, dissolve it with a small amount of dichloromethane, then transfer it into a 10mL volumetric flask, dilute to the mark with ethanol, and mix At this time, a probe stock solution with a concentration of 1 mmol / L was obtained. And take 1mL of the stock solution and add it to 100mL of ethanol, first add some deionized water, then add 5mL of PBS buffer solution (pH=7.4), continue to add deionized water to make the volume to 200mL, and obtain a probe of 5 μmol / L test fluid.
[0028] Mercury ion stock solution: Accurately weigh 32.46mg mercury nitrate (Hg(NO 3 ) 2 ), add deionized water to dissolve, and after the sample is completely dissolved, transfer it into a 10mL volumetric flask, set the volume to the mark, and mix well. The concentration of this solution is 10mmol / L. Take 1mL of the above solution an...
Embodiment 3
[0029] Embodiment 3: the mensuration of probe fluorescence spectrum
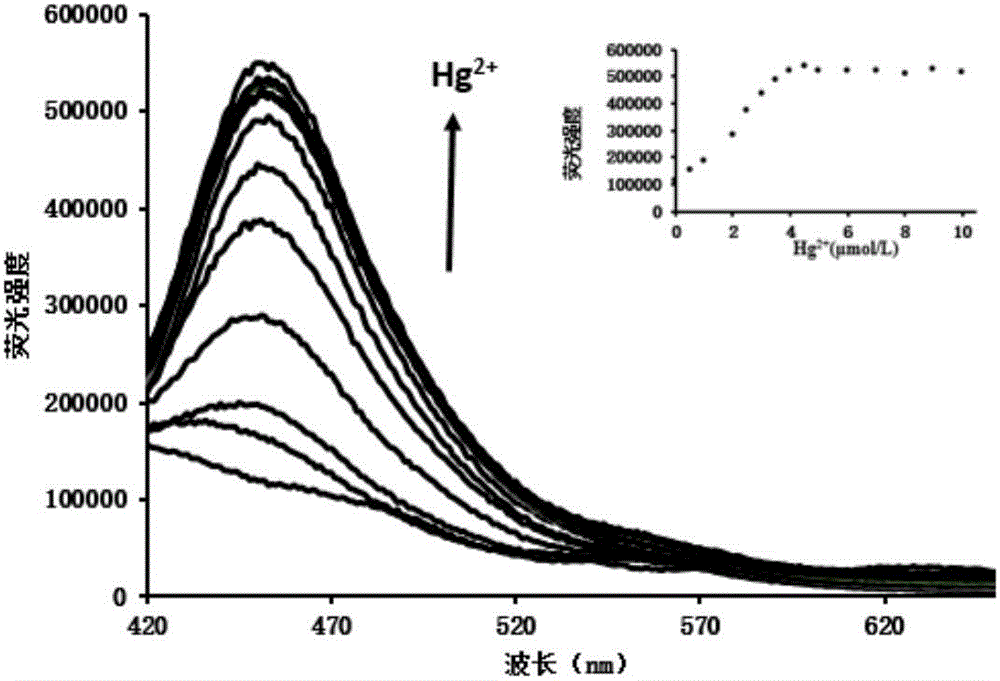
[0030] Take 15 10mL colorimetric tubes, and then number them, add 5μmol / L probe test solution 5mL, and then add 1mmol / L mercury ions in order of 0μL, 2.5μL, 5μL, 10μL, 12.5μL, 15μL , 17.5 μL, 20 μL, 22.5 μL, 25 μL, 30 μL, 35 μL, 40 μL, 45 μL, 50 μL, shake well. Take 4mL of the solutions in the above-mentioned 15 colorimetric tubes in a cuvette, and measure the fluorescence spectrum at 456nm.
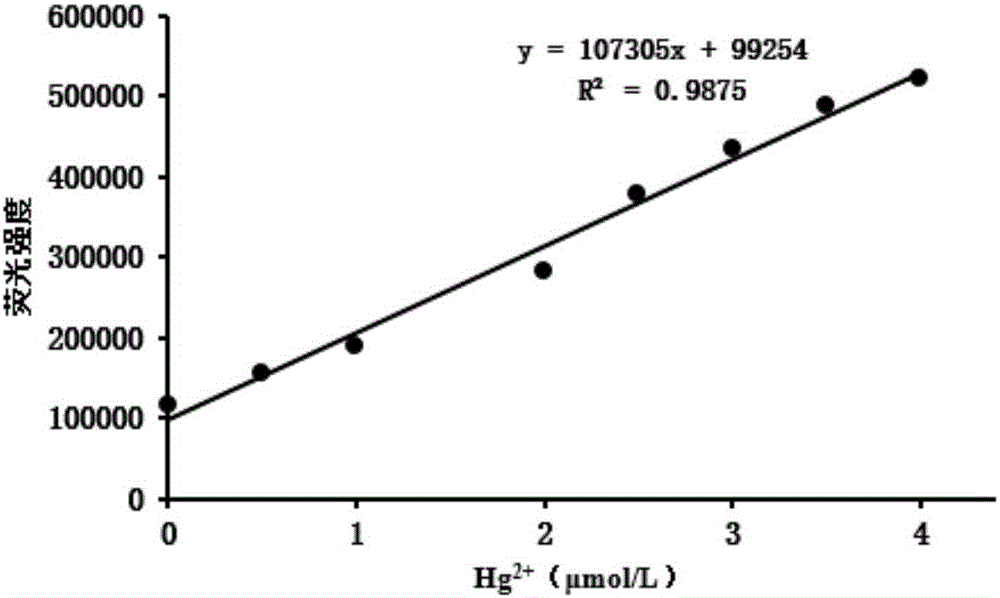
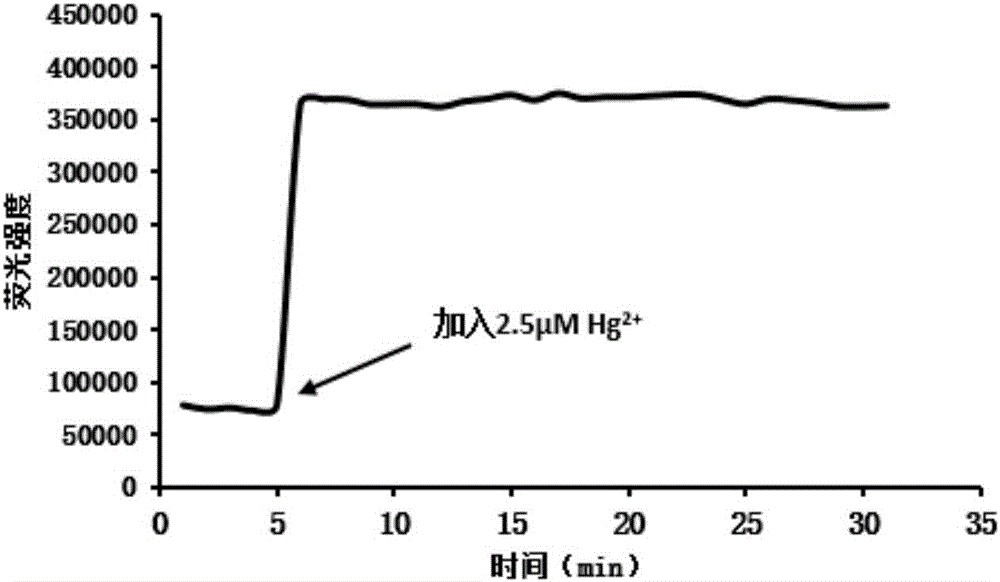
[0031] In the probe synthesized in embodiment 1, add the change of fluorescence intensity of different concentrations of mercury ions, such as figure 1 shown. Along with the increase of mercury ion concentration, its fluorescence intensity is also increasing, when the concentration of mercury ion is greater than 4 μ mol / L, the fluorescence intensity of the synthesized probe in embodiment 1 is basically stable, and it can be seen that the concentration of mercury ion is in When the concentration of mercury ions is 0-4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com