1,2-disubstituted bis ruthenium face chirality ligand and method for synthesizing same
A technology of ruthenocene faces and chiral ligands, which is applied in 1 field and can solve problems such as low ligand reactivity and poor asymmetry induction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
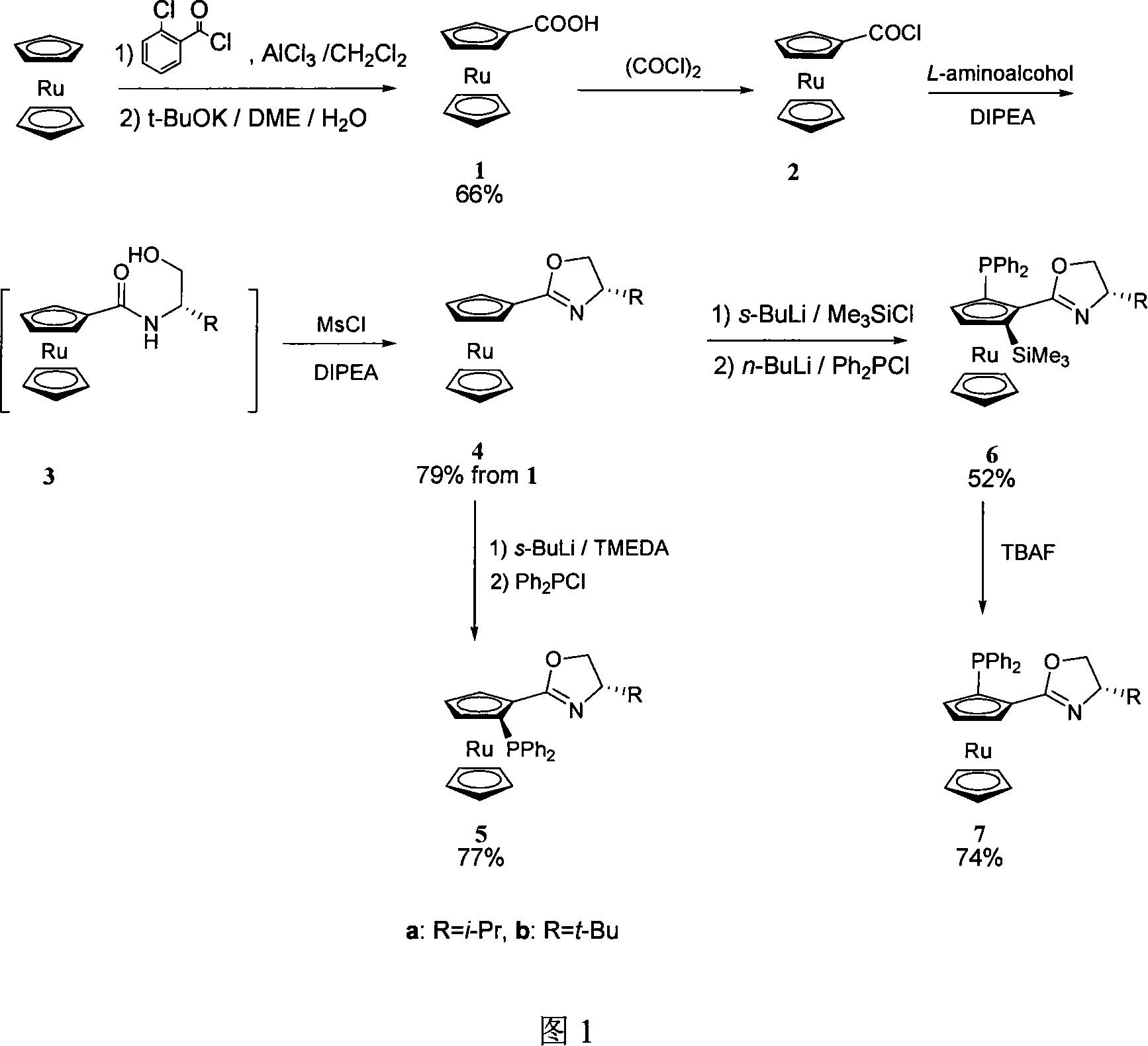
[0031] 1, the preparation of 1-dicarboxyruthenocene (1)
[0032] Add ruthenocene (0.23g, 1mmol), m-chlorobenzoyl chloride (0.18g, 1mol) and dichloromethane (5mL) into a 250mL two-necked flask. Cool down to 0-2°C, add anhydrous aluminum trichloride (0.14 g, 1 mmol) in batches, wait for the temperature to rise naturally and stir overnight. Cool down to 0-2°C, add water (2mL) carefully, stir for 2h, then dilute the system with dichloromethane (20mL), wash with 10% sodium hydroxide solution, water and saturated brine successively, and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was subjected to column chromatography (ethyl acetate / petroleum ether=1 / 20) to obtain an acylated product (51.5 mg, y=13.9%).
[0033] 1 H NMR (400MHz, CDCl 3 ): δ4.64(s, 5H), 4.83(tJ=2Hz, 2H), 5.01(tJ=2Hz, 2H), 7.28-7.55(m, 4H), 7.99-8.05(m, 1H).
[0034] 2-Chlorobenzoylruthenocene (8.2g, 22mmol) and potassium tert-butoxide (9.9g, 88mmol) were adde...
Embodiment 2
[0044] 1, the preparation of 1-dicarboxyruthenocene (1)
[0045] Into a 250 mL two-necked flask were added ruthenocene (0.23 g, 1 mmol), m-chlorobenzoyl chloride (2.7 g, 15 mol) and dichloromethane (5 mL). Cool down to 0-2°C, add anhydrous aluminum trichloride (0.42g, 3mmol) in batches, wait for the temperature to rise naturally and stir overnight. Cool down to 0-2°C, add water (2mL) carefully, stir for 2h, then dilute the system with dichloromethane (20mL), wash with 10% sodium hydroxide solution, water and saturated brine successively, and anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was subjected to column chromatography (ethyl acetate / petroleum ether=1 / 20) to obtain an acylated product (168 mg, y=65.4%).
[0046] 1 H NMR (400MHz, CDCl 3 ): δ4.64(s, 5H), 4.83(t J=2 Hz, 2H), 5.01(t J=2 Hz, 2H), 7.28-7.55(m, 4H), 7.99-8.05(m, 1H) .
[0047] 2-Chlorobenzoylruthenocene (8.2g, 22mmol) and potassium tert-butoxide (9.9g, 8...
Embodiment 3
[0057] 1, the preparation of 1-dicarboxyruthenocene (1)
[0058] Add ruthenocene (11.6 g, 50 mmol), m-chlorobenzoyl chloride (63 mL, 0.5 mol) and dichloromethane (100 mL) into a 250 mL two-necked flask. Cool down to 0-2°C, add anhydrous aluminum trichloride (8.7 g, 65 mmol) in batches, wait for the temperature to rise naturally and stir overnight. Cool down to 0-2°C, add water (20mL) carefully, stir for 2h, then dilute the system with toluene (200mL), wash with 10% sodium hydroxide solution, water and saturated brine successively, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was subjected to column chromatography (ethyl acetate / petroleum ether=1 / 20) to obtain an acylated product (13.5 g, y=73%).
[0059] 1 H NMR (400MHz, CDCl 3 ): δ4.64(s, 5H), 4.83(tJ=2Hz, 2H), 5.01(tJ=2Hz, 2H), 7.28-7.55(m, 4H), 7.99-8.05(m, 1H).
[0060]2-Chlorobenzoylruthenocene (8.2g, 22mmol) and potassium tert-butoxide (9.9g, 88mmol) were added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com