New synthesizing method of chlorodiphenylphosphine
A technology of diphenylphosphine chloride and phenylphosphine dichloride, which is applied in the synthesis field of diphenylphosphine chloride, can solve the problems of environmental protection, product yield and purity need to be improved, and high cost, and can speed up the reaction rate , The effect of simplifying production operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
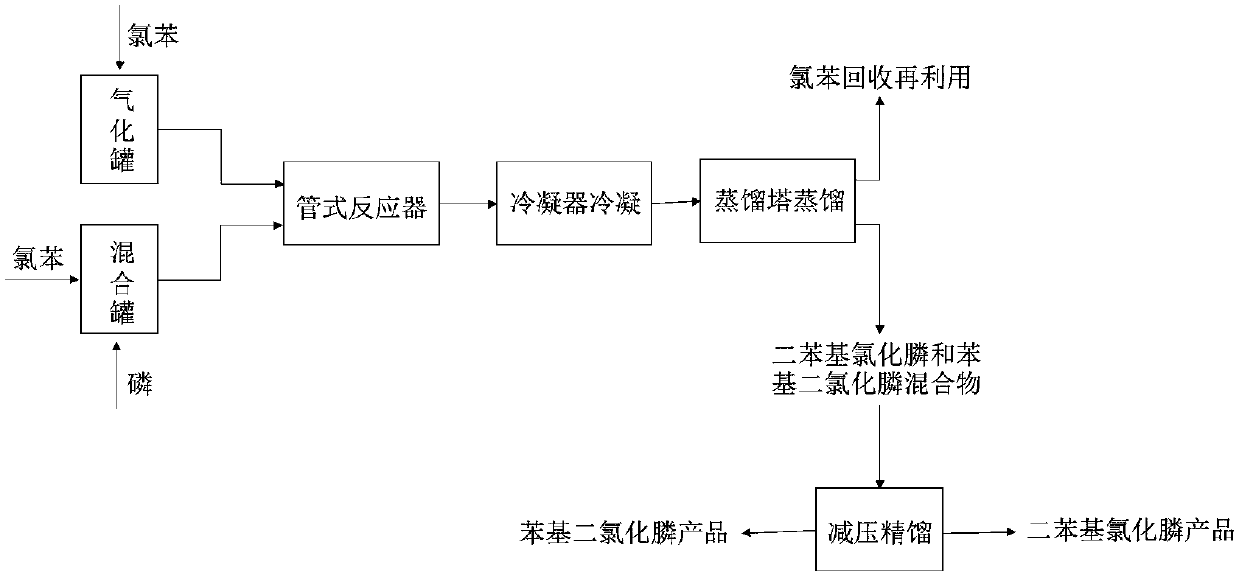
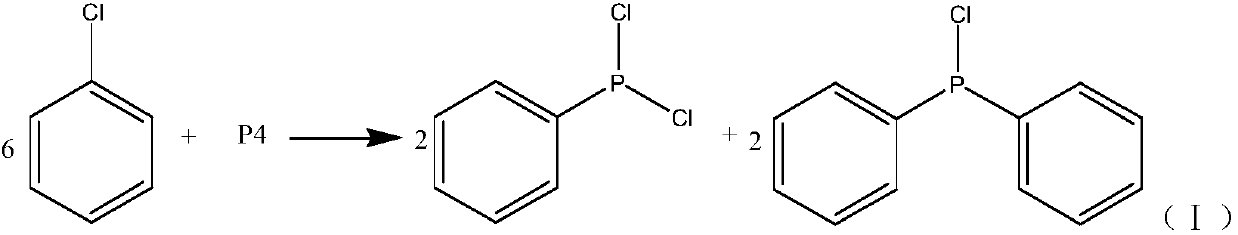
[0027] A new synthetic method of diphenylphosphine chloride, comprising the following steps:
[0028] (1) Raw material preparation: add 13 mol chlorobenzene to the gasification tank with the temperature set at 290°C to vaporize the chlorobenzene for later use; add 2 mol chlorobenzene and 1 mol phosphorus to the mixing tank with the temperature set at 125°C to make them fully mixed Get the mixture and set aside;
[0029] (2) Reaction in the tubular reactor: the gasified chlorobenzene is introduced into the tubular reactor, and the flow rate is controlled by a gas flow meter so that the flow rate of gaseous chlorobenzene in the tubular reactor is 4L / min, and the gaseous chlorine The residence time of benzene in the tubular reactor is 8s, and the mixed solution is injected into the tubular reactor with a metering pump, and the flow rate is controlled by a liquid flow meter so that the flow rate of the mixed solution in the tubular reactor is 4ml / min. The residence time of the mi...
Embodiment 2
[0033] A new synthetic method of diphenylphosphine chloride, comprising the following steps:
[0034] (1) Raw material preparation: add 4mol chlorobenzene to the gasification tank with the temperature set at 280°C to vaporize the chlorobenzene for later use; add 1mol chlorobenzene and 1mol phosphorus to the mixing tank with the temperature set at 120°C to make them fully mixed Get the mixture and set aside;
[0035] (2) Reaction in the tubular reactor: the gasified chlorobenzene is introduced into the tubular reactor, and the flow rate is controlled by a gas flow meter so that the flow rate of gaseous chlorobenzene in the tubular reactor is 3L / min, and the gaseous chlorine The residence time of benzene in the tubular reactor is 5s, and the mixed solution is pumped into the tubular reactor with a metering pump, and the flow rate is controlled by a liquid flow meter so that the flow rate of the mixed solution in the tubular reactor is 2ml / min. The residence time of the mixed so...
Embodiment 3
[0038] A new synthetic method of diphenylphosphine chloride, comprising the following steps:
[0039] (1) Raw material preparation: add 20mol chlorobenzene to the gasification tank with the temperature set at 300°C to vaporize the chlorobenzene for later use; add 5mol chlorobenzene and 1mol phosphorus to the mixing tank with the temperature set at 130°C to make them fully mixed Get the mixture and set aside;
[0040] (2) Reaction in the tubular reactor: the gasified chlorobenzene is introduced into the tubular reactor, and the flow rate is controlled by a gas flow meter so that the flow rate of gaseous chlorobenzene in the tubular reactor is 7L / min, and the gaseous chlorine The residence time of benzene in the tubular reactor is 10s, and the mixed solution is injected into the tubular reactor with a metering pump, and the flow rate is controlled by a liquid flow meter so that the flow rate of the mixed solution in the tubular reactor is 6ml / min. The residence time of the mixt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com