Novel method for synthesizing chlorodiphenylphosphine by one-step process
A new technology of diphenylphosphine chloride, which is applied in the field of synthesis of diphenylphosphine chloride, can solve the problems of heavy pollution, complicated process steps, high-temperature disproportionation environment, etc., and achieve the effect of realizing recovery, increasing income and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
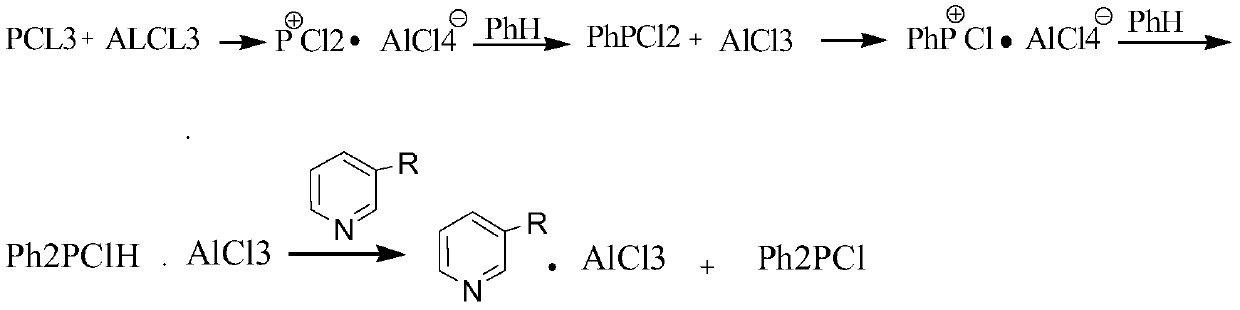
[0033] Add 1.86mol PH and 0.9mol anhydrous AlCl3 into a dry four-necked flask with a stirrer, immediately install the reflux condenser and tail gas receiver, replace the air in the system with nitrogen, and add PCl3 dropwise within 45min while stirring 0.9mol, after dropping, slowly raise the temperature to the reflux of the reaction mixture, after the AlCl3 is completely dissolved, raise the temperature to 140~150°C within 2h. Continue to react in this temperature range for 6h, cool to 35°C, dropwise add 1.8mol Dilute it with phosphorus chloride, then add 0.96 mol of pyridine dropwise at 25 to 40 to obtain a light yellow viscous substance, which is extracted with 500ml of phosphorus trichloride each time, and continuously extracted several times. The low boiling point was removed to obtain 127.5g of crude product. The 140-142°C fraction was collected at 0.4kPa to obtain 90.2g of colorless viscous liquid with a yield of 45.40%. The infrared spectrum of the product Ph2PCl was co...
specific Embodiment 2
[0035] Add 1.86mol phH and 0.9mol anhydrous AlCl3 into a dry four-necked flask with a stirrer, immediately install a reflux condenser and a tail gas receiver, replace the air in the system with nitrogen, and add PCl3 dropwise within 45 minutes while stirring 0.9mol, after dropping, slowly raise the temperature to the reflux of the reaction mixture, after the AlCl3 is completely dissolved, raise the temperature to 140~150°C within 2h. Continue to react in this temperature range for 6h, cool to 35°C, dropwise add 1.8mol Dilute it with phosphorus chloride, then add 0.96 mol of 2-methylpyridine dropwise at 25-40 to obtain a light yellow viscous substance, extract it with 500ml phosphorus trichloride each time, and extract it several times continuously. The solvent was removed under reduced pressure to obtain 127.5 g of the crude product. The 140-142 ° C fraction was collected at 0.4 kPa to obtain 102 g of a colorless viscous liquid with a yield of 510%. The infrared spectrum of the...
specific Embodiment 3
[0037] Add 1.86mol phH and 0.9mol anhydrous AlCl3 into a dry four-necked flask with a stirrer, immediately install a reflux condenser and a tail gas receiver, replace the air in the system with nitrogen, and add PCl3 dropwise within 45 minutes while stirring 0.9mol, after dropping, slowly raise the temperature to the reflux of the reaction mixture, after the AlCl3 is completely dissolved, raise the temperature to 140~150°C within 2h. Continue to react in this temperature range for 6h, cool to 35°C, dropwise add 1.8mol Dilute it with phosphorus chloride, then add 0.96 mol of lutidine dropwise at 25-40 to obtain a light yellow viscous substance, extract with 500ml phosphorus trichloride each time, and extract continuously several times, and evaporate the solvent at normal pressure , the low boiling point was removed under reduced pressure to obtain 127.5g of crude product. The 140-142°C fraction was collected at 0.4kPa to obtain 103.5g of colorless viscous liquid with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com