Environment-friendly synthesis method for diphenyl phosphine chloride
A technology of diphenylphosphine chloride and a synthesis method, which is applied in chemical instruments and methods, aluminum compounds, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as pollution of the environment, and reduce pollution and production time short, yield-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
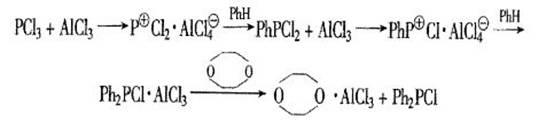
[0029] 1. Add 137.7g (1.0mol) of phosphorus trichloride, 78g (1.0mol) of benzene and 160.2g (1.2mol) of aluminum trichloride into the three-necked flask respectively, pass in nitrogen protection, stir vigorously, and heat up to 140- 150°C, keep reflux (a large amount of white smoke is released at this time). When the white smoke disappears, start to cool down to room temperature.
[0030] 2. Post-processing operations:
[0031] After adding heptane to the above reaction solution and stirring for 0.5 h, β-triethyl chlorophosphate was added dropwise, and the addition was completed after 0.5 h. Stir for 1.0h and let stand for 1.0h. Separate the lower β-triethyl chlorophosphate layer, and distill the heptane layer under reduced pressure. The crude diphenylphosphine chloride (CDPP) was obtained with a content of about 98% and a small amount of complexes, and the yield was 92.5-95.5%. The crude product was distilled under high vacuum to obtain pure diphenylphosphine chloride (CD...
Embodiment 2
[0036] 1. Add 137.7g (1.0mol) of phosphorus trichloride, 78g (1.0mol) of benzene and 173.3g (1.3mol) of aluminum trichloride into a three-necked flask respectively, pass in nitrogen protection, stir vigorously, and heat up to 140- 150°C, keep reflux (a large amount of white smoke is released at this time). When the white smoke disappears, start to cool down to room temperature.
[0037] 2. Post-processing operations:
[0038] After adding heptane to the above reaction solution and stirring for 0.5 h, β-triethyl chlorophosphate was added dropwise, and the addition was completed after 0.5 h. Stir for 1.0h and let stand for 1.0h. Separate the lower β-triethyl chlorophosphate layer, and distill the heptane layer under reduced pressure. The crude diphenylphosphine chloride (CDPP) was obtained with a content of about 98% and a small amount of complexes, and the yield was 92.5-95.5%. The crude product was distilled under high vacuum to obtain pure diphenylphosphine chloride (CDPP...
Embodiment 3
[0042] 1. Add 137.7g (1.0mol) of phosphorus trichloride, 78g (1.0mol) of benzene and 133.3g (1.0mol) of aluminum trichloride into the three-necked flask respectively, pass in nitrogen protection, stir vigorously, and heat up to 140- 150°C, keep reflux (a large amount of white smoke is released at this time). When the white smoke disappears, start to cool down to room temperature.
[0043] 2. Post-processing operations:
[0044] After adding heptane to the above reaction solution and stirring for 0.5 h, β-triethyl chlorophosphate was added dropwise, and the addition was completed after 0.5 h. Stir for 1.0h and let stand for 1.0h. Separate the lower β-triethyl chlorophosphate layer, and distill the heptane layer under reduced pressure. The crude diphenylphosphine chloride (CDPP) was obtained, with a content of about 98%, and a small amount of complexes, with a yield of 85.5-91.5%. The crude product was distilled under high vacuum to obtain pure diphenylphosphine chloride (CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com