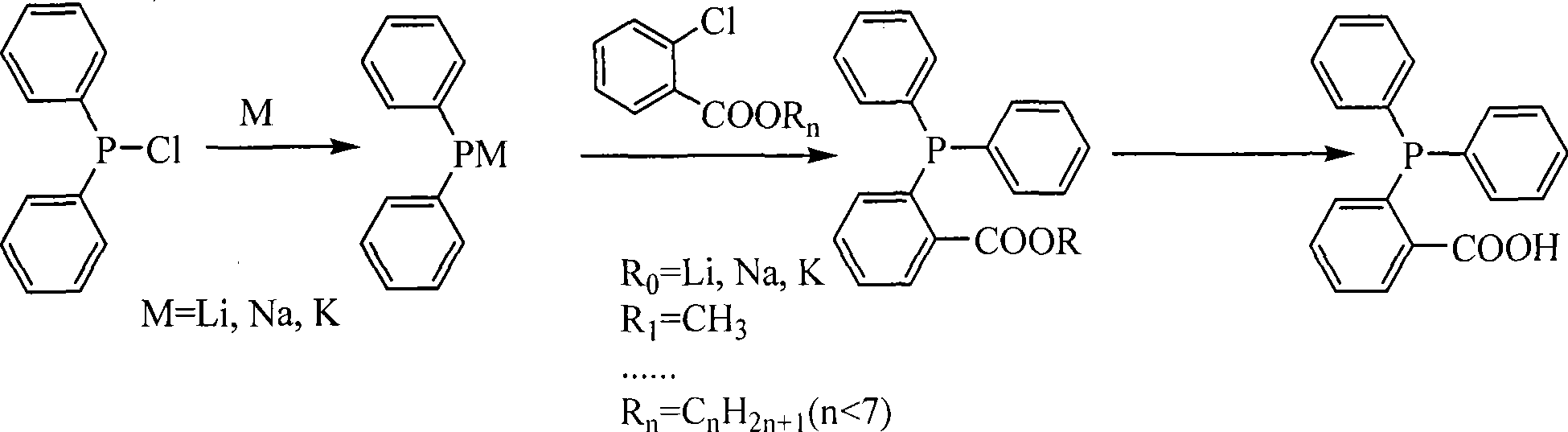
Process for synthesizing O-diphenylphosphinolbenzoic acid
The technology of a diphenylphosphine and a synthesis method is applied in the synthesis field of O-diphenylphosphine benzoic acid, can solve the problems of liquid ammonia environmental pollution, high product cost, many impurities, etc., and achieves simple technological process, convenient operation, good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 50ml tetrahydrofuran, 1.0g sodium metal, and 4.4g diphenylphosphine chloride into a 250ml three-necked flask, and react at 20°C for 15 hours to completely react diphenylphosphine chloride to form sodium diphenylphosphine, and lower the temperature to 0°C , add 3.1g of methyl o-chlorobenzoate, react for 4h to generate methyl diphenylphosphinebenzoate, add concentrated hydrochloric acid for hydrolysis, concentrate to obtain 3.1g of O-diphenylphosphinebenzoate, HPLC=99.1%, yield 50.9 %.
Embodiment 2
[0032] Add 50ml of tetrahydrofuran, 1.1g of sodium metal, and 4.4g of diphenylphosphine chloride into a 250ml three-necked flask, and react at 35°C for 2.5 hours to complete the reaction of diphenylphosphine chloride to form sodium diphenylphosphine, and lower the temperature to 0 ℃, added to 100ml tetrahydrofuran system containing 6.3g of lithium o-chlorobenzoate, reacted for 3h to generate lithium diphenylphosphinebenzoate, added concentrated hydrochloric acid for hydrolysis, concentrated to obtain 3.1g of O-diphenylphosphinebenzoate, HPLC=98.9 %, yield 50.5%.
Embodiment 3
[0034] Add 65ml tetrahydrofuran, 1.1g metal sodium, 4.4g diphenylphosphine chloride to a 250ml three-necked flask, and react at 35°C for 2.5 hours to complete the reaction of diphenylphosphine chloride to form sodium diphenylphosphine, and lower the temperature to 0 ℃, add it into 100ml tetrahydrofuran system containing 7.6g sodium o-chlorobenzoate, react for 3h to generate sodium diphenylphosphinebenzoate, add dilute sulfuric acid (5%-30%) for hydrolysis, and concentrate to obtain O-diphenylphosphinebenzoic acid 3.2g, HPLC=98.5%, yield 52.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com