Patents
Literature
34 results about "Chlorobenzoates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
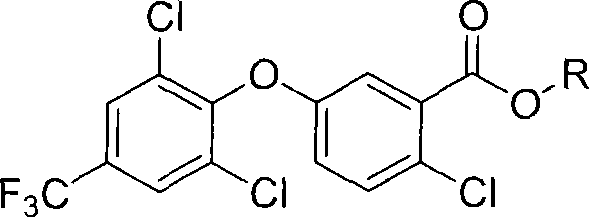
Benzoic acid or benzoic acid esters substituted with one or more chlorine atoms.
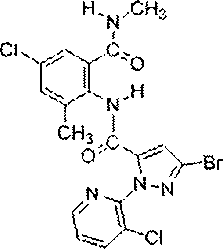
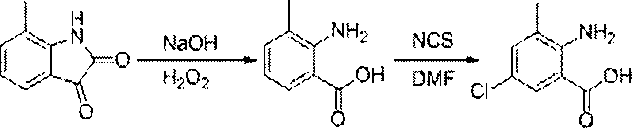
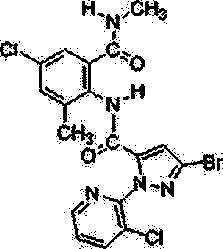
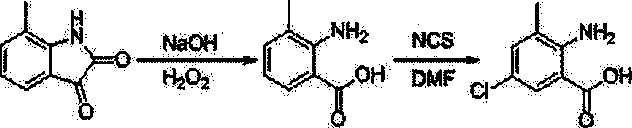
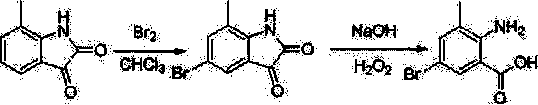
Chlorantraniliprole preparation method
The invention discloses a chlorantraniliprole preparation method. A technical scheme adopted by the invention is characterized in that the chlorantraniliprole preparation method comprises the follow synthetic steps: 1, 2-amino-3-methyl-5-chlorobenzoate synthesis; 2, 3-bromo-1-(3-chloropyridine-2-pyridyl)-1H-pyrazole-5-formic acid synthesis; and 3, chlorantraniliprole synthesis. The invention concretely relates to synthetic methods of the key chlorantraniliprole intermediates comprising 2-amino-3-methyl-5-chlorobenzoate and 3-bromo-1-(3-chloropyridine-2-pyridyl)-1H-pyrazole-5-formic acid. The synthetic method of the pesticide chlorantraniliprole has the advantages of low cost, high yield, strong operability, realization of the industrialized production, and the like.
Owner:HENAN NORMAL UNIV
Diamond resin honing stone for ultra-precisely machining cylinder sleeve platform anilox roller and preparation method thereof
InactiveCN102205525AGuaranteed accuracyImprove grinding effectAbrasion apparatusGrinding devicesChlorobenzoatesEpoxy
The invention relates to a diamond resin honing stone for ultra-precisely machining a cylinder sleeve platform anilox roller and a preparation method thereof. The diamond resin honing stone comprises the following raw materials in percentages by weight: 20-30% of epoxy resin, 5-15% of polyurethane, 2-6% of W7 diamond micron powder, 35-45% of electrolytic copper powder, 5-15% of cerium oxide powder and 4-12% of isobutyl 3,5-diamino-p-chlorobenzoate as a solidifying agent. The preparation method comprises the following steps of: mixing the raw materials according to percentages by weight, pouring and forming to prepare the diamond resin honing stone for ultra-precisely machining the engine cylinder sleeve platform anilox roller. The engine cylinder sleeve platform anilox roller which is precisely honed by utilizing the diamond resin honing stone prepared by the preparation method disclosed by the invention has uniform deep grooves, the uniform width and distance and high platform precision, and an engine cylinder sleeve reaches EU III and IV emission standards.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
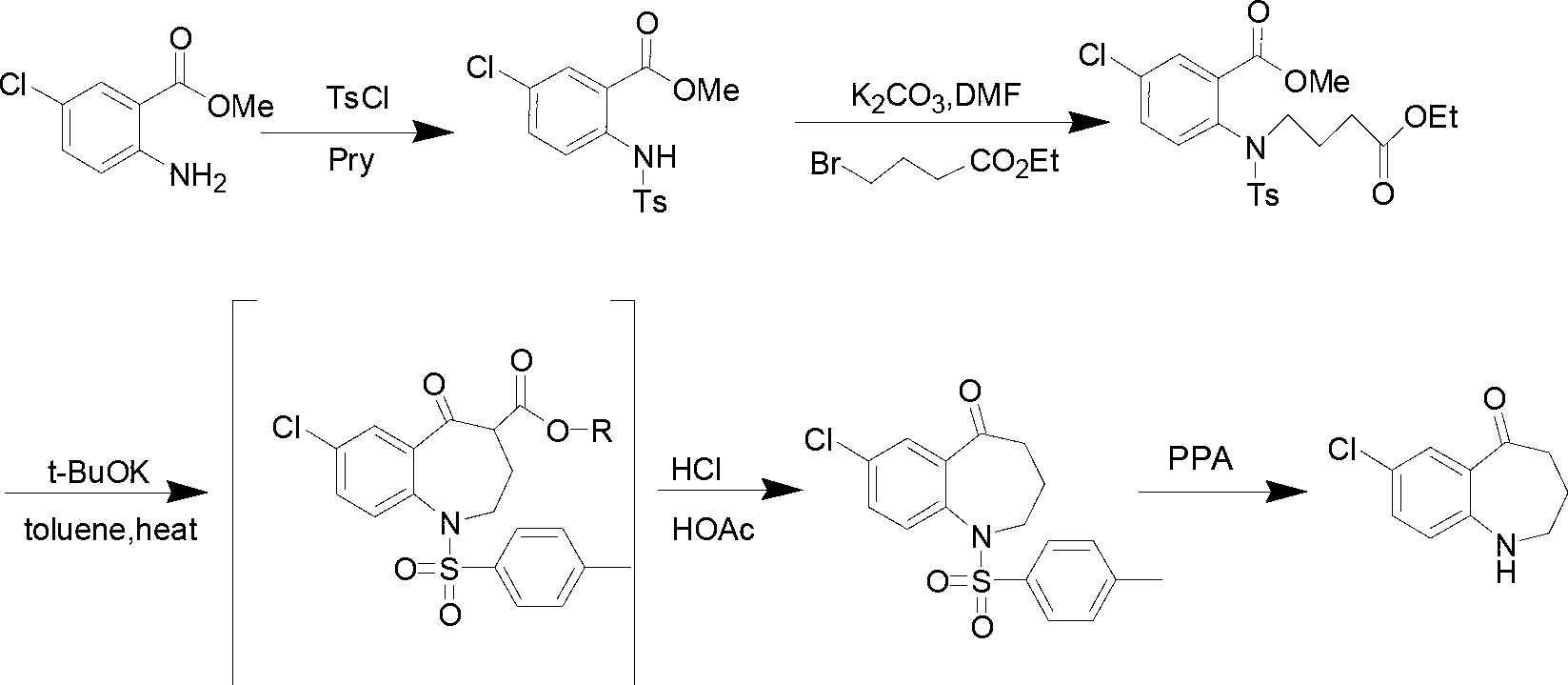
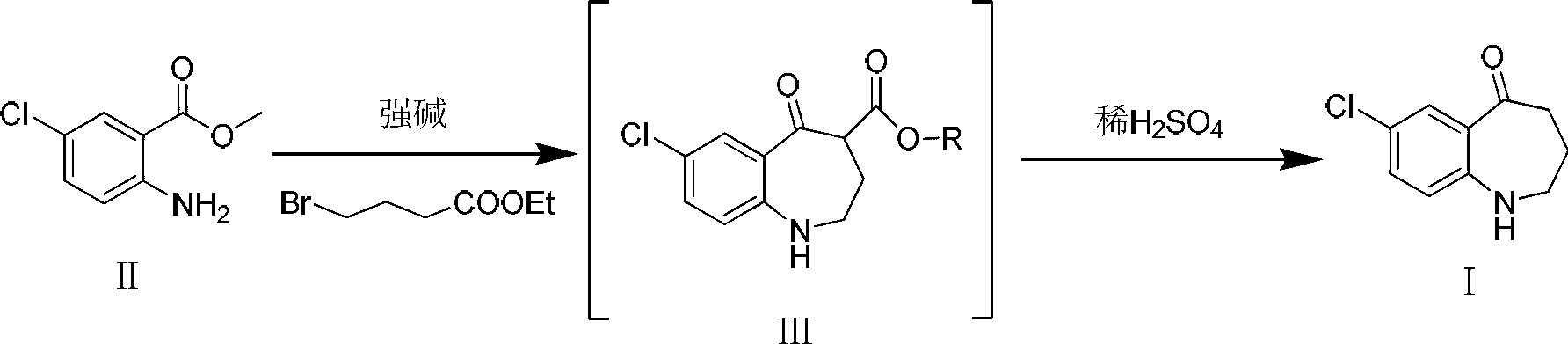
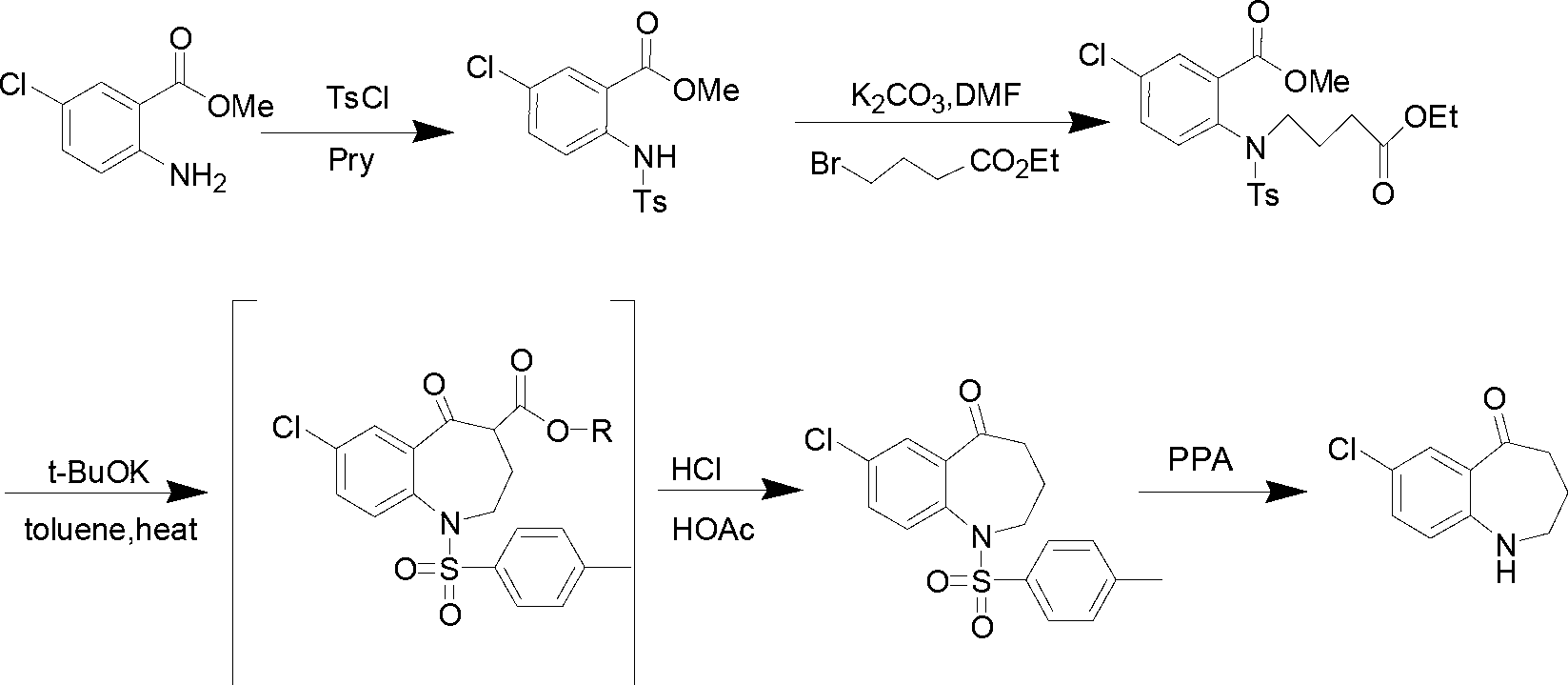
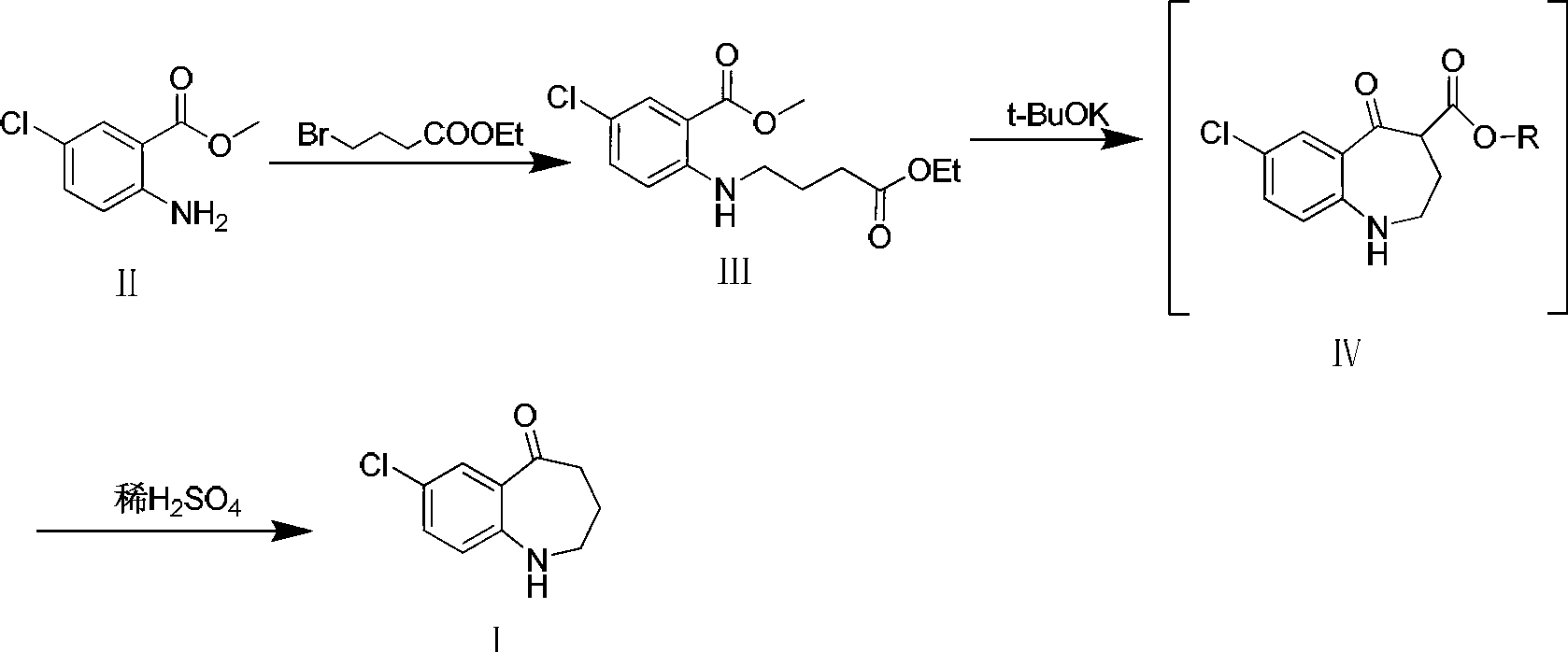
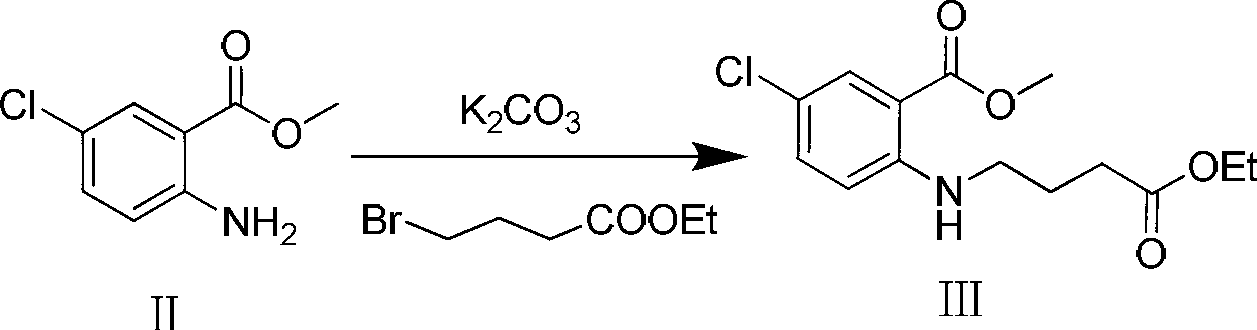
Preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine
The invention relates to a preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine, which is an important intermediate for preparing arginine vasopressin V2 receptor antagonist Tolvaptan. The preparation method comprises the following steps: with methyl 2-amido-5-chlorobenzoate and ethyl 4-bromobutyrate as starting raw materials, reacting at a low temperature under the effect of an acid binding agent to generate secondary amine first, then carrying out Dieckman condensation reaction at a raised temperature to generate an intermediate mixture III, and finally carrying out hydrolysis to obtain the target compound 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine. Compared with the existing method, the method provided by the invention has the advantages that the reaction steps are reduced, the two-step reaction is simplified to one-step reaction through temperature control, so the operation is simple, the processing is convenient, the product purity is high, and the yield is also greatly improved, thus the production cost can be reduced, and the benefits are increased.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
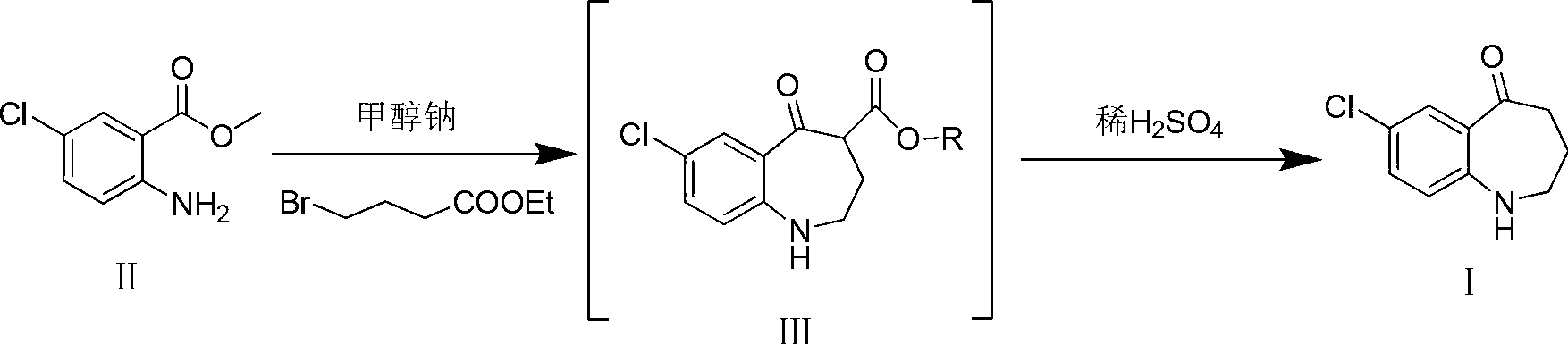
Preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine
The invention relates to a method for preparing 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine, which is an important intermediate for preparing arginine vasopressin V2 receptor antagonist Tolvaptan. The preparation method comprises the following steps: with methyl 2-amido-5-chlorobenzoate and ethyl 4-bromobutyrate as starting raw materials, reacting under the effect of an acid binding agent to generate secondary amine, and then carrying out Dieckman condensation and hydrolysis reaction to obtain the target compound 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine. Compared with the existing method, the method provided by the invention has the advantages that the reaction steps are reduced, the operation is easy and simple, the product purity is high, and the yield is also greatly improved, thus the production cost can be reduced and the benefits are increased.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Prepn process of 3-amino-4-cetyl chlorobenzoate
ActiveCN1974543AImprove catalytic performanceQuick responseOrganic compound preparationAmino-carboxyl compound preparationChlorobenzoatesOrganic solvent
The present invention relates to the preparation process of 3-amino-4-cetyl chlorobenzoate. Compound 3-amino-4-cetyl chlorobenzoate is prepared with 3-amino-4- chlorobenzoic acid and cetanol as main material, and tin tetrachloride, stannous chloride or butyl titanate as catalyst, and through solvent reflux dewatering and esterification. The preparation process includes the following steps: setting 3-amino-4- chlorobenzoic acid and cetanol into organic solvent, heating to dissolve and adding catalyst via stirring, reflux dewatering, recovering solvent after reaction and adding refined organic solvent, filtering to obtain solid, re-crystallizing in refined organic solvent and stoving to obtain 3-amino-4-cetyl chlorobenzoate product. The present invention has great production capacity and high product purity, and is suitable for industrial production.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
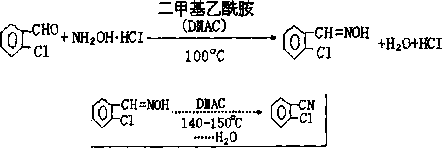
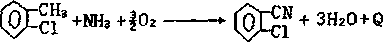
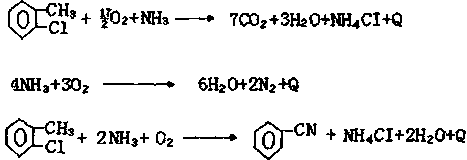
Synthetic method for 2-chlorobenzonitrile
ActiveCN110423207ARealize cleaner productionAvoid complex processOrganic compound preparationCarboxylic acid amides preparationChlorobenzoates2-chlorobenzamide
A synthetic method for 2-chlorobenzonitrile is disclosed and includes the following steps: performing a reaction on o-chlorobenzoic acid and ammonia to generate ammonium o-chlorobenzoate, high temperature dehydration is performed to produce 2-chlorobenzamide, and the high temperature dehydration is performed again to generate the 2-chlorobenzonitrile. In a production process, especially ultrasonicor microwave catalyzed high temperature dehydration is utilized to produce the 2-chlorobenzonitrile, the yield is up to 93%, and the purity of products can reach 97%. And the method has the characteristics of short process route, high reaction yield, good product purity, safe and clean whole reaction process, and easy environmentally friendly treatment. Production of waste water of the 2-chlorobenzonitrile can be effectively reduced, clean production of the 2-chlorobenzonitrile is achieved, unreacted raw material o-chlorobenzoic acid and intermediates can be recovered and then recycled and utilized, and the production cost can be significantly reduced. Moreover, the method can avoid the defects that traditional process equipment is complex, is high in operation requirements and is low inproduct yield, has the characteristics of less "three wastes" and less pollution, is another ideal way to achieve industrial production, and has strong market competitiveness.
Owner:三门峡环宇生化科技有限公司
Chlorantraniliprole preparation method
The invention discloses a chlorantraniliprole preparation method. A technical scheme adopted by the invention is characterized in that the chlorantraniliprole preparation method comprises the follow synthetic steps: 1, 2-amino-3-methyl-5-chlorobenzoate synthesis; 2, 3-bromo-1-(3-chloropyridine-2-pyridyl)-1H-pyrazole-5-formic acid synthesis; and 3, chlorantraniliprole synthesis. The invention concretely relates to synthetic methods of the key chlorantraniliprole intermediates comprising 2-amino-3-methyl-5-chlorobenzoate and 3-bromo-1-(3-chloropyridine-2-pyridyl)-1H-pyrazole-5-formic acid. The synthetic method of the pesticide chlorantraniliprole has the advantages of low cost, high yield, strong operability, realization of the industrialized production, and the like.
Owner:HENAN NORMAL UNIV
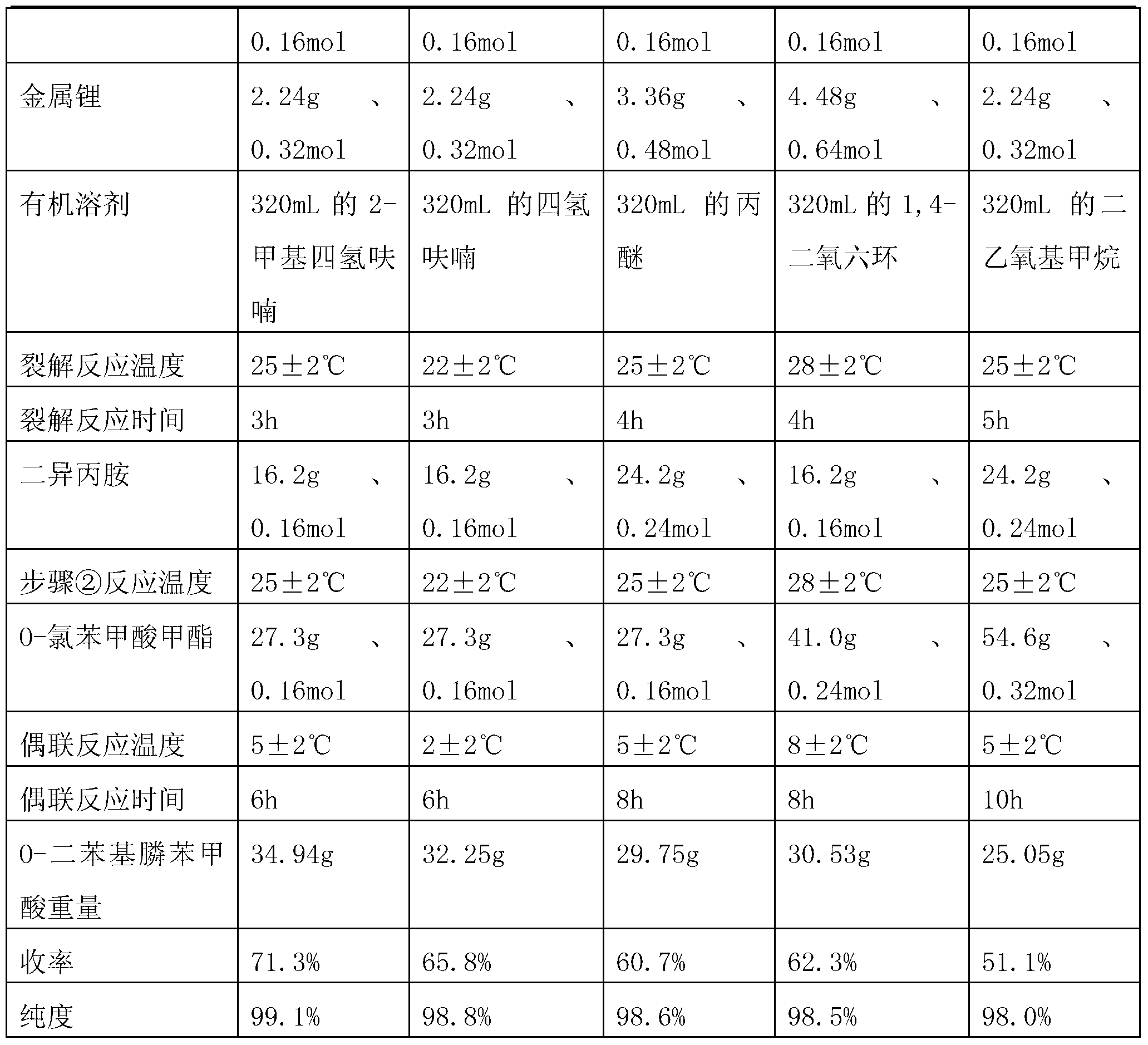
Preparation method of O-diphenylphosphine benzoic acid
InactiveCN103319530AInhibitionHigh reaction yieldGroup 5/15 element organic compoundsBenzoic acidFormate
The invention discloses a preparation method of O-diphenylphosphine benzoic acid. The preparation method comprises the following steps of: (1) carrying out cracking reaction on alkali metal and triphenylphosphine in an organic solvent to generate diphenylphosphine alkali metal salt and phenyl alkali metal salt; (2) slowly adding diisopropylamine, and enabling diisopropylamine to react with the phenyl alkali metal salt completely; (3) slowly adding O-chlorobenzol formate or O-bromobenzol formate, and enabling the diphenylphosphine alkali metal salt and the O-chlorobenzol formate or the O-bromobenzol formate to carry out coupling reaction and generate O-diphenylphosphino benzoate; (4) firstly hydrolyzing under the alkaline condition, obtaining O-diphenylphosphino sodium benzoate, acidizing and neutralizing under the acid condition, and obtaining the O-diphenylphosphine benzoic acid. The preparation method disclosed by the invention has the advantages that phenyllithium produced by cracking is completely reacted by adding the diisopropylamine, so that the generation of side reaction is effectively suppressed, and the reaction yield is greatly increased; since the triphenylphosphine is adopted as a reaction material, compared with the chlorodiphenylphosphine, the production cost is greatly reduced.
Owner:常州化工研究所有限公司
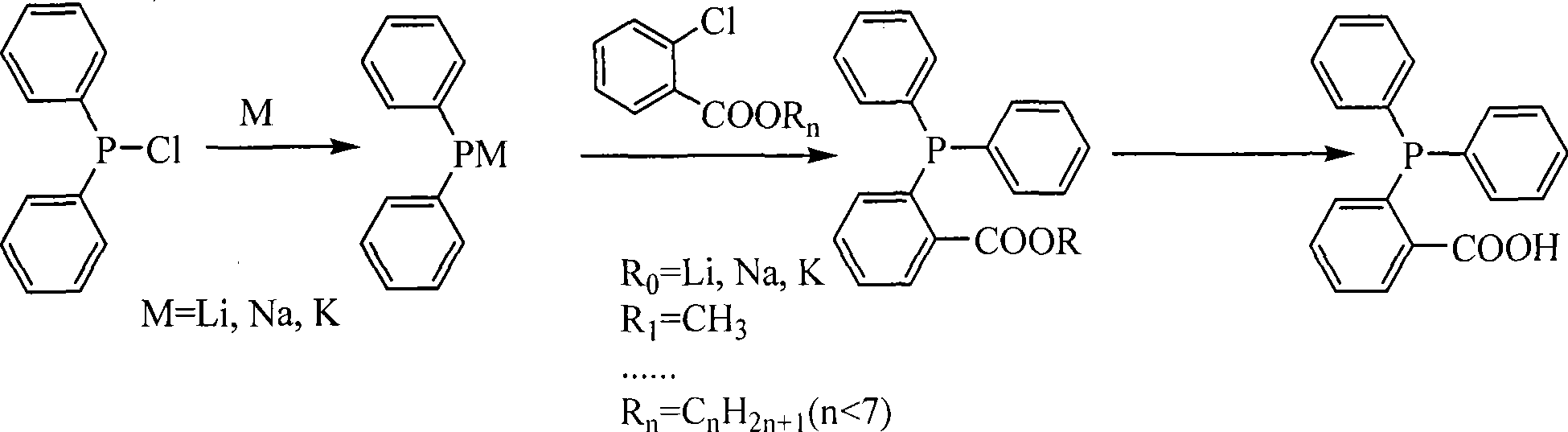
Process for synthesizing O-diphenylphosphinolbenzoic acid
ActiveCN101481388AGuaranteed supplyReduce manufacturing costGroup 5/15 element organic compoundsPotassiumHydrolysis
The invention relates to a synthetic method of o-diphenylphosphinolbenzoic acid. The synthetic method comprises the following steps: taking chlorodiphenylphosphine as a starting material, adding the chlorodiphenylphosphine and an alkali metal to a solvent for cracking to generate diphenylphosphine alkali salt, then adding ortho-chlorobenzoate or ortho-chlorobenzoic acid ether for reaction to generate diphenylphosphine benzoate or diphenylphosphine benzoic ether, and obtaining the o-diphenylphosphinolbenzoic acid after hydrolysis. The solvent is tetrahydrofuran, 2-methyltetrahydrofuran or an ether solvent such as n-butyl ether or 1,4-dioxane and the like, the alkali metal for cracking can be lithium, sodium or potassium, the ortho-chlorobenzoate can be lithium ortho-chlorobenzoate, sodium ortho-chlorobenzoate and lithium ortho-chlorobenzoate, and the ortho-chlorobenzoic acid ether can be ortho-chlorobenzoic methyl ester, ortho-chlorobenzoic acid ethyl ester, ortho-chlorobenzoic acid propyl ester, ortho-chlorobenzoic acid butyl ester, ortho-chlorobenzoic acid pentyl ester and the like. The synthetic method can prepare the o-diphenylphosphinolbenzoic acid above 0 DEG C at normal pressure with safe and stable operation, can save a large amount of energy sources and is suitable for large-scale production.
Owner:SINOMAX SOLUTIONS CO LTD
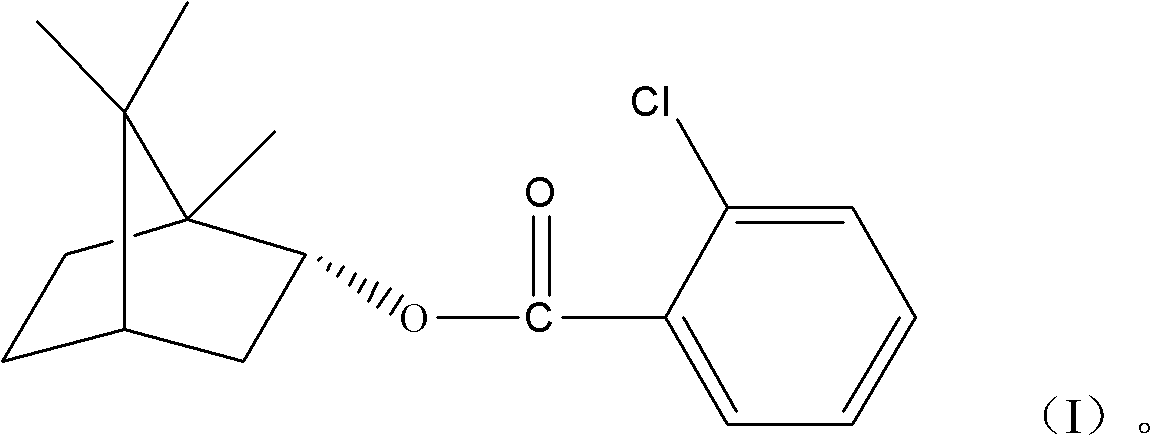
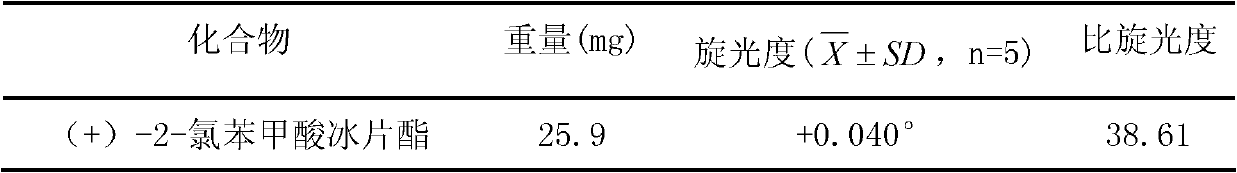
(+)-2-bornyl chlorobenzoate and its preparation method and application
InactiveCN102285886AGood permeation of the blood-brain barrierOrganic active ingredientsNervous disorderChemical structureBenzoic acid
The present invention relates to a kind of bornyl benzoate, its chemical structure is shown in following formula (I), and chemical name is (+)-2-chlorobornyl benzoate. The synthetic method of bornyl benzoate of the present invention is that (+)-borneol, 1.04 times of borneol quality 2-chlorobenzoic acid, 0.067 times of borneol quality p-toluenesulfonic acid are added in toluene, at 110 It was obtained by reacting at ℃ for 6 hours. The (+)-2-chlorobornyl benzoate of the present invention has low toxicity and the effect of penetrating the blood-brain barrier, and can promote medicines to penetrate the blood-brain barrier.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
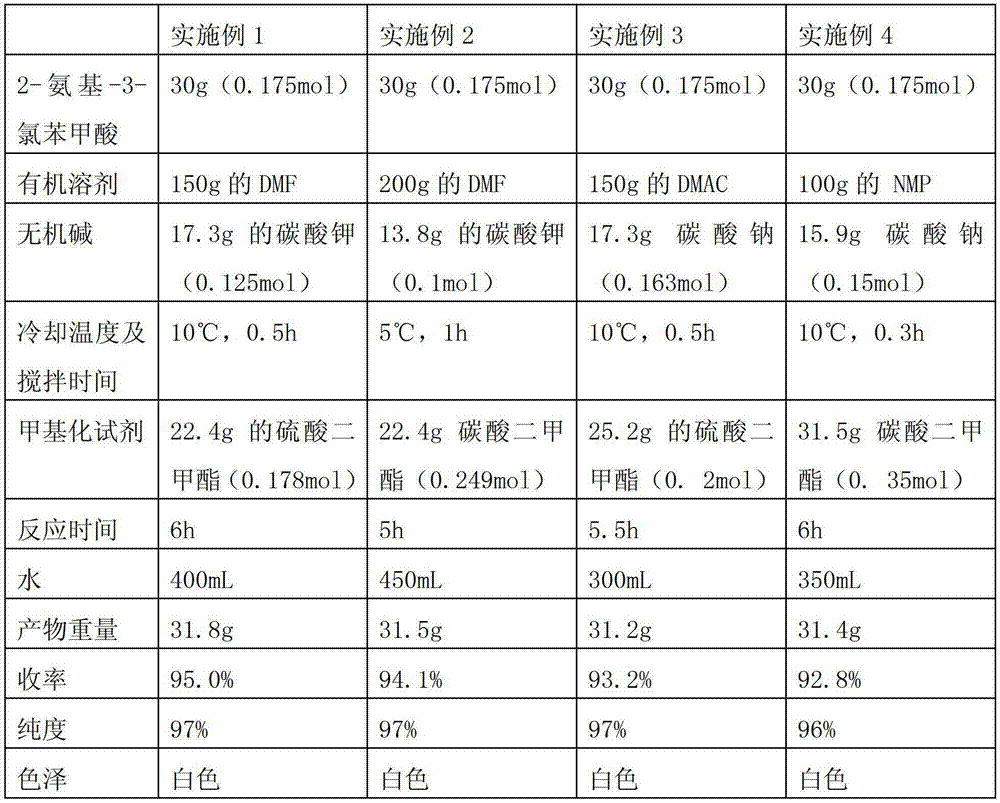
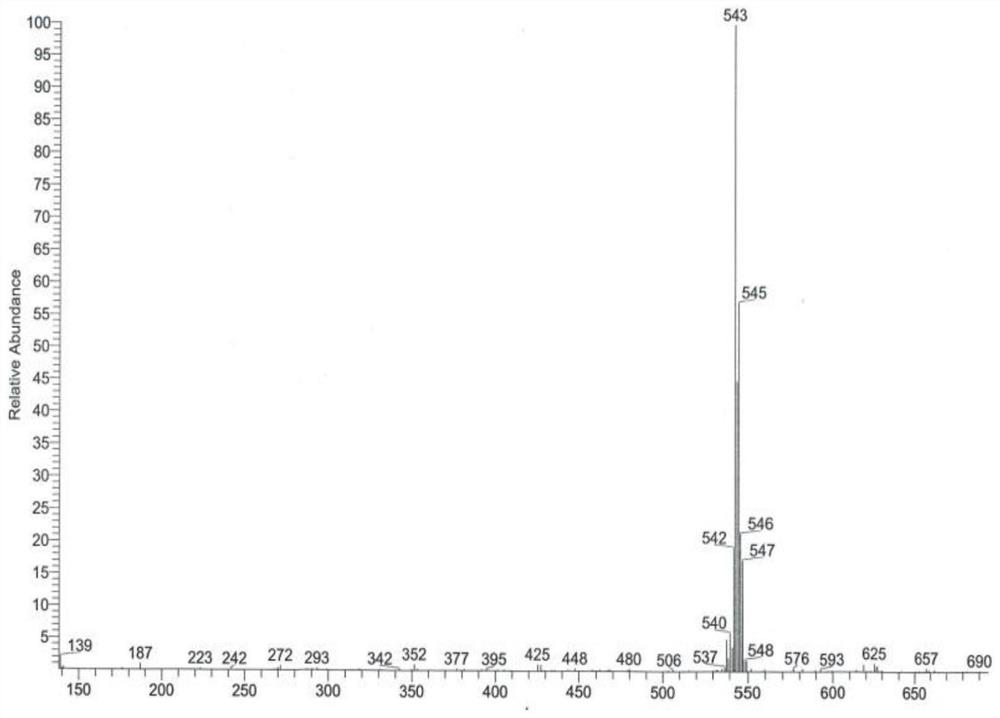
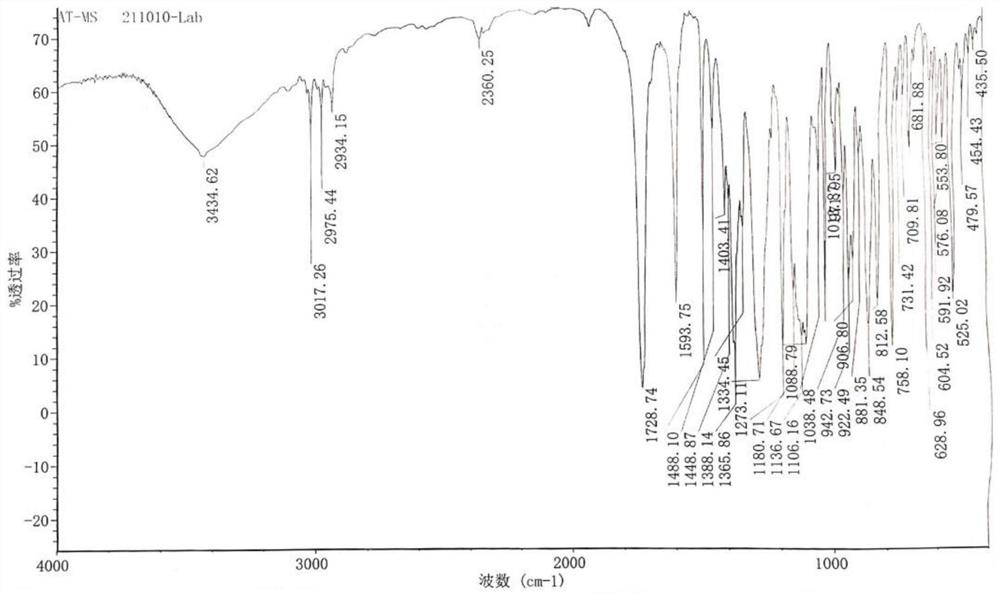
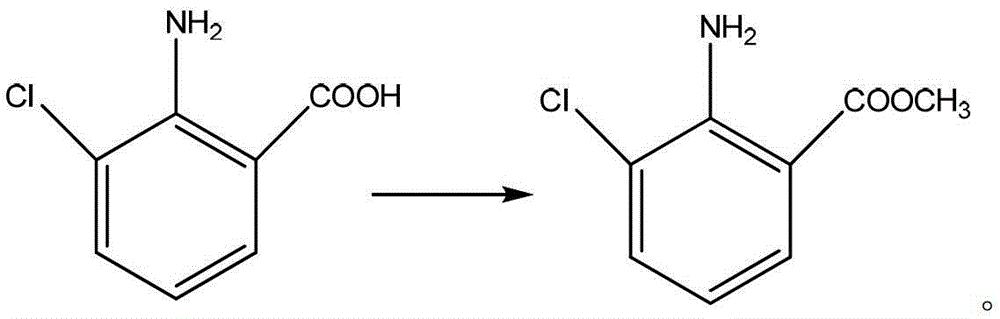
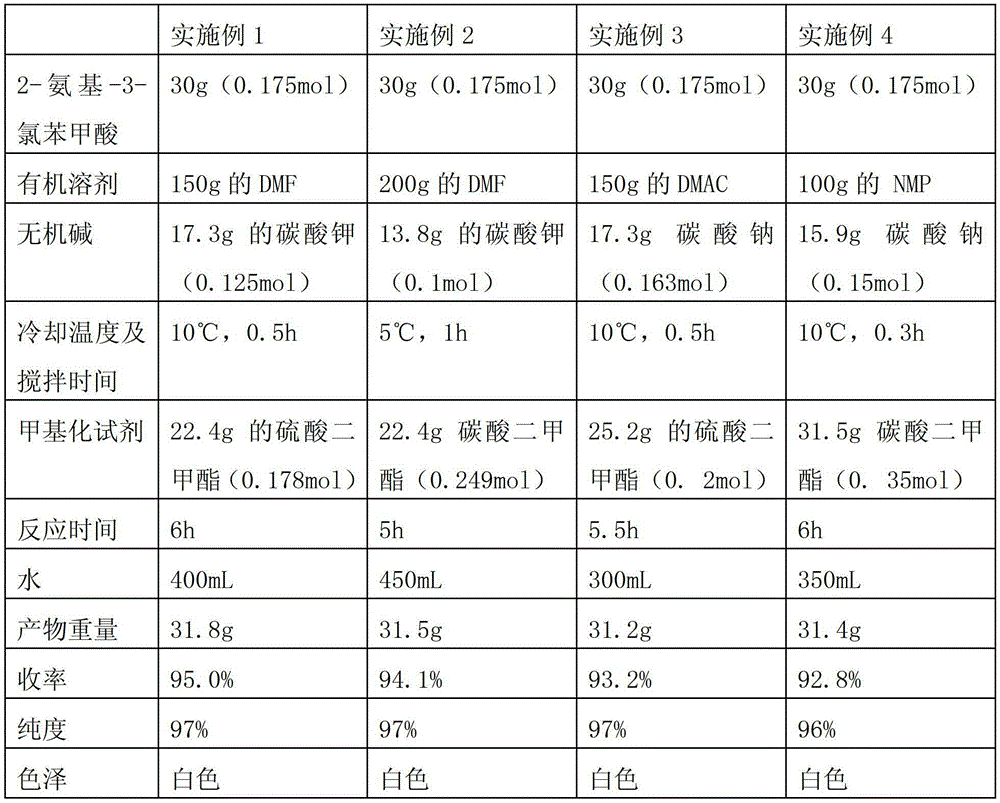
Preparation method of 2-amino-3-chlorobenzoic methyl ester
ActiveCN103193666AHigh yieldGood colorOrganic compound preparationAmino-carboxyl compound preparationChlorobenzoatesOrganic solvent
The invention discloses a preparation method of 2-amino-3-methyl chlorobenzoate. The preparation method comprises the following steps of: (1) adding inorganic acid to 2-amino-3-methyl chlorobenzoate dissolved in organic solvent at the room temperature; (2) cooling the solution to 5 DEG C to 10 DEG C and stirring the solution for 0.1 hour to 1 hours after adding the inorganic acid, and dropwise adding methylation reagent slowly; (3) stirring and reacting for 4 hours to 8 hours at the room temperature after adding the methylation reagent; and (4) filtering the solution, washing the filter cake with water and drying the washed filter cake to obtain the 2-amino-3-methyl chlorobenzoate. According to the preparation method of the 2-amino-3-methyl chlorobenzoate, the methylation reagent and the 2-amino-3-methyl chlorobenzoate are used for carrying out an esterification reaction, so that the reaction process is simple, the operation is easy, the safety is good, the cost is low, and particularly, the purity of the obtained product is higher and the yield is higher, and therefore, the preparation method is very suitable for industrial production. Besides, after the reaction is finished, enough water is used for separating out the product from the reacted materials, so that the yield is further improved; moreover, the separated product is good in color.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
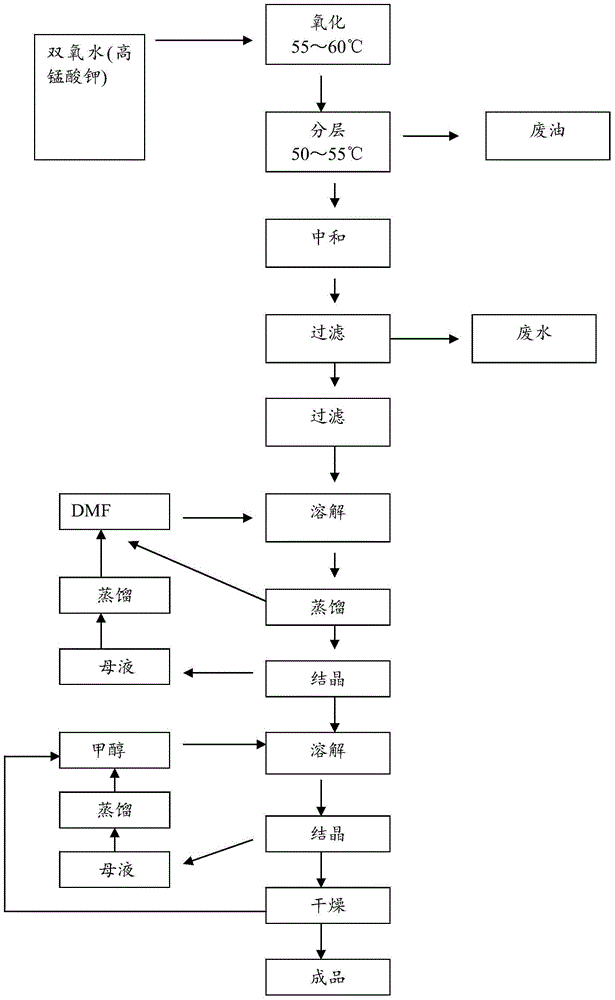
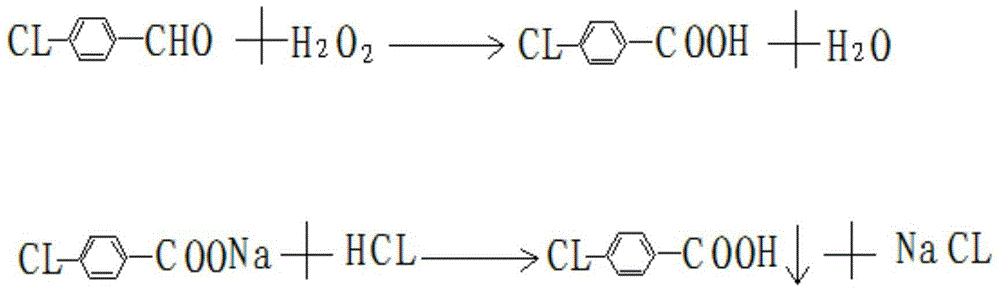
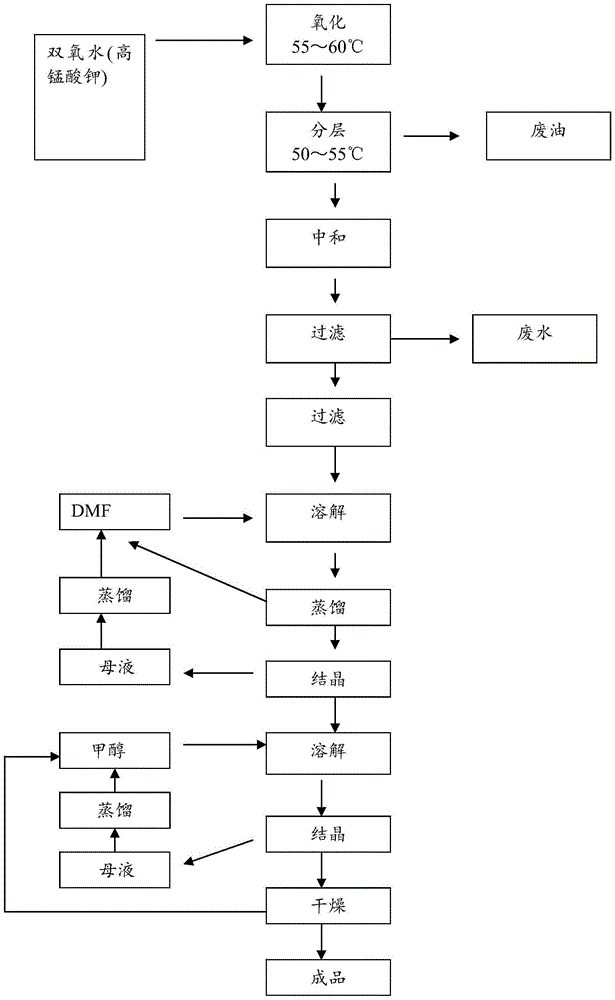
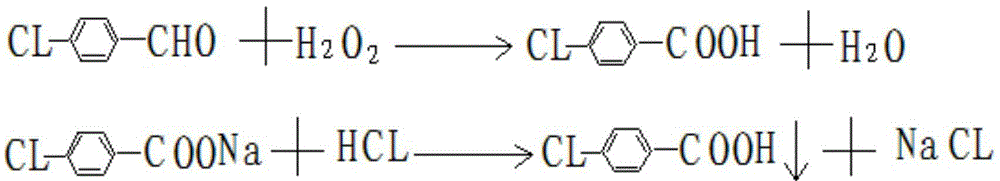
Method for extracting para-chlorobenzoic acid from ketene wastewater
ActiveCN104892405AHigh purityOrganic compound preparationCarboxylic preparation by oxidationN dimethylformamideImpurity
The invention discloses a method for extracting para-chlorobenzoic acid from ketene wastewater, which comprises the following steps: oxidizing sodium para-chlorophenylmethoxide in wastewater to obtain sodium para-chlorobenzoate, stratifying to separate part of organic matters, neutralizing with hydrochloric acid, crystallizing out para-chlorobenzoic acid, purifying by dissolving organic impurities in the crude product into DMF (N,N-dimethylformamide) and methanol to obtain a refined para-chlorobenzoic acid product, and drying to obtain the product. The purity of the product is greater than 99%, which is higher than that of the para-chlorobenzoic acid in the market.
Owner:内蒙古沙洲化学科技有限公司
Preparation method for 3-amino-4-hexadecyl chlorobenzoate
InactiveCN110183336AReduce the temperatureHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChlorobenzoatesOrganic solvent
The invention relates to a preparation method for 3-amino-4-hexadecyl chlorobenzoate. The method comprises the following steps: adding 3-amino-4-chlorobenzoic acid and n-hexadecanol into a water-carrying agent, carrying out heating to 50 DEG C, maintaining a constant temperature for 0.5 to 1 h, then adding a catalyst namely p-toluenesulfonic acid, continuing heating to 140 to 170 DEG C, carrying out a reaction under a refluxing state for 6 to 12 h, and stopping heating; adding an organic solvent into a reaction solution obtained in the previous step, then carrying out cooling crystallization,washing and filtering, and drying a filter cake so as to obtain a crude product; collecting a filtrate; adding an organic solvent into the crude product, carrying out dissolving under heating, and carrying out cooling crystallization and filtering; and drying a filter cake so as to obtain a finished product namely the 3-amino-4-hexadecyl chlorobenzoate. The preparation method provided by the invention improves the utilization rate of raw materials, improves the yield of a product, reduces the treatment amount of an industrial filtrate, reduces the cost of industrial production and is more favorable for industrial production.
Owner:HEBEI UNIV OF TECH
Herbicide containing tralkoxydim and preparation method thereof
The invention discloses an herbicide containing tralkoxydim and a preparation method thereof. The herbicide comprises the following components (by weight): 10-20 parts of tralkoxydim, 15-30 parts of methyl 2-amino-3-chlorobenzoate, 5-15 parts of bensulfuron-methyl, 8-12 parts of 2,3-difluoro-5-chloropyridine, 10-20 parts of 2-chloro-4-diethylamine-6-isopropylamine-1,3,5-triazine, 10-14 parts of acetochlor emulsifiable concentrates, 10-20 parts of atrazine, 1-3 parts of an organic silicon defoamer, 5-15 parts of mefenacet, 20-30 parts of sodium hexametaphosphate, 5-15 parts of sodium nitrate, 10-20 parts of citric acid, 20-30 parts of methyl isopropyl ketone, 5-15 parts of cyhalofop-butyl, 7-10 parts of bispyribac-sodium, 5-15 parts of bentazone, and 1-3 parts of a pesticide adjuvant. The prepared herbicide has the advantages of high efficiency, low toxicity, low residue, low cost and good control effect on grassy and broadleaf weeds.
Owner:NANJING PHARMATECHS
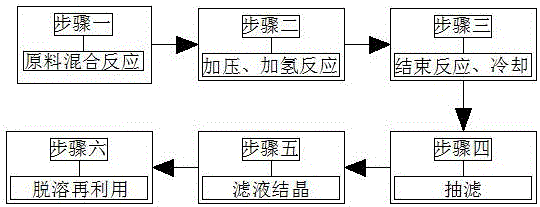
Preparation method of isobutyl 3,5-diamino-4-chlorobenzoate
InactiveCN106478436AReduce manufacturing costEasy to separateOrganic compound preparationAmino-carboxyl compound preparationHydrogen pressureNitrogen gas
A preparation method of isobutyl 3,5-diamino-4-chlorobenzoate comprises the following basic steps: step 1, putting raw material ester compound, a solvent, a catalyst Raney nickel and a dehalogenation inhibitor into a high pressure reaction kettle at one time, sealing, and adopting 0.2-0.3 MPa of nitrogen gas and hydrogen gas for replacing gas in the high pressure reaction kettle for 3 times respectively; step 2, after completely sealing in the step 1, adding the hydrogen gas pressure to 3.0 MPa, increasing the temperature of the high pressure reaction kettle, carrying out a reaction, and timely replenishing the hydrogen gas pressure to ensure that the pressure in the high pressure reaction kettle in the reaction process is maintained at 3.0 MPa; step 3, adding hydrogen gas to maintain the pressure at 3.0 MPa in the step 2, carrying out a reaction for about 1.5-2 hours, finishing the reaction after the system absorbs hydrogen gas no longer, and overall cooling; step 4, after the step 3 is finished, recycling the catalyst Raney nickel; step 5, carrying out reduced pressure concentration of a filtrate to be viscous, putting into a refrigerator, and cooling and crystallizing at low temperature; and step 6, desolventizing the solvent alcohol and the dehalogenation inhibitor, recycling and reusing. The method is low in cost, safe and feasible.
Owner:中农发河南农化有限公司
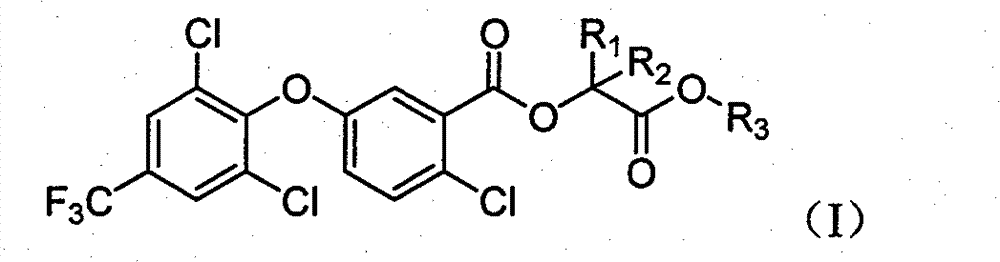
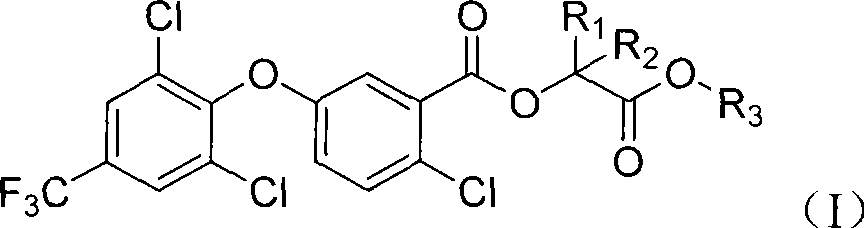
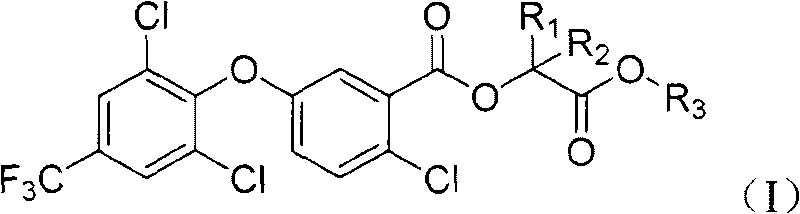
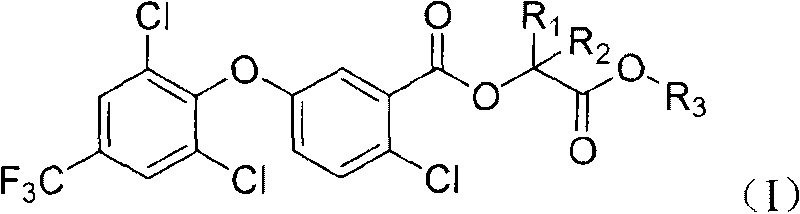
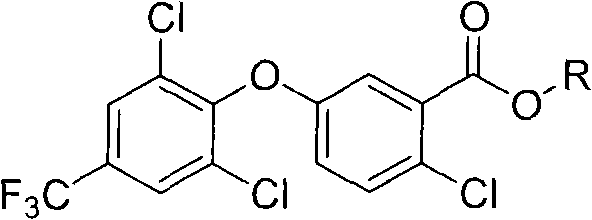
2-chloro-benzoate compound and application thereof
ActiveCN101747202AImprove herbicidal activityEffective controlBiocideOrganic chemistryChlorobenzoates2-Chlorobenzoic acid
The invention discloses a 2-chloro-benzoate compound which has a novel structure and is shown as the general formula (I), wherein R1 is selected from H or methyl, R2 is selected from H or methyl, and R3 is selected from C1-C6 alkyl or benzyl. When R1 is selected from H and R2 is selected from methyl, carbon atoms connected with R1 and R2 in the general formula are in an S or R structural type. Orthe invention provides a compound which contains different contents of S and R structural types. The compound which is shown as the general formula (I) has extraordinary weeding activity after seedling.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Compound of 1-pyrimidine ketone group-4-chlorine-5-benzoic ethers and preparation method thereof
InactiveCN1687037AHas herbicidal activityEffective controlBiocideOrganic chemistryKetonePhenyl group
The present invention relates to a 1-pyrimidone group-4-chlo-5-benzoate compound and its preparation method. Said invention also provides the general formula of said compound. Said compound can be used as weedicide, and has high herbicidal activity.
Owner:NANKAI UNIV
Environment-friendly waterproof architectural decorative coating
InactiveCN103497683ALittle degree of degradationExtended service lifePolyurea/polyurethane coatingsChlorobenzoatesEmulsion
The invention discloses an environment-friendly waterproof architectural decorative coating which is composed of the following components in parts by weight: 30-45 parts of elastic emulsion, 1-3 parts of wetting agent, 1-2 parts of dispersant, 1-5 parts of polyether glycol, 25 parts of toluene diisocyanate, 2-5 parts of chlorobenzoate, 2-3 parts of white carbon black, 2 parts of kaolin, 2 parts of pigment, 3 parts of thickener and 9 parts of dispersant. The product has the advantages of small degradation degree, high stability and long service life; and all the production raw materials are environment-friendly, and thus, no waste water, waste gas, waste slag or pollution is generated in the production and construction process.
Owner:QINGDAO LONGMEI ELECTROMECHANICAL TECH
Universal herbicide for flowers and preparation method thereof
InactiveCN109380422AReduce in quantityIt will not affectBiocideDead animal preservationChlorobenzilateZephyranthes candida
The invention discloses a universal herbicide for flowers. The universal herbicide comprises the following components by mass: 12-16 parts of oriental plane leaves, 14-16 parts of the leaves of Cinnamomum camphora, 18-24 parts of pine needle water, 8-14 parts of bamboo leaves, 9-13 parts of quicklime, 12-14 parts of bentazone, 3-6 parts of a dispersant, 4-8 parts of Sophora alopecuroide, 7-9 partsof methyl chlorobenzoate, 2-4 parts of bensulfuron-methyl, 3-5 parts of fulvic acid and 10-14 parts of a safener. Most raw materials of the herbicide of the invention are natural plants, and the universal herbicide is prepared by refining mutually-promoting-and-restricting components and the like of plants, so the universal herbicide is safe, has low toxicity, specially kills weeds, is free of harm to flowers and plants, and especially has obvious effect on flowers and trees like Zephyranthes candida and Ophiopogon japonicus and other are particularly effective.
Owner:张旭东
A method for extracting p-chlorobenzoic acid in enone wastewater
ActiveCN104892405BHigh purityOrganic compound preparationCarboxylic preparation by oxidationN dimethylformamideImpurity
Owner:内蒙古沙洲化学科技有限公司
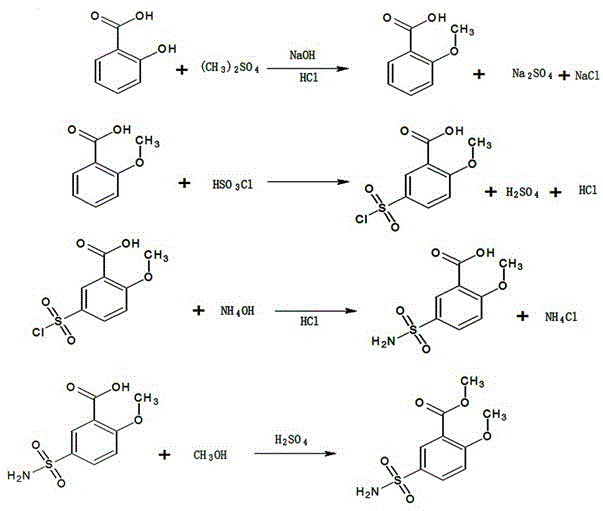
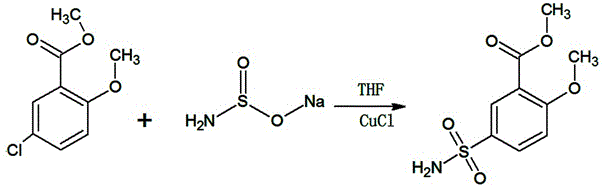
Method for synthesizing 2-methoxy-5-aminosulfonylmethyl benzoate by one-step method
InactiveCN105481733AThe original process is shortHigh yieldOrganic compound preparationSulfonic acid amide preparationSulfonateActivated carbon
The invention provides a method for synthesizing 2-methoxy-5-aminosulfonylmethyl benzoate by a one-step method. The method is characterized by adding 2-methoxy-5-methyl chlorobenzoate, sodium amino sulfonate, a solvent and a catalyst to a reaction device and controlling the temperature at 45-60 DEG C to react for 8-16 hours; adding activated carbon for decoloration after reaction is completed and removing activated carbon, the catalyst and a byproduct sodium chloride through filtration; carrying out vacuum concentration on the filtrate, thus obtaining 2-methoxy-5-aminosulfonylmethyl benzoate, wherein the mole ratio of 2-methoxy-5-methyl chlorobenzoate to sodium amino sulfonate is 1 to (1-1.1). The method for synthesizing 2-methoxy-5-aminosulfonylmethyl benzoate by the one-step method has the advantages that the original technological process is short; the yield is high; the quality is good; no three wastes polluting the environment are generated, so that the method is very environment-friendly and is suitable for large-scale industrial production.
Owner:苏州诚和医药化学有限公司
A kind of method of joint production o-sulfonate sodium benzaldehyde and o-chlorobenzoic acid
ActiveCN104262208BLow costSimple production processPreparation from carboxylic acid saltsSulfonic acids salts preparationSulfite saltBenzaldehyde
The invention discloses a method for combined production of o-benzaldehyde sulfonic acid sodium salt and o-chlorobenzoic acid. The method comprises that o-chlorobenzaldehyde, sodium sulfite, a surfactant and water are mixed, the mixture is heated to a temperature of 160-210 DEG C under the seal condition and undergoes a sulfonation reaction, the reaction product is cooled and crystallizes, o-benzaldehyde sulfonic acid sodium salt is obtained by filtration, the filtrate is concentrated and re-crystallizes, the crystals are subjected to recovered by secondary filtration to form o-benzaldehyde sulfonic acid sodium salt, and the secondary filtration mother liquid is added with an acid so that a pH value is adjusted to less than or equal to 3, then the mother liquid is heated to a temperature of 80-105 DEG C, then is cooled to a temperature of 10-40 DEG C and then is treated to form o-chlorobenzoic acid. The method for combined production of o-benzaldehyde sulfonic acid sodium salt and o-chlorobenzoic acid utilizes the low-price surfactant as a catalyst, realizes o-benzaldehyde sulfonic acid sodium salt one-step synthesis, simplifies a production process, realizes combined production of o-chlorobenzoic acid by acidification recrystallization, reduces waste water organic matter content and realizes waste recovery and recycle.
Owner:ZHEJIANG HONGDA CHEM
Preparation method of alpha-configuration aroyl bromo-sugar
ActiveCN114031651AHigh yieldRaise the ratioSugar derivativesSugar derivatives preparationFuranBenzoic acid
The invention belongs to the technical field of organic synthesis, and provides a preparation method of alpha-configuration aroyl bromo-sugar. Beta-configuration (2R)-3, 5-bis(4-chlorobenzoate)-2-deoxy-2-fluoro-2-methyl-beta-D-erythro-furanpentose is used as a raw material, and is subjected to methylsulfonylation to prepare a mixture containing alpha-sulfonylated sugar and beta-sulfonylated sugar, the proportion of the beta-sulfonylated sugar is increased through transformation, the property of the obtained SN2 configuration inversion feed liquid obtained through SN2-configuration inversion under the action of lithium bromide is stable, the configuration of the alpha-configuration aroyl bromo-sugar is not converted any more, and the yield of the alpha-configuration aroyl bromo-sugar is improved. In addition, the preparation method provided by the invention is more suitable for industrial application.
Owner:JIANGSU COBEN PHARMA CO LTD +2
The preparation method of 2-amino-3-chlorobenzoic acid methyl ester
ActiveCN103193666BHigh yieldGood colorOrganic compound preparationAmino-carboxyl compound preparationChlorobenzoatesOrganic solvent
Owner:江苏省农用激素工程技术研究中心有限公司 +1
Preparation process of 3-amino-4-cetyl chlorobenzoate
ActiveCN100463900CImprove catalytic performanceQuick responseOrganic compound preparationAmino-carboxyl compound preparationChlorobenzoatesTetrachloride
The present invention relates to the preparation process of 3-amino-4-cetyl chlorobenzoate. Compound 3-amino-4-cetyl chlorobenzoate is prepared with 3-amino-4- chlorobenzoic acid and cetanol as main material, and tin tetrachloride, stannous chloride or butyl titanate as catalyst, and through solvent reflux dewatering and esterification. The preparation process includes the following steps: setting 3-amino-4- chlorobenzoic acid and cetanol into organic solvent, heating to dissolve and adding catalyst via stirring, reflux dewatering, recovering solvent after reaction and adding refined organic solvent, filtering to obtain solid, re-crystallizing in refined organic solvent and stoving to obtain 3-amino-4-cetyl chlorobenzoate product. The present invention has great production capacity and high product purity, and is suitable for industrial production.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate
The invention provides a method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate. The method comprises the following steps: a, preparing a compound 2 from a cyclic imido group substituted compound 1, wherein the cyclic imido group is any one of phthalimide, succimide and maleimide; b, carrying out an amination reaction on a primary amine and the compound 2to generate a compound 3; and c, reacting the compound 3 with 4-chlorobenzoic acid to obtain a compound 4. A reaction route is shown in the description. The synthesis method is friendly to the environment, and is suitable for large-scale industrial production.
Owner:SHANGHAI ARK BIOSCI CO LTD
2-chloro-benzoate compound and application thereof
The invention discloses a 2-chloro-benzoate compound which has a novel structure and is shown as the general formula (I), wherein R1 is selected from H or methyl, R2 is selected from H or methyl, and R3 is selected from C1-C6 alkyl or benzyl. When R1 is selected from H and R2 is selected from methyl, carbon atoms connected with R1 and R2 in the general formula are in an S or R structural type. Or the invention provides a compound which contains different contents of S and R structural types. The compound which is shown as the general formula (I) has extraordinary weeding activity after seedling.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Diamond resin honing stone for ultra-precisely machining cylinder sleeve platform anilox roller and preparation method thereof
InactiveCN102205525BGuaranteed accuracyImprove grinding effectAbrasion apparatusGrinding devicesChlorobenzoatesEpoxy
The invention relates to a diamond resin honing stone for ultra-precisely machining a cylinder sleeve platform anilox roller and a preparation method thereof. The diamond resin honing stone comprises the following raw materials in percentages by weight: 20-30% of epoxy resin, 5-15% of polyurethane, 2-6% of W7 diamond micron powder, 35-45% of electrolytic copper powder, 5-15% of cerium oxide powder and 4-12% of isobutyl 3,5-diamino-p-chlorobenzoate as a solidifying agent. The preparation method comprises the following steps of: mixing the raw materials according to percentages by weight, pouring and forming to prepare the diamond resin honing stone for ultra-precisely machining the engine cylinder sleeve platform anilox roller. The engine cylinder sleeve platform anilox roller which is precisely honed by utilizing the diamond resin honing stone prepared by the preparation method disclosed by the invention has uniform deep grooves, the uniform width and distance and high platform precision, and an engine cylinder sleeve reaches EU III and IV emission standards.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
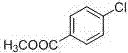
Synthetic method of methyl 4-chlorobenzoate
InactiveCN107324996AEfficient synthesisEfficient use ofElectrolysis componentsOrganic compound preparationTetramethylammonium iodideTetramethylammonium bromide
The invention discloses a synthetic method of methyl 4-chlorobenzoate. The synthetic method comprises the following steps: mixing 1,4-dichlorobenzene with N,N-dimethylformamide or acetonitrile and tetraethylammonium iodide or tetraethylammonium bromide or tetraethylammonium chloride or tetraethylammonium tetraborate or tetrabutylammonium iodide or tetrabutylammonium bromide or tetrabutylammonium chloride to form electrolyte, introducing carbon dioxide into the electrolyte at the room temperature for 30 minutes, carrying out electrolysis by virtue of a constant current (carbon dioxide is continuously introduced during the electrolysis until the electrolysis is finished), and carrying out esterification and post-treatment, so as to obtain methyl 4-chlorobenzoate. The synthetic method has the beneficial effects that a reaction system is simple and easy to control; rice C1 resource CO2 is taken as one of the raw material and is cheap, easily available and low in cost, the environmental pollution is avoided, and a novel path is opened for the research of environment-friendly synthesis of methyl 4-chlorobenzoate; and the synthetic method presents excellent application prospects in the industries of chemical engineering and electrons and is a process route with high industrial synthesis values.
Owner:LIAOCHENG UNIV
Environment-friendly energy-saving degradable high polymer biological material
The invention discloses an environment-friendly energy-saving degradable high polymer biological material which is composed of the following components in parts by weight: 25-30 parts of modified natural high polymer material, 1-3 parts of wetting agent, 1-2 parts of dispersant, 1-5 parts of polyether glycol, 25 parts of toluene diisocyanate, 2-5 parts of chlorobenzoate, 2-3 parts of silica white, 2 parts of kaolin, 2 parts of pigment, 3 parts of thickener, 5-9 parts of film-forming assistant, 2 parts of ceramic powder and 1 part of perfume. The environment-friendly degradable high polymer material is free of any substance harmful to the environment, and has the advantages of high degradation capacity, high strength, simple technique and low cost.
Owner:QINGDAO LONGMEI ELECTROMECHANICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate](https://images-eureka.patsnap.com/patent_img/639eed41-5a1a-4325-aa30-f8c01ea923e1/HDA0001145412350000011.png)
![Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate](https://images-eureka.patsnap.com/patent_img/639eed41-5a1a-4325-aa30-f8c01ea923e1/FDA0001145412330000011.png)
![Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate Method for synthesizing p-methoxybenzyl [3-(aminomethyl)-oxetane-3-yl]carbamate p-chlorobenzoate](https://images-eureka.patsnap.com/patent_img/639eed41-5a1a-4325-aa30-f8c01ea923e1/DEST_PATH_GDA0001170870360000021.png)