Preparation method of isobutyl 3,5-diamino-4-chlorobenzoate
A technology of isobutyl chlorobenzoate and diamino, applied in the field of preparation of chemical raw materials, can solve the problems of large environmental pollution, difficult treatment, high production cost, etc., and achieve the effects of reducing production cost, increasing reaction rate, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
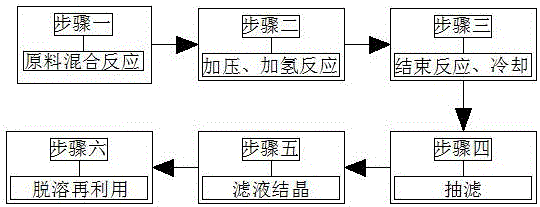
[0025] 1. Raw materials: 20g of esterified product, 100mL of isobutanol, 4g of Raney nickel, 1g of morpholine.
[0026] 2. Operation process:
[0027] 1) Put 20g of the esterified product, 4g of Raney nickel, 100mL of isobutanol, and 1g of morpholine into the autoclave to seal in turn, and replace the autoclave with 0.2-0.3MPa nitrogen and hydrogen for 3 times respectively;
[0028] 2) Fill the hydrogen pressure to 3.0MPa, raise the temperature to 90-100℃ and keep it warm, and replenish the pressure to 3.0MPa in time when the pressure in the kettle drops to 2.0Mpa;
[0029] 3) Continue to replenish and maintain the pressure in the kettle at 2.0-3.0MPa to react for 2.5 hours, the system will no longer absorb hydrogen, the reaction is over, and cool down;
[0030] 4) Suction filtration, solid Raney nickel recycling;
[0031] 5) The filtrate was concentrated under reduced pressure to a viscous state, placed in the refrigerator, and frozen for crystallization.
[0032] Experime...
Embodiment 2
[0034] 1. Raw materials: 20g of esterified product, 100mL of ethanol, 4g of Raney nickel, 1g of morpholine.
[0035] 2. Operation process:
[0036] 1) Put 20g of the esterified product, 4g of Raney nickel, 100mL of ethanol, and 1g of morpholine into the autoclave to seal, and use 0.2-0.3MPa nitrogen and hydrogen to replace the autoclave 3 times;
[0037] 2) Fill the hydrogen pressure to 3.0MPa, raise the temperature to 90-100℃ and keep it warm, and replenish the pressure to 3.0MPa in time when the pressure in the kettle drops to 2.0Mpa;
[0038] 3) Continue to replenish and maintain the pressure in the kettle at 2.0-3.0MPa to react for 1.5 hours, the system will no longer absorb hydrogen, the reaction is over, and cool down;
[0039] 4) Suction filtration, solid Raney nickel recycling;
[0040] 5) The filtrate was concentrated under reduced pressure to a viscous state, placed in the refrigerator, and frozen for crystallization.
[0041] Experimental results: the reaction is...
Embodiment 3
[0043] 1. Raw materials: 20g of esterified product, 100mL of ethanol, 2.5g of Raney nickel, 1g of morpholine.
[0044] 2. Operation process:
[0045] 1) Put 20g of esterified product, 2.5g of Raney nickel, 100mL of ethanol, and 1g of morpholine into the autoclave to seal, and use 0.2-0.3MPa nitrogen and hydrogen to replace the autoclave 3 times;
[0046] 2) Fill the hydrogen pressure to 3.0MPa, raise the temperature to 90-100℃ and keep it warm, and replenish the pressure to 3.0MPa in time when the pressure in the kettle drops to 2.0Mpa;
[0047] 3) Continue to replenish and maintain the pressure in the kettle at 2.0-3.0MPa to react for 1.5 hours, the system will no longer absorb hydrogen, the reaction is over, and cool down;
[0048] 4) Suction filtration, solid Raney nickel recycling;
[0049] 5) The filtrate was concentrated under reduced pressure to a viscous state, placed in the refrigerator, and frozen for crystallization.
[0050] Experimental results: the reaction wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com