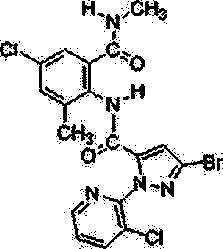
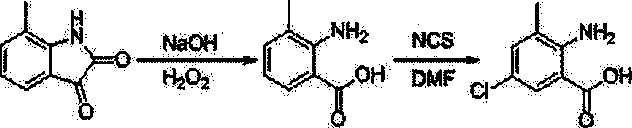
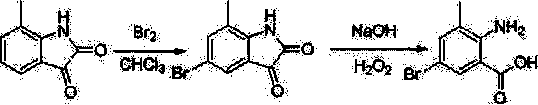
Chlorantraniliprole preparation method
A technology for chlorantraniliprole and chlorobenzoic acid, which is applied in the field of preparation of chlorantraniliprole, can solve the problems of long reaction route, low cost and the like, and achieves the effects of reduced dosage, mild conditions and easy conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of N-2-methylbenzene-2-isonitrosoacetamide (2)
[0037]
[0038] Solution A: A 250 ml three-neck round bottom flask, equipped with a condensing device and a thermometer, added hydroxylamine hydrochloride (3.5 g, 50 mmol), anhydrous sodium sulfate (23.7 g, 167 mmol), chloral hydrate (2.7 g, 16.3 mmol), 67 ml of water. Heat to 40°C with vigorous stirring to dissolve. Solution B: o-toluidine (1.8 g, 16.8 mmol) was added dropwise to a solution containing 15 ml of water and 1.5 ml of concentrated hydrochloric acid. Add solution B to solution A at one time, stir rapidly and heat to reflux. After 1-2 min, the mixture turns milky white, accompanied by a small amount of white precipitates of brown by-products. Remove the oil bath, and cool the ice bath to room temperature (20 ° C), after standing overnight at room temperature, the precipitate was obtained by suction filtration, washed with ice water, and dried with phosphorus pentoxide to obtain 2.24 g of a yellow...
Embodiment 2
[0040] Synthesis of 7-methylindole-2,3-dione (3) Synthesis
[0041]
[0042] In the 50 ml three-neck round-bottomed flask equipped with a condenser tube and a thermometer, add 26.5 ml of concentrated sulfuric acid, heat to 60 ° C, and add N-2-methylbenzene-2-isonitrosoacetamide ( 6.7 g, 37.6 mmol), keep the temperature between 60-70 ° C, the solution turns purple, after the addition, it is warming up to 80 ° C and stirred for 10 min, the reaction is stopped, cooled at room temperature, and the reaction solution is poured under stirring On 160 g of crushed ice, let it stand overnight, and filter with suction to obtain a rust-colored precipitate, dissolve the rust-colored precipitate in 12 ml of hot water, add NaOH solution (that is, dissolve 1.4 g of sodium hydroxide in 5 ml of H 2 O) to make it dissolve, then adjust the pH to 3 with 4 M hydrochloric acid solution, and precipitate occurs, filter, and the filter residue is a black substance, continue to adjust the pH of the f...
Embodiment 3
[0044] Synthesis of 5-chloro-7-methylindole-2,3-dione
[0045]
[0046] (1) Add 1.2 g (7.5 mmol) 7-methylindole-2,3-dione and 18 ml glacial acetic acid into a 50 ml three-neck flask, stir for 5 minutes, the solution turns wine red, but there are still some solids insoluble. Add 1.5 ml (18.5 mmol) of sulfuryl chloride dropwise to the reaction solution at room temperature, heat in an oil bath, and the solid dissolves at an internal temperature of 65 °C, continue heating to an internal temperature of 118 °C, reflux for 3 hours, stop the reaction, cool to room temperature and pour into crushed ice, extracted with dichloromethane, dried, and rotary evaporated to obtain 1.24 g of a brick-red solid, with a yield of 85.1%. 1 H NMR (400 MHz, DMSO-d 6 ) : δ = 2.17 (s, 3H, CH 3 ), 7.37 (s, 1H, Ar-H), 7.51 (s, 1H, Ar-H), 11.20 (s, 1H, NH).
[0047] (2) Add 1.2 g (7.5 mmol) 7-methylindole-2,3-dione and 18 ml glacial acetic acid into a 50 ml three-neck flask, stir for 5 minutes, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com