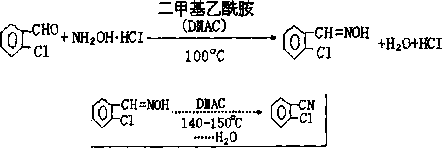
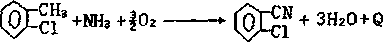
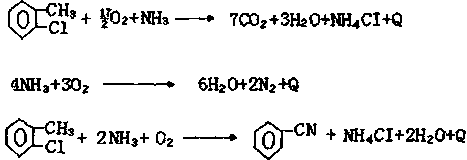
Synthetic method for 2-chlorobenzonitrile
A technology of o-chlorobenzonitrile and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, purification/separation of carboxylic acid nitrile, etc., can solve the problem of high price of o-chlorobenzaldoxime, influence on yield and product quality, The effect is not very ideal and other problems, to achieve the effect of easy environmental protection treatment, avoiding complicated equipment, and strong market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 200 g of o-chlorobenzoic acid with a content of 98.0% to a 500ml four-necked flask equipped with a thermometer, an ammonia pipe and a distillation column, turn on the power supply of the heating mantle to raise the temperature, and when the temperature rises to 145°C, it basically melts, and then turn on the liquid The ammonia gas valve and the pressure reducing valve of the ammonia steel cylinder pass ammonia, open and stir, and the early stage reaction is controlled at 140~180 ℃, and reacts for 1~2h, taking 2 h as an example, stop passing ammonia. The molar ratio of o-chlorobenzoic acid to ammonia is 1:1.5. The content of ammonium o-chlorobenzoate in the reactant was detected by high performance liquid chromatography to be 97.2%. During the reaction process, the unreacted and escaped ammonia gas is absorbed by water to generate ammonia water.
[0041] Slowly raise the temperature, and react at 230-250° C. for 1-5 hours. Taking the reaction for 1.5 hours as an exa...
Embodiment 2
[0045] Add 200 g of o-chlorobenzoic acid with a content of 98.0% to a 500ml four-necked flask equipped with a thermometer, an ammonia pipe and a distillation column, turn on the power supply of the heating mantle to raise the temperature, and when the temperature rises to 145°C, it basically melts, and then turn on the liquid The ammonia gas valve and the pressure reducing valve of the ammonia steel cylinder are connected to the ammonia, and the stirring is turned on. The initial reaction is controlled at 140-180° C., and the reaction is 1-2 hours. Taking the reaction for 2 hours as an example, the ammonia flow is stopped. The molar ratio of o-chlorobenzoic acid to ammonia is 1:1.5. The content of ammonium o-chlorobenzoate in the reactant was detected by high performance liquid chromatography to be 97.1%. During the reaction process, the unreacted and escaped ammonia gas is absorbed by water to generate ammonia water.
[0046] Slowly raise the temperature, and react at 230~25...
Embodiment 3
[0050] Add 3000g of o-chlorobenzoic acid with a content of 98.0% to a 10L ultrasonic constant temperature kettle equipped with a thermometer probe, an ultrasonic generator probe, an ammonia pipe and a distillation column, turn on the heating power supply, and basically melt when the temperature rises to 145°C , start stirring, then open the ammonia gas valve and the pressure reducing valve of the liquid ammonia steel cylinder to pass through the ammonia, the early stage reaction is controlled at 140~180°C, react for 1~2h, take the reaction 2 h as an example to stop passing through the ammonia. The molar ratio of o-chlorobenzoic acid to ammonia is 1:1.2. The content of ammonium o-chlorobenzoate in the reactant was detected by high performance liquid chromatography to be 97.3%. During the reaction process, the unreacted and escaped ammonia gas is absorbed by water to generate ammonia water.
[0051] Slowly raise the temperature, turn on the ultrasonic generator at the same time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com