Patents
Literature
223 results about "Chlorobenzoic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
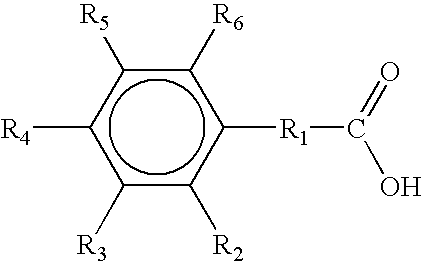
2-Chlorobenzoic acid is an organic compound with the formula ClC6H4CO2H. It is one of three isomeric chlorobenzoic acids, the one that is the strongest acid. This white solid is used as a precursor to a variety of drugs, food additives, and dyes.
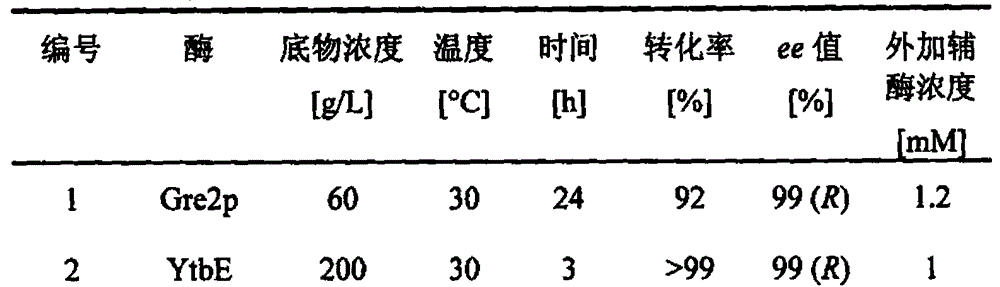
Carbonyl reductase mutant as well as gene and application thereof
InactiveCN104099305AImprove thermal stabilityIncreased reductase activityBacteriaOxidoreductasesMethyl o-chloromandelateMandelic acid
The invention relates to a carbonyl reductase CgKR1 mutant, a coding gene of the mutant, a recombinant expression vector containing the gene of the carbonyl reductase mutant, a recombinant expression transformant, a recombinase, a preparation method of the recombinase, and an application of the carbonyl reductase mutant to asymmetric reduction of ketonic ester for preparation of optically pure chiral hydroxyl ester, such as catalysis of o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare (R)-o-chloro mandelic acid methyl ester. Compared with wild enzymes, the carbonyl reductase mutant has the advantages that the thermal stability is substantially improved, and the catalytic activity of part of the mutant to the o-chlorobenzoic acid formyl methyl ester is also obviously improved. The multiple mutants can be applied to catalysis of the ketonic ester for asymmetric reduction to prepare the optical purely-chiral hydroxyl ester, such as catalysis of the o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare the optically pure (R)-o-chloro mandelic acid methyl ester. The carbonyl reductase mutants have the very good industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
5-bromine-2-chlorobenzaldehyde preparation method
InactiveCN104744227ABad for recyclingHigh selectivityOrganic compound preparationHydroxy compound preparationBenzoic acidChlorobenzene
The invention relates to a preparation method of 5-bromine-2-chlorobenzaldehyde, which takes 2-chlorobenzoic acid as a raw material, 2-chlorobenzoic acid is reacted to NBS / H2SO4 to generate 5-bromine-2-chlorobenzoic acid, and then 5-bromine-2-chlorobenzoic acid is reduced to 5-bromine-2-chlorobenzyl alcoholunder condition of NBS / H2SO4, and a target compound 5-bromine-2-chlorobenzaldehyde can be obtained by oxidation under condition of NaClO / TEMPO.
Owner:PORTON FINE CHEM
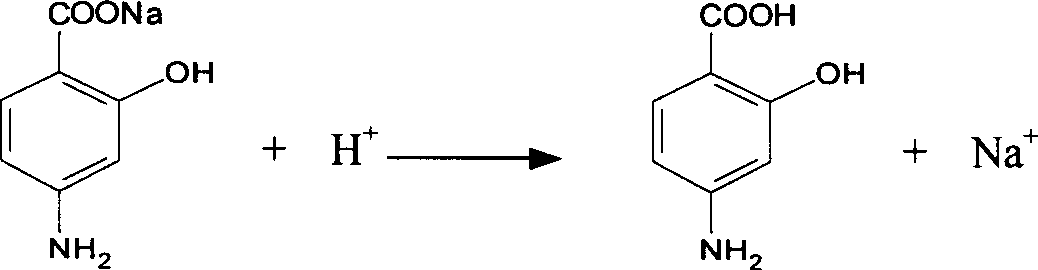
Process for reclaiming o-chlorobenzoic acid from mother liquor or waste water
InactiveCN101519348AReduce processing loadEasy to recycleCarboxylic compound separation/purificationTreatment burdenDesorption
The invention discloses a process for reclaiming o-chlorobenzoic acid from mother liquor or waste water, which comprises that: alkalescent ion exchange resin or macroporous absorbent resin is loaded into an adsorption column; after conventional pretreatment, mother liquor of o-chlorobenzoic acid or waste water containing o-chlorobenzoic acid to be treated flows through the adsorption column, the adsorption temperature is controlled to be between 5 and 40 DEG C and the flow rate is controlled to be between 2 and 16 BV / h, and o-chlorobenzoic acid is exchanged or adsorbed on the resin; and after the resin is adsorbed by the adsorption column to saturation, 2 to 4 percent NaOH solution or KOH solution or an organic solvent of methanol, ethanol, acetone or aether and the like is used for desorption to generate sodium o-chlorobenzoic acid water solution or methanol, ethanol, acetone or aether water solution of o-chlorobenzoic acid, the flow rate is controlled to be between 1 and 3 BV / h and the temperature is controlled to be between 35 and 90 DEG C, and the methanol, ethanol, acetone or aether water solution of o-chlorobenzoic acid is distilled and separated to obtain the o-chlorobenzoic acid and the organic solvent. The reclaiming method is simple, the recovery rate of the o-chlorobenzoic acid reaches 92 to 96 percent, and the method has good economic benefit, reclaims the resources and simultaneously lightens the treatment burden of the waste water.
Owner:NANJING UNIV
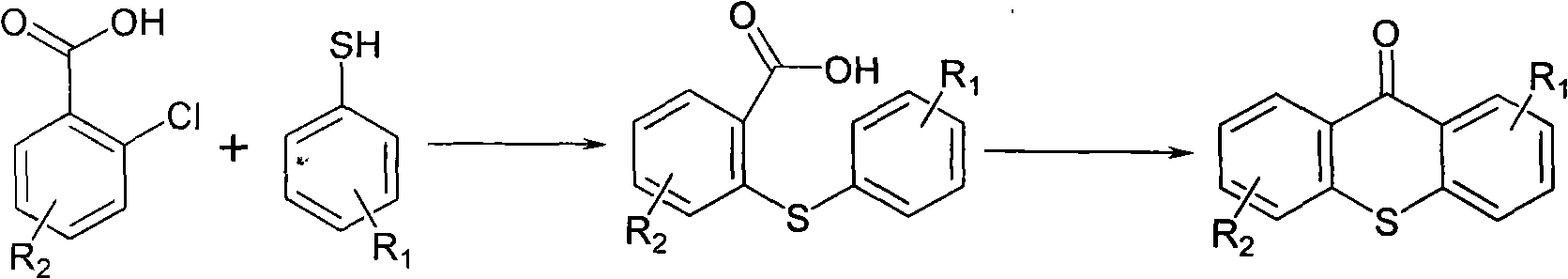
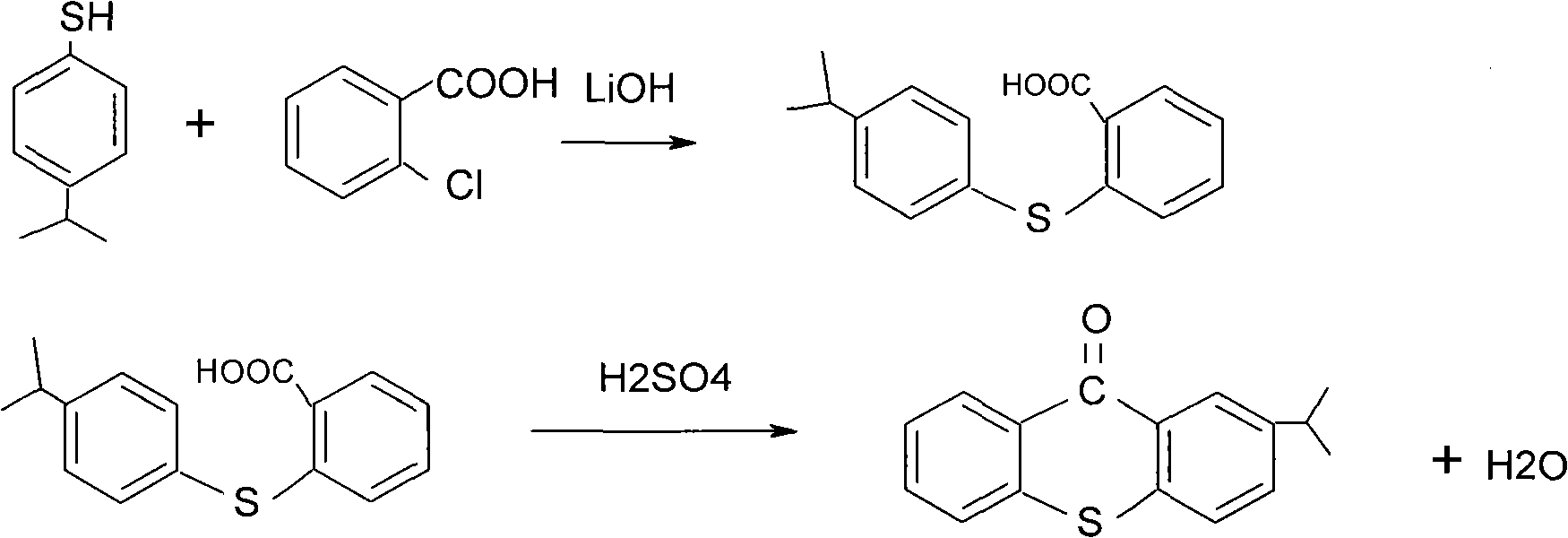
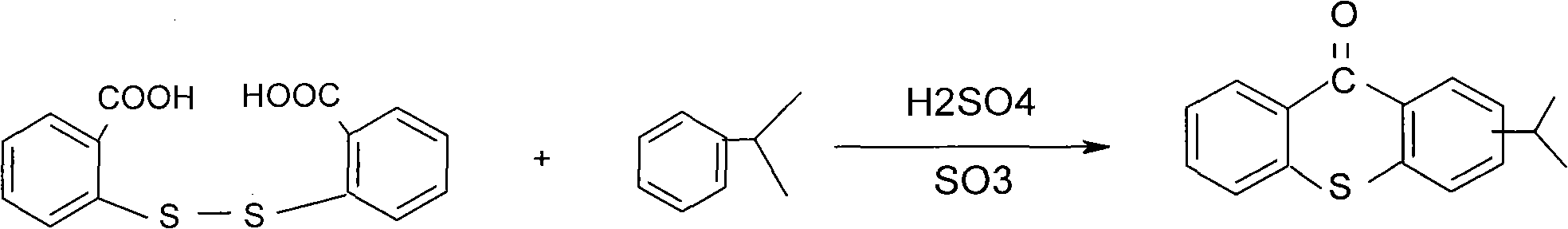
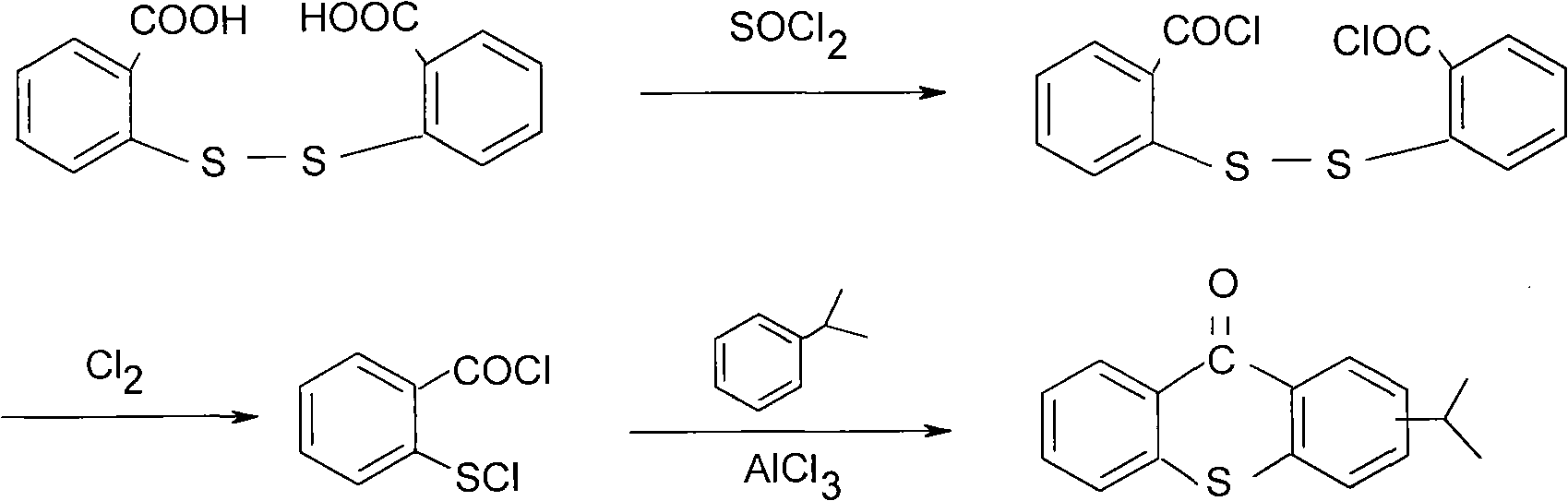
Preparation method of 2-isopropyl thioxanthone and derivatives thereof
The invention relates to a preparation method of 2-isopropyl thioxanthone and derivatives thereof, comprising the following steps of: carrying out condensation reaction on a derivative of o-chlorobenzoic acid (C7H5ClO2) and a derivative of 4-isopropyl thiophenol (C9H12S) in an organic solvent, and carrying out dehydration cyclization reaction on condensation products under the catalysis of concentrated sulfuric acid. The 2-isopropyl thioxanthone prepared by the invention can be used as a high-efficiency radical II-type photo initiator. The invention has easy operation, low cost, environmental protection and high purity; the purity of the product can reach more than 99 percent; the product can be commercially prepared and produced on a large scale to meet the current ever-increasing market demand.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
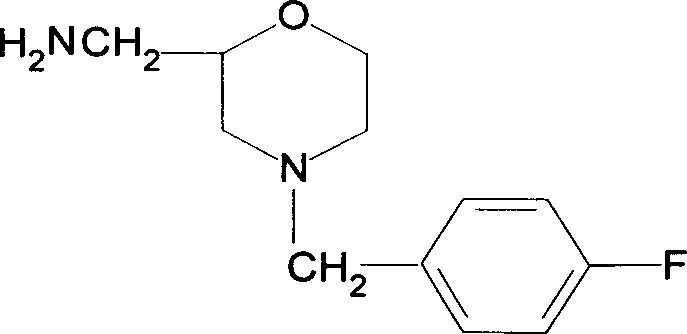
Synthesis of Important intermediate for mosapride citrate
InactiveCN1526700AWide variety of sourcesRealize localizationOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideMorpholine
The present invention relates to the preparation process of important intermediates for Mosapride citrate, 2-oxethyl-4-actamino-5-chlorobenzoic acid and 4-(4-fluorobenzyl-2- aminomethyl morpholine. The intermediate 2-oxethyl-4-actamino-5-chlorobenzoic acid is prepared with amino salicylic acid as initial material and through acidification with hydrochloric acid, esterification with methol, acetylation with acetic anhydride, ethylation, NCS chlorination and alkali hydrolysis. The intermediate 4-(4-fluorobenzyl-2- aminomethyl morpholine is prepared with p-fluorobenzaldehyde and phthalimide as initial material and through dropping at 70-90 deg.c, maintaining at 125-145 deg.c, and post-treatment in ice bath in 2-10 deg.c while adding acetic anhydride through stirring for 10-20hr. The present invention can raise the yield of Mosapride citrate and lower its production cost.
Owner:LUNAN BETTER PHARMA
Preparation process of 2-isopropylthioxanthone
InactiveCN101570534AReduce manufacturing costIncrease productivityOrganic chemistryCalcium hydroxideTetralin
The invention relates to a preparation process of 2-isopropylthioxanthone of light trigger, in particular to a process for preparing 2-isopropylthioxanthone by using 4-isopropylbenzenethiol and 2-chlorobenzoic acid as the main raw material through the reaction steps of condensation, exsolution, neutralization, cyclization, hydrolyzation, recrystallization, and the like. The preparation process is characterized in that the 4-isopropylbenzenethiol and the 2-chlorobenzoic acid are used as the raw materials and are condensed by using tetraline as solvent under the alkaline (lithium hydroxide) condition, the mol ratio of the 4-isopropylbenzenethiol, the 2-chlorobenzoic acid and the lithium hydroxide is 1:1-1.5:2-2.5, and preference for 1:1.05:2.1; the condensation temperature is 180-190 DEG C, the product is prepared by the reaction steps of exsolution, neutralization, cyclization, hydrolyzation, recrystallization, and the like after the reaction is performed for 6h. The invention has easily obtained raw materials, can improve the performability of the process, can greatly lower the production cost because the produced lithium hydroxide of the side product can be sold through the treatment and has high purity of produced products without isomer.
Owner:江苏省中兴化工有限公司
Method for preparing 9-oxo-10(9H)-acridineacetic acid
ActiveCN103396362ASmooth responseResponse is smooth and easy to controlOrganic chemistryBenzoic acidPtru catalyst
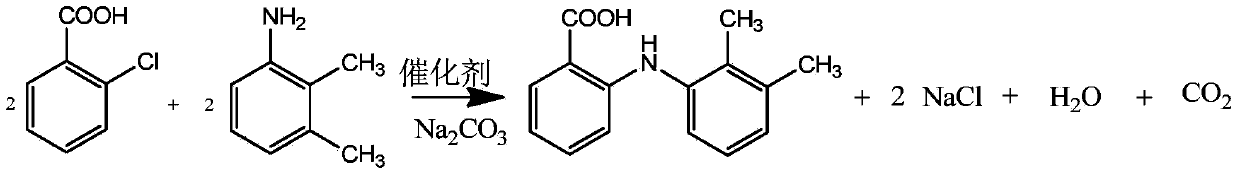
The invention discloses a method for preparing 9-oxo-10(9H)-acridineacetic acid, and belongs to the field of organic synthesis. The method comprises the following steps: (1) under the effects of a metal carbonate, a catalyst and a solvent, performing a condensation reaction on o-chlorobenzoic acid (a) and aniline (b) to prepare an intermediate c; (2) under the effects of toluene and p-toluenesulfonic acid, performing a cyclization reaction on the intermediate c by refluxing and dehydrating to prepare an intermediate d; (3) dissolving the intermediate d in dimethyl formamide , successively adding 60% NaH, KI and ethyl chloroacetate, reacting at room temperature to obtain a wet intermediate e; and (4) hydrolyzing the wet intermediate d with NaOH to obtain a crude product f, and refining the crude product f to obtain the 9-oxo-10(9H)-acridineacetic acid product. The method has the advantages of low cost consumption, small pollution during reaction, smooth and easy-controlling reaction and high product yield, and has better industrial application value.
Owner:ZHENGZHOU SIGMA CHEM
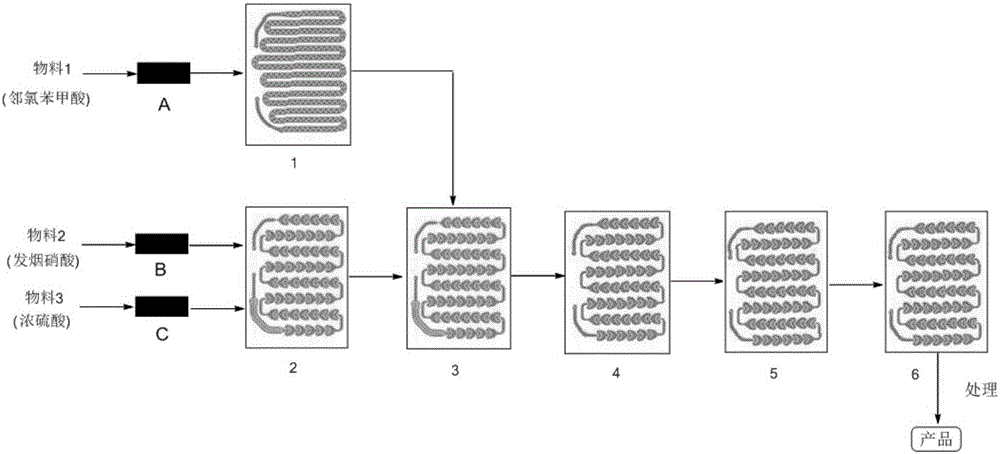
Method for synthesizing 2-chloro-5-nitrobenzoic acid through microchannel reactor
InactiveCN106674016AAvoid infringementAvoid corrosionNitro compound preparationIntrinsic safetyReaction temperature
The invention provides a method for synthesizing 2-chloro-5-nitrobenzoic acid through a microchannel reactor. The method comprises the steps of dissolving a raw material o-chlorobenzoic acid into concentrated sulfuric acid to obtain a material 1, and entering a preheating module; adopting fuming nitric acid and the concentrated sulfuric acid as a material 2 and a material 3, and entering another preheating module; preheating the material 1, the material 2 and the material 3, entering a reaction module group for reacting, collecting effluent reaction liquid, processing to obtain a crude product, and refining to obtain the product 2-chloro-5-nitrobenzoic acid. According to the method provided by the invention, the reaction time is shortened to a few minutes to a few seconds, the production energy consumption is reduced, and the reaction efficiency is remarkably improved. High-efficient mass and heat transfer efficiency ensures the reaction temperature to maintain within a setting range, the possibilities of temperature out of control, temperature runaway and even sharp reaction overflow or explosion caused by over-high local concentration do not exist, the intrinsic safety problem of the nitration reaction is solved fundamentally, and the reaction yield and the product purity are remarkably improved.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
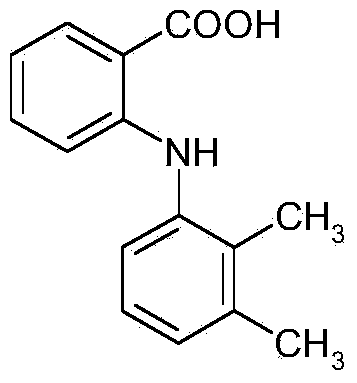
Process for preparing mefenamic acid
ActiveCN101475505AImprove productivityLow yieldOrganic chemistryOrganic compound preparationDimethylaniline N-oxideAnti-inflammatory analgesics
The invention belongs to the technical field of anti-inflammatory drug production, and relates to a method for preparing mefenamic acid. The method comprises: adding o-chlorobenzoic acid and 2,3-dimethylaniline into a system which is formed by a non-protonic polar solvent and a dehydrant, performing condensation reaction in the presence of an acid-binding agent, a catalyst and a phase-transfer catalyst, and obtaining mefenamic sodium; acidifying the mefenamic sodium and obtaining coarse mefenamic acid products; and refining the coarse mefenamic acid products in an organic solvent and water and obtaining finished mefenamic acid products. The method improves the production efficiency of the mefenamic acid and reduces the production cost of the mefenamic acid.
Owner:BAOJI TIANXIN PHARM CO LTD
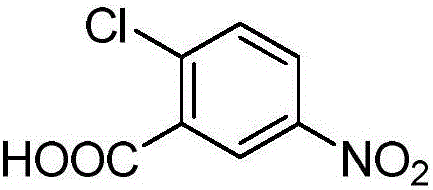
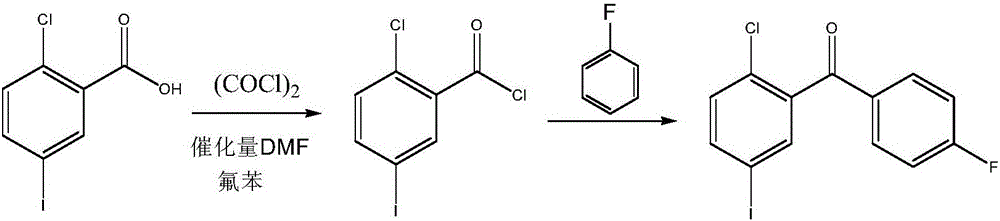
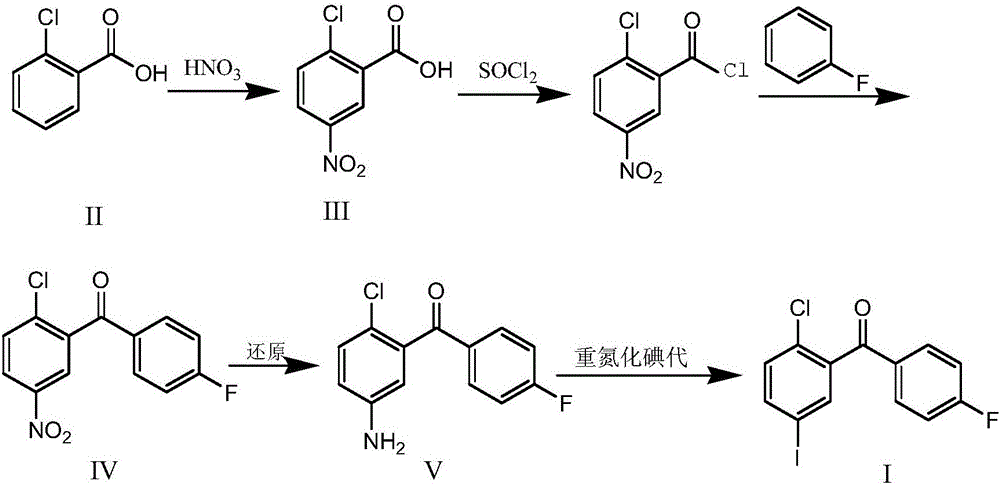
Synthesis method for (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone
ActiveCN106699570AEasy to getIncrease profitOrganic compound preparationCarbonyl compound preparationSandmeyer reactionNitration
The invention discloses a synthesis method for (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone. According to the method, with cheap o-chlorobenzoic acid as a starting material, (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone is obtained by nitration, Friedel-Crafts acylation and reduction and finally by Sandmeyer reaction for iodination. The materials used by the method are cheap and easy to obtain, and the method adopting Sandmeyer reaction for iodination can increase the iodine utilization rate, and has the characteristics of simplicity in operation, high yield, high purity and suitability for industrialized production. (The chemical formula is shown in the specification).
Owner:SHANDONG BOYUAN PHARM CO LTD
Production method of mefenamic acid
ActiveCN102344384AIncrease profitAvoid pollutionOrganic chemistryOrganic compound preparationSodium bicarbonateDimethylaniline N-oxide
The invention discloses a production method of mefenamic acid, which comprises the following steps of: (1) adding water and o-chlorobenzoic acid into a reaction kettle, evenly mixing, and dropwise adding a sodium hydroxide solution while stirring, thereby obtaining a mixed solution; (2) adding solid sodium bicarbonate into the mixed solution in the step (1), adding chalcanthite, 2,3-dimethylaniline and cetyl trimethyl ammonium chloride, and carrying out reflux reaction; (3) after the reflux reaction in the step (2) is finished, dropwise adding hydrochloric acid, and filtering to obtain the crude product; (4) washing the crude product in the step (3) with water, filtering, and drying under reduced pressure to obtain the crude product; and (5) adding dimethyl formamide and water to dissolvethe crude product in the step (4), adding activated carbon to carry out reflux decolorization, filtering, cooling to room temperature to crystallize, filtering, recrystallizing, washing with water after the filtration for the last recrystallization, and drying under reduced pressure to obtain the fine mefenamic acid product. The invention can lower the production cost, easily implement industrialproduction, and overcome the defects in the prior art.
Owner:DEZHOU BOCHENG PHARMA
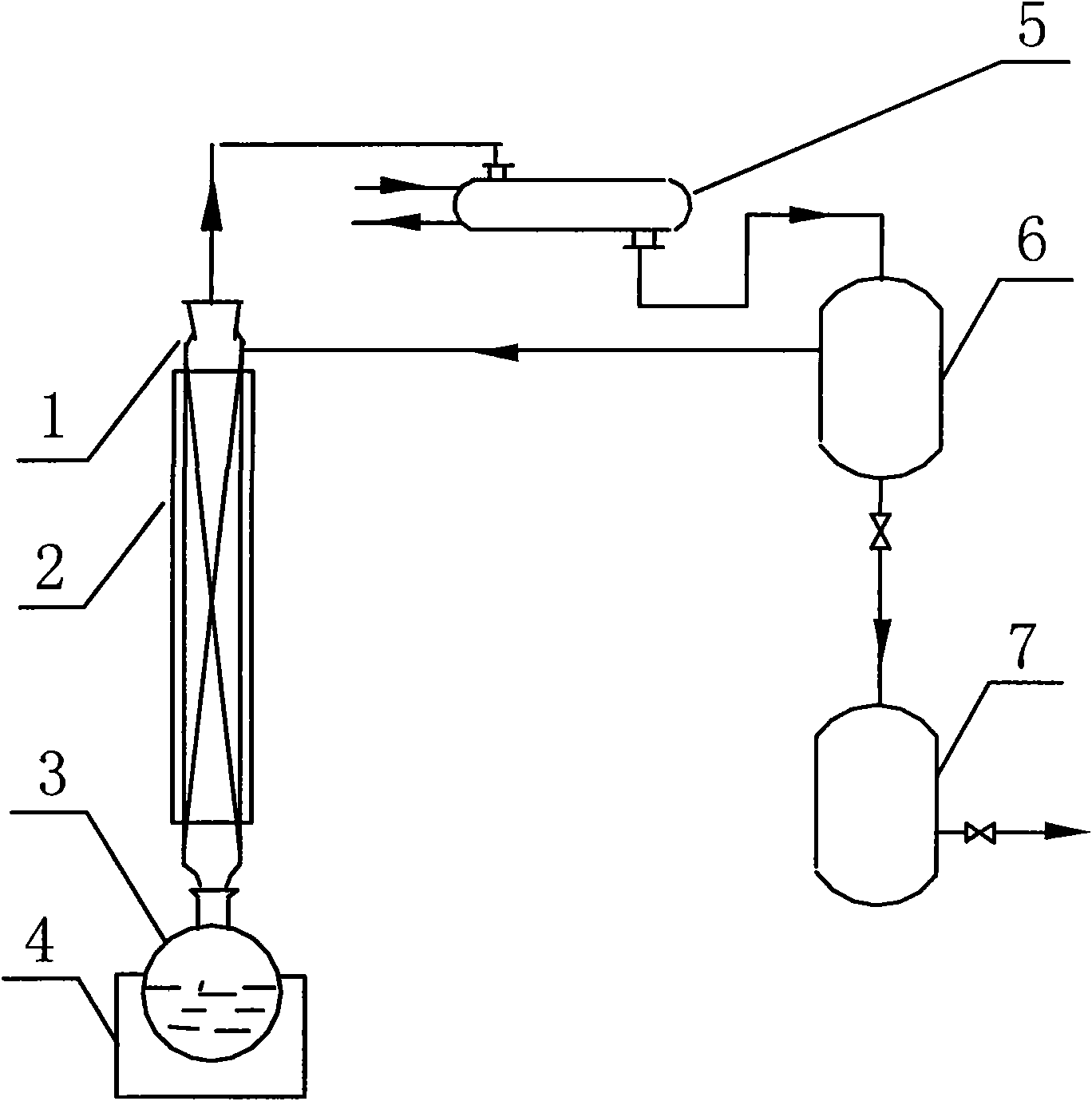
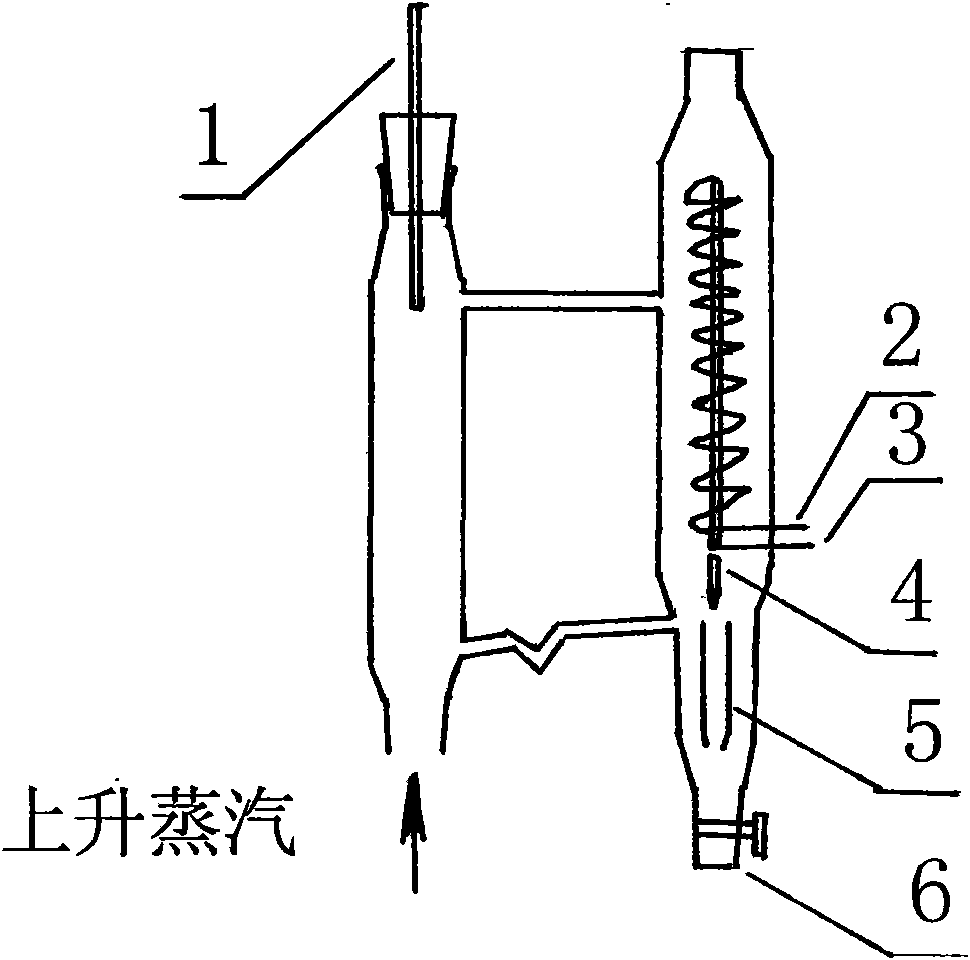
Device for purifying formic acid solution with interval azeotropic distillation and method thereof
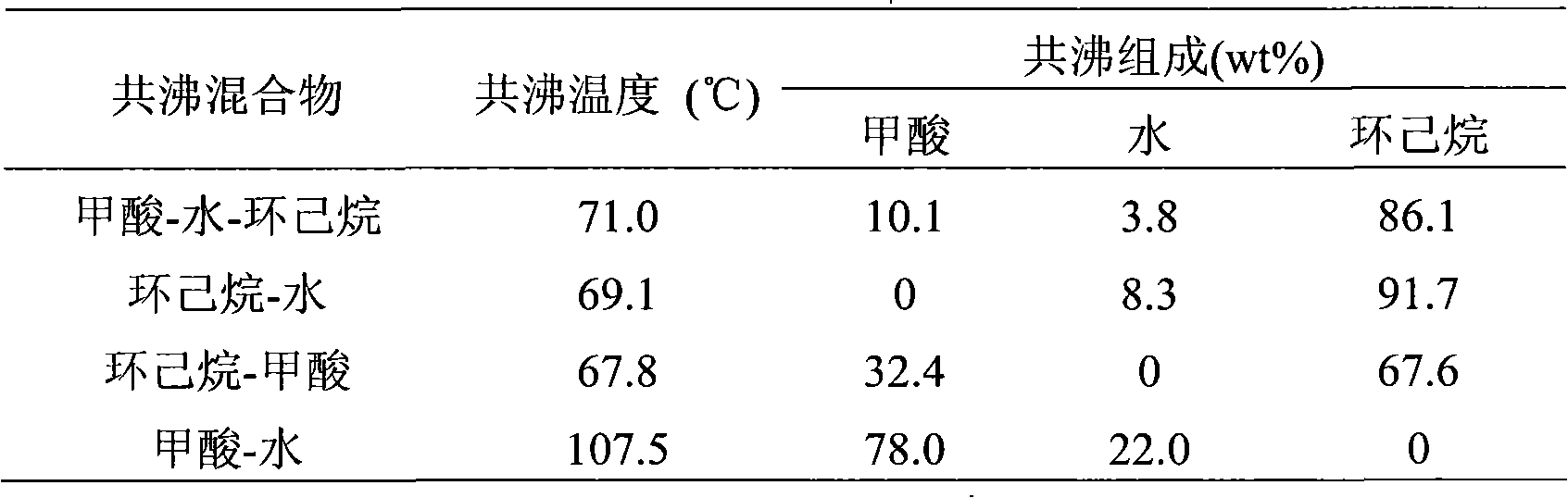
InactiveCN101596371ALow priceAbundant resourcesCarboxylic compound separation/purificationFractional distillationHigh concentrationChlorobenzene
The invention relates to a device for purifying formic acid solution with interval azeotropic distillation and a method thereof. The device comprises a rectification column body, an insulating layer, a tower pot, a heating jacket, a condenser, a phase separator and a product storage tank, wherein, the insulating layer is arranged outside the column body, the lower end of the column body is connected with the tower pot, the upper end is respectively connected with the condenser and the phase separator, and the phase separator is respectively connected with the condenser and the product storage tank. The method comprises the following steps: firstly, cyclohexane is used as entrainer, and dilute formic acid water solution with mass concentration being 10% is initially concentrated to 30%; then, chlorobenzene is used as entrainer, the top of the tower continuously produces azeotrope of chlorobenzene and water; when the top of the tower reaches 95 DEG C-102 DEG C, the mixture of chlorobenzene, formic acid and water is produced, and the mass fraction of the formic acid is 60-70% after phase separating; at last, pressurization rectification is used, the pressure is controlled at 4atm, and the tower pot can obtain 80-85% high-concentration formic acid solution. The invention has the characteristics of reasonable process flow, simple device structure, high efficiency and low cost and is suitable for industrial continuous operation.
Owner:TIANJIN UNIV
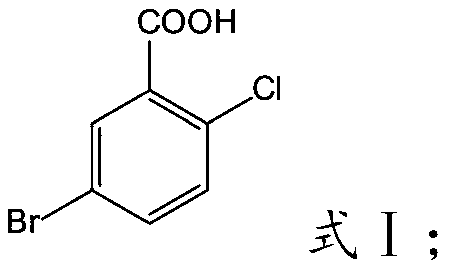
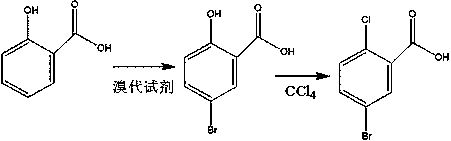
Preparation method of 5-bromo-2-chlorobenzoic acid
InactiveCN110590541AFast preparation methodMild responseOrganic compound preparationCarboxylic compound preparationOrganic solventBromine
The invention relates to the technical field of preparation of 5-bromo-2-chlorobenzoic acid, and in particular relates to a preparation method of the 5-bromo-2-chlorobenzoic acid. The preparation method of the 5-bromo-2-chlorobenzoic acid provided by the invention comprises the following steps: mixing 2-chlorobenzoic acid, bromine, a catalyst and an organic solvent, and performing a bromination reaction to obtain the 5-bromo-2-chlorobenzoic acid. The preparation method provided by the invention has short steps, mild reaction conditions, low costs, a high yield, and high purity of the product;and at the same time, according to the description of embodiments, the purity by HPLC of the 5-bromo-2-chlorobenzoic acid prepared by using the preparation method is >=99.5%, and the molar yield is >=80.9%.
Owner:吕东
New post-treatment technology of 5-bromo-2-chlorobenzoic acid
InactiveCN107162894AOrganic compound preparationCarboxylic compound preparationHigh concentration2-Chlorobenzoic acid
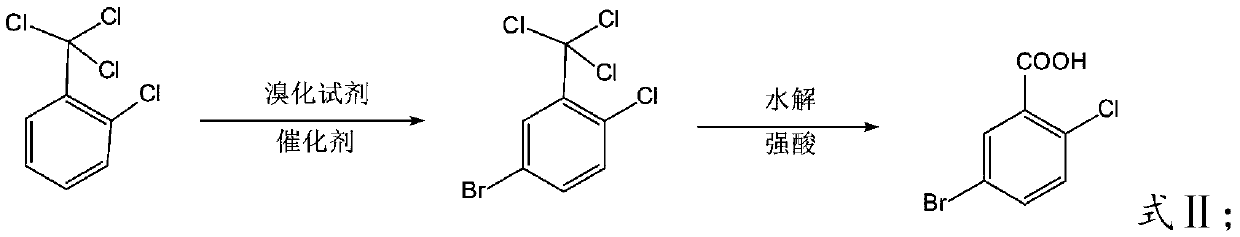
The invention relates to a new post-treatment technology of 5-bromo-2-chlorobenzoic acid. The technology comprises the following steps: 1, reacting 2-chlorobenzotrichloride with bromine under the action of a catalyst to prepare 2-chloro-5-bromobenzotrichloride, adding an organic acid, carrying out a heating reaction, and concentrating the obtained reaction product after the reaction ends to recover the organic acid; and 2, adding water to a concentrate obtained in step 1 to precipitate crystals, and filtering the obtained solution to obtain crude 5-bromo-2-chlorobenzoic acid. The technology solves the problem of the wastewater problem of large amounts of H2SO4, HCl, ferric chloride or ferric bromide, other high-concentration acids and salts generated in the reaction and the hidden safety trouble problem in the prior art, is safe and environmentally-friendly, and is economical and practical due to the concentrated and recovered organic acid which can be directly repeatedly used.
Owner:NANTONG NABAIYUAN CHEM +1
Synthetic method of 5-bromo-2-chlorobenzoic acid
ActiveCN108250060AReduce pollutionReduce manufacturing costOrganic compound preparationCarboxylic compound preparationBenzoic acid2-Chlorobenzoic acid
The invention discloses a synthetic method of 5-bromo-2-chlorobenzoic acid. The method comprises the following steps of by taking salicylic acid as a starting material, performing bromination reactionto obtain 2-hydroxyl-5-bromo-benzoic acid, and then performing chlorination reaction to obtain the 5-bromo-2-chlorobenzoic acid, wherein a bromination system adopted by the bromination reaction is tetrabutyl ammonium bromide / oxygen / sodium metavanadate, a catalyst is aluminium tribromide, and a solvent is 1,4-dioxane; and a chlorinating agent is carbon tetrachloride, and a catalyst is molybdenum hexacarbonyl. The method provided by the invention adopts the cheap salicylic acid which is wide in source as the starting material and prepares the 5-bromo-2-chlorobenzoic acid through two-step reaction of bromination and chlorination, is novel in synthetic route, relatively low in production cost, relatively small in environmental pollution and very suitable for industrial mass production. According to the method, through the bromination reaction and the chlorination reaction, by selecting proper reagents, relatively high reaction yield and product purity can be obtained.
Owner:江苏尚莱特医药化工材料有限公司
Stabilized subtilisin composition
InactiveUS8071345B2Composition is stableOrganic detergent compounding agentsHydrolasesSubtilisinAmylase
The present invention relates to a liquid detergent composition comprising a surfactant, a subtilisin and a protease stabilizer that is 3-chlorobenzoic acid, 4-chlorobenzoic acid, 3-chlorophenylacetic acid, 3,5-dichlorobenzoic acid, 3-(3-chlorophenyl)propionic acid or their corresponding salts. The composition can further comprise a another enzyme that is a lipase, an amylase, a cellulase or mixtures thereof. The stabilizer can be present at a concentration of 0.001 to 20% w / w. The concentration of the subtilisin can be at least 1.5 g / L.
Owner:NOVOZYMES AS
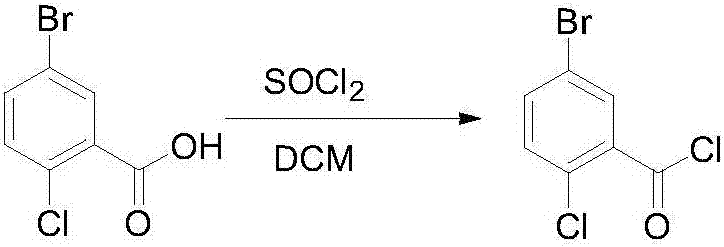
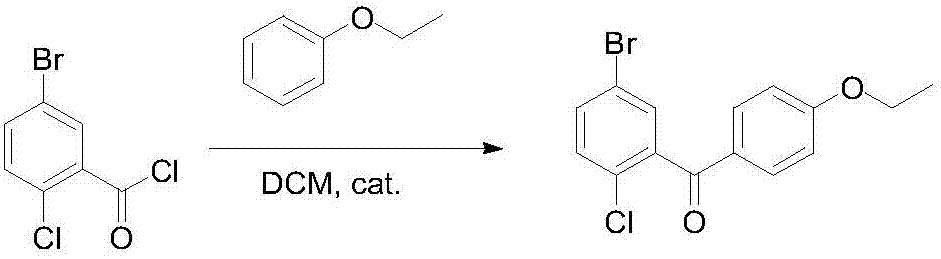
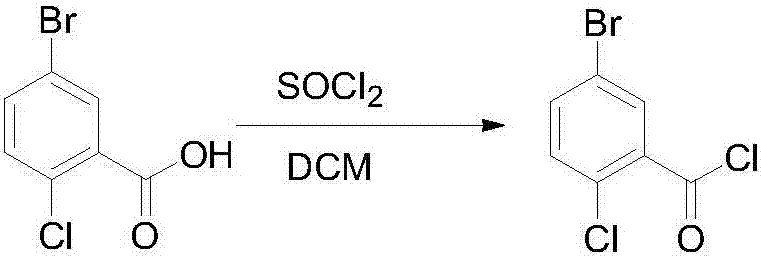
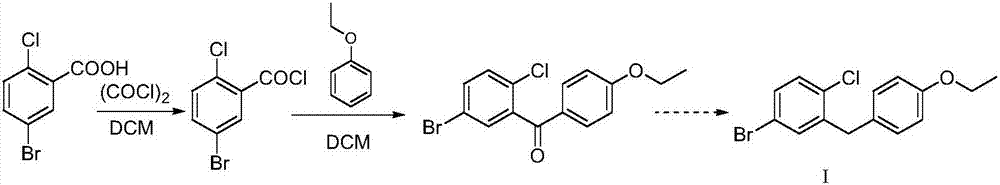
Novel method for synthesizing dapagliflozin intermediate compound
InactiveCN107417515AEasy to operateReact cleanOrganic compound preparationCarbonyl compound preparation by condensation2-Chlorobenzoic acidSolid acid
The invention discloses a novel method for synthesizing a dapagliflozin intermediate compound. The method comprises the following steps: (1) by taking dichloromethane as a solvent and pyridine as a catalyst, reacting 5-bromo-2-chlorobenzoic acid and thionyl chloride to obtain 5-bromo-2-chlorobenzoyl chloride; (2) by taking dichloromethane as a solvent and solid acid as a catalyst, reacting phenetole and the 5-bromo-2-chlorobenzoyl chloride to obtain 5-bromo-2-chloro-4-ethoxydiphenylketone; and (3) by taking THF as a solvent, adding the 5-bromo-2-chloro-4-ethoxydiphenylketone concentrated solution obtained in the step (2); by taking acetic acid and aluminum trichloride as catalysts, adding sodium borohydride, and performing a reduction reaction; and after the reaction is completed, adding a saturated saline solution, and performing quenching at 25 DEG C or below to obtain 5-bromo-2-chloro-4-ethoxydiphenylmethane. The novel method disclosed by the invention has the advantages of cheap and available raw materials, simple and easy operation, no discharge of three wastes, high reaction yield and the like.
Owner:上海常丰生物医药科技有限公司
Synthesis method of UV absorbent ethylhexyl triazone
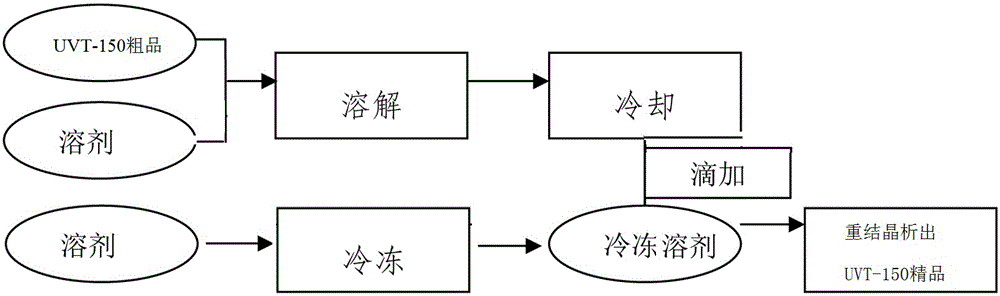
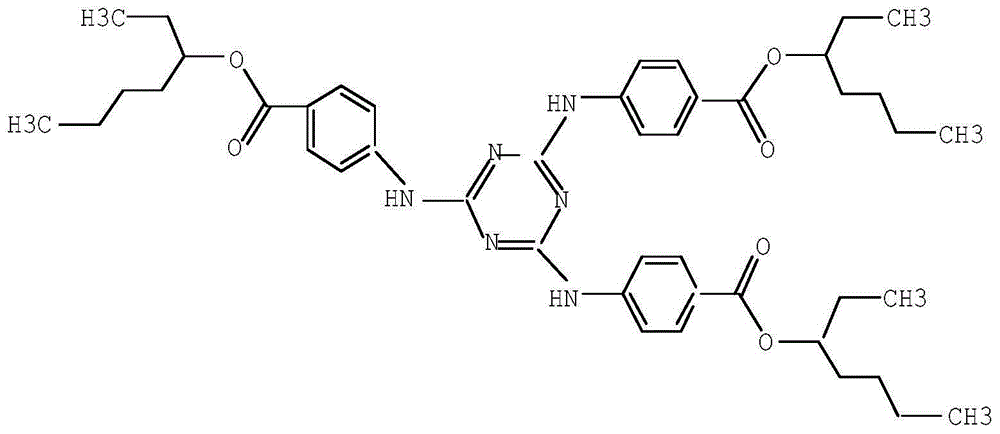
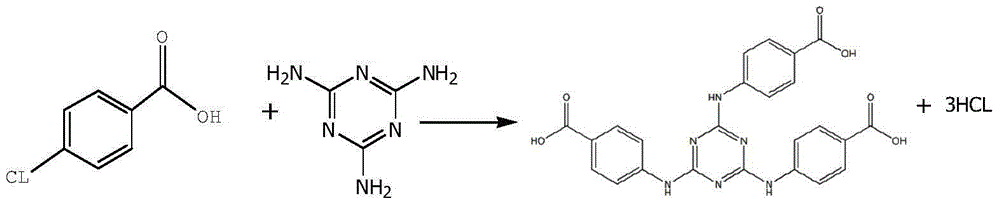
ActiveCN105061345ASimple process routeRaw materials are easy to getOrganic chemistrySynthesis methodsDistillation
The invention discloses a synthesis method of a UV absorbent ethylhexyl triazone. The synthesis method comprises that melamine and 4-chlorobenzoic acid as initial raw materials undergo a trisubstitution reaction to produce 2,4,6-tris[(p-carboxyphenyl)amino]-1,3,5-triazine (H3TATAB), 2,4,6-tris[(p-carboxyphenyl)amino]-1,3,5-triazine (H3TATAB) and isooctanol undergo an esterification reaction to produce an ethylhexyl triazone crude product, the ethylhexyl triazone crude product is subjected to distillation desolvation and crystallization and the crystals are dried to form an ethylhexyl triazone finished product. The synthesis method has the characteristics of simple production route, easily available raw materials, mild reaction conditions, unique crystallization method, less three wastes and high product purity and is suitable for industrial production.
Owner:宜都市华阳化工有限责任公司
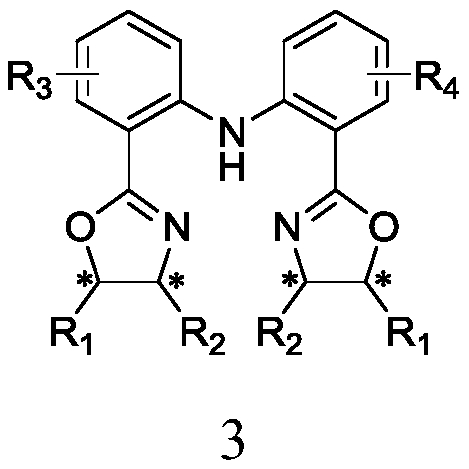
C2-symmetry-removing diphenylamine-type chiral bisoxazoline ligands as well as synthetic method and application thereof
ActiveCN110229114AThe synthesis method is simpleMild conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNickel saltAlcohol
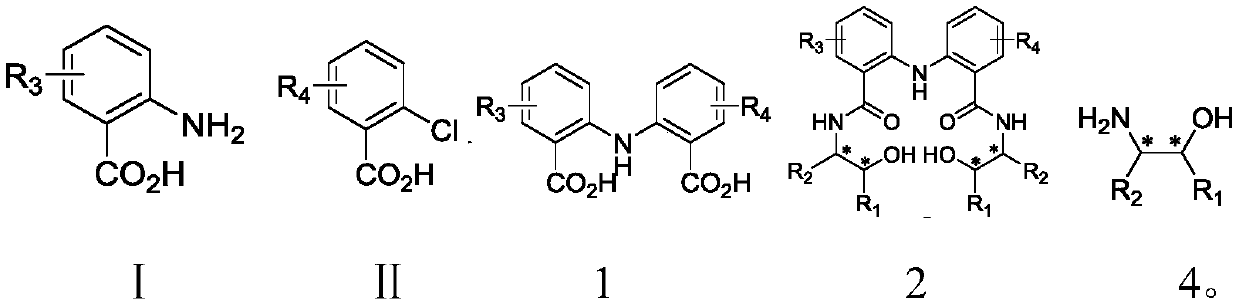
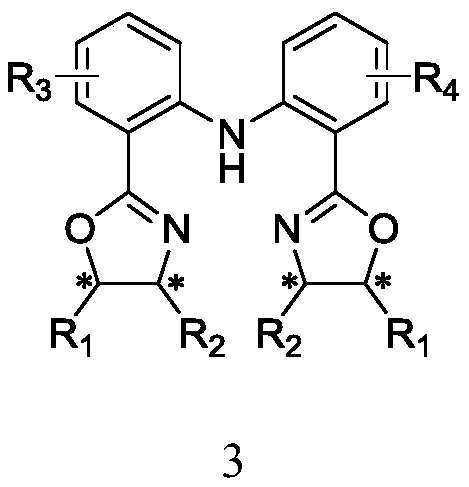
The invention discloses C2-symmetry-removing diphenylamine-type chiral bisoxazoline ligands represented by a formula 3 shown in the description, a synthetic method of the ligands and an application ofthe ligands in an asymmetric catalytic reaction. According to the ligands provided by the invention, different groups are introduced to a diphenylamine skeleton to remove C2-symmetry to realize precise regulation and control of an ''electron effect'' of the ligand skeleton; and the ligands are prepared by the steps of preparing a compound represented by a formula 1 shown in the description by using an anthranilic acid derivative and an o-chlorobenzoic acid derivative as starting materials, performing a reaction on the compound represented by the formula 1 and a chiral amino alcohol compound represented by a formula 4 shown in the description to obtain beta-bishydroxyamide represented by a formula 2 shown in the description, and performing condensation on the beta-bishydroxyamide to obtainthe C2-symmetry-removing diphenylamine-type chiral bisoxazoline ligands represented by the formula 3. The invention also provides the application of a catalyst formed by performing coordination on the C2-symmetry-removing diphenylamine-type chiral bisoxazoline ligands, a copper salt, a zinc salt, a nickel salt, an iron salt or a rhodium salt in the asymmetric catalytic reaction.
Owner:ZHEJIANG UNIV OF TECH
Technique for reclaiming byproduct o-chlorobenzoic acid of o-chlorobenzaldehyde production
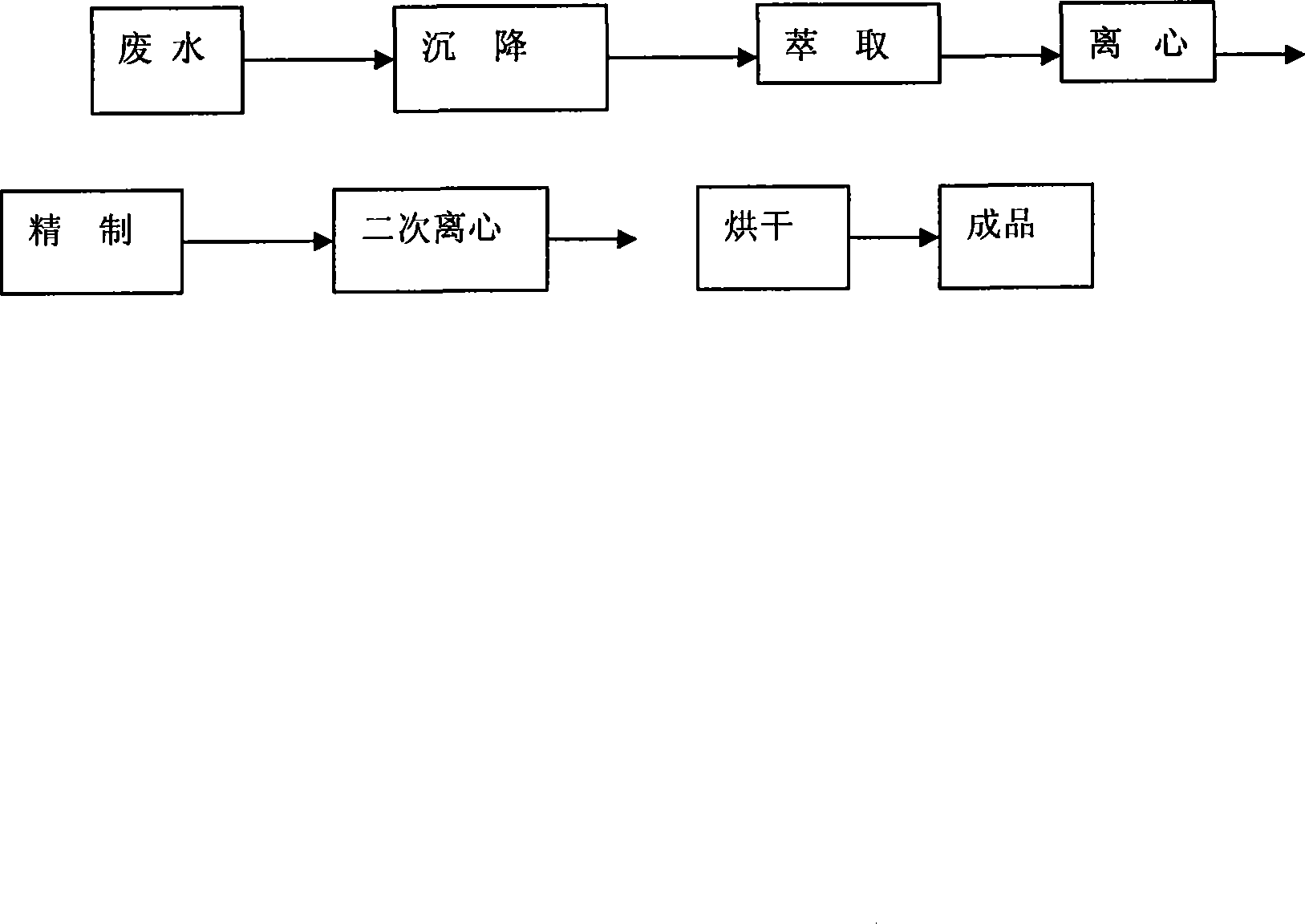
ActiveCN101215232AReduce recycling costsNo polluting emissionsCarboxylic compound separation/purificationWastewaterChlorobenzoic Acids
Disclosed is a technique for recycling byproduct of o-chlorobenzoic acid in the production of o-chlorobenzaldehyde, comprising procedures of extracting, centrifugal filtration, refining, twice centrifugal filtration and drying. Employing the invention, purity of products is not less than 99.5%, content of heavy metal is not more than 5PPM, and drying shrinkage is not more than 0.5%, thereby the recycling cost of the o-chlorobenzoic acid is decreased largely. Further, the invention has no pollution because waste water is reused.
Owner:江苏中超新材料科技有限公司
Mefenamic acid short-process synthesis preparation and refining method
InactiveCN103420863AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationDimethylaniline N-oxideOrganic solvent
A mefenamic acid synthesis preparation and refining method comprises the following steps: 1, adding o-chlorobenzoic acid and acid-binding agent at appropriate ratio in DMF for stirring; 2, adding a certain amount of 2,3-dimethyl aniline, and performing condensation reaction with a catalyst of suitable ratio at a certain temperature to obtain a mefenamic acid crude product; 3-6, allowing the mefenamic acid crude product to be subjected to refining including bleaching and crystallization in an organic solvent to obtain the mefenamic acid finished product, wherein the acid-binding agent adopts o-chlorobenzoic acid and sodium carbonate, the molar ratio of the o-chlorobenzoic acid to 2,3-dimethyl aniline to sodium carbonate to DMF to catalyst is 1:(2-3):(0.5-1):(5-6):0.05:0.15, and the temperature is 120-130 DEG C. Compared with the traditional process, the method has the advantages of high target product productivity, simple process procedures, mild reaction conditions, simple purifying process, high product purity and suitability for industrialized production; and the primary solvent N-dimethyl formamide can be recycled, so that the production cost is further reduced.
Owner:JIANGSU BEIHEDE CHEM
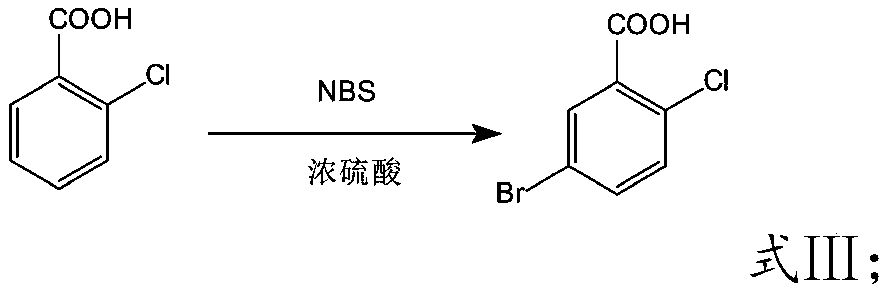
Preparation method of high-selectivity 5-bromo-2-chlorobenzoic acid
ActiveCN110002989ASimple processLow costOrganic compound preparationCarboxylic compound preparationBromineOrganic synthesis
The invention discloses a preparation method of 5-bromo-2-chlorobenzoic acid and belongs to the technical field of organic synthesis. According to the preparation method, 2-chlorobenzoic acid is takenas a raw material and is subjected to a mono-bromination reaction in an NBS / sulfuric acid system, 5-bromo-2-chlorobenzoic acid is prepared, and a catalyst inhibiting production of 4-bromo-2-chlorobenzoic acid is added in the reaction process. The process is simple, raw materials are cheap and easy to obtain, the cost is low, the yield is high, and the high-purity 5-bromo-2-chlorobenzoic acid product is obtained through one-time refining after the reaction; the catalyst can effectively inhibit production of impurities such as 4-bromo-2-chlorobenzoic acid and the like, and the impurity contentof the obtained product is low.
Owner:河北合佳医药科技集团股份有限公司
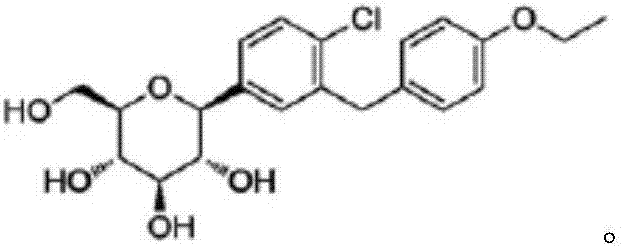
Preparation method of Dapagliflozin intermediate used for treating II-type diabetes
InactiveCN107200683AHigh yieldGood reserve supportOrganic compound preparationCarbonyl compound preparation by condensationTert-butyldimethylsilyl chloride2-Chlorobenzoic acid
The invention discloses a preparation method of a Dapagliflozin intermediate used for treating II-type diabetes. The preparation method comprises the following steps: 1) performing a reaction on 5-bromine-2-chlorobenzoic acid and oxalyl chloride in anhydrous dichloromethane under the catalysis of DMF (dimethyl formamide), so as to obtain 5-bromine-2-chloro-benzoyl chloride; 2) under the condition that tert-Butyldimethylsilyl chloride exists, performing a reaction on 5-bromine-2-chloro-benzoyl chloride obtained in step 1) and phenetole under the catalysis of ferric trichloride, so as to obtain 5-bromine-2-chloro-4'-ethyoxyl benzophenone. According to the preparation method provided by the invention, no ortho-by-product is generated, and the yield of a target product is high, so that the good support of storage of raw materials is provided for Dapagliflozin. Additionally, the preparation method is mild in conditions and short in reaction time, therefore, the preparation method is suitable for industrial production and promotion.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
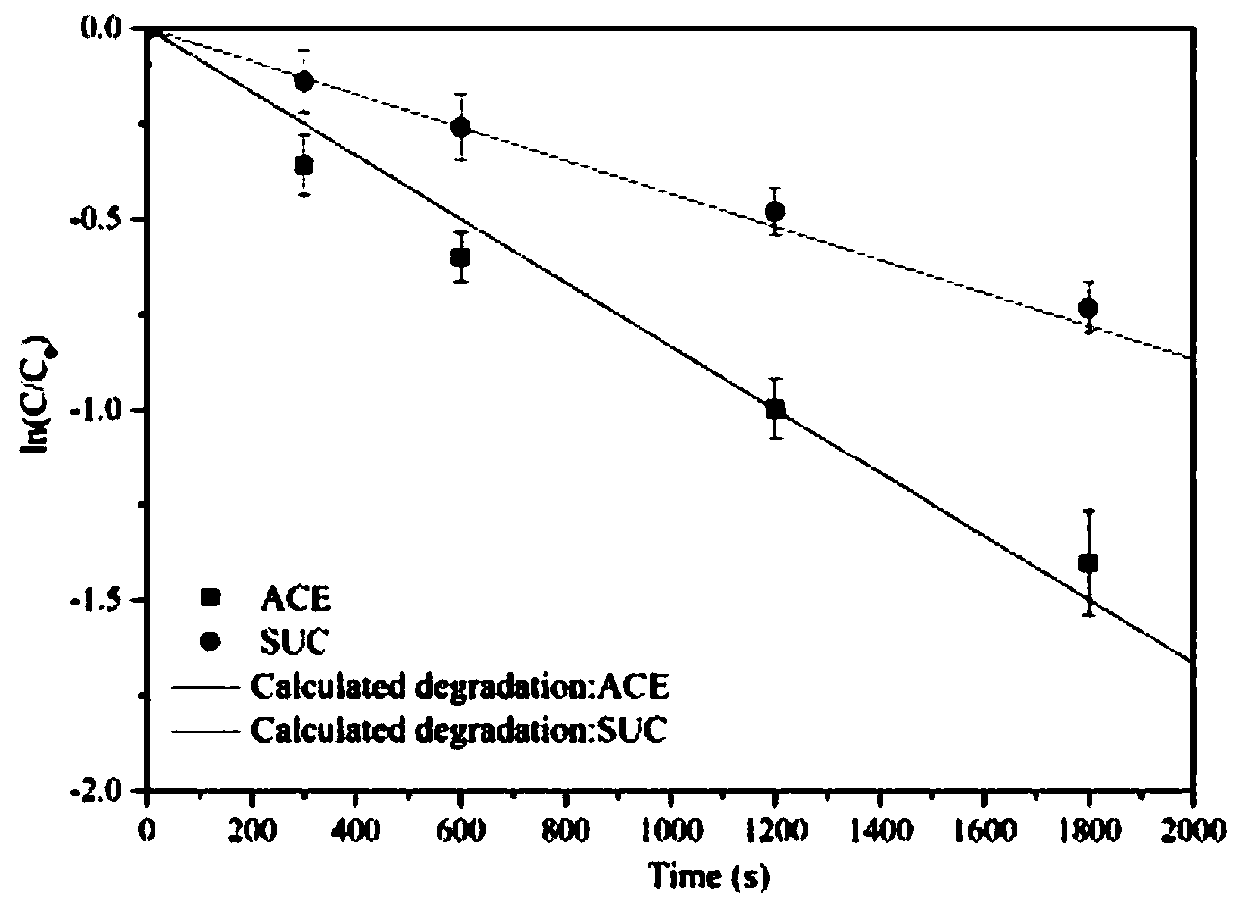
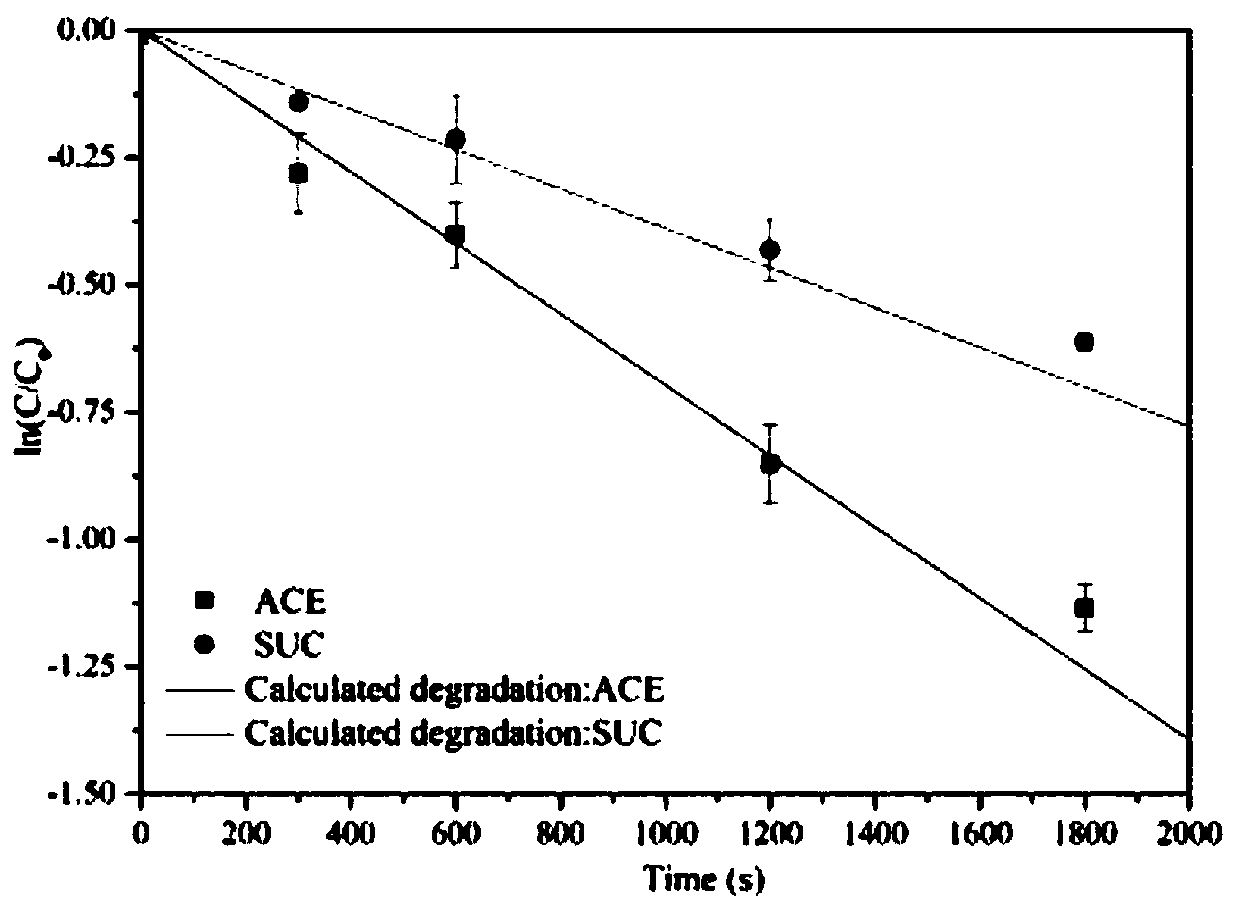
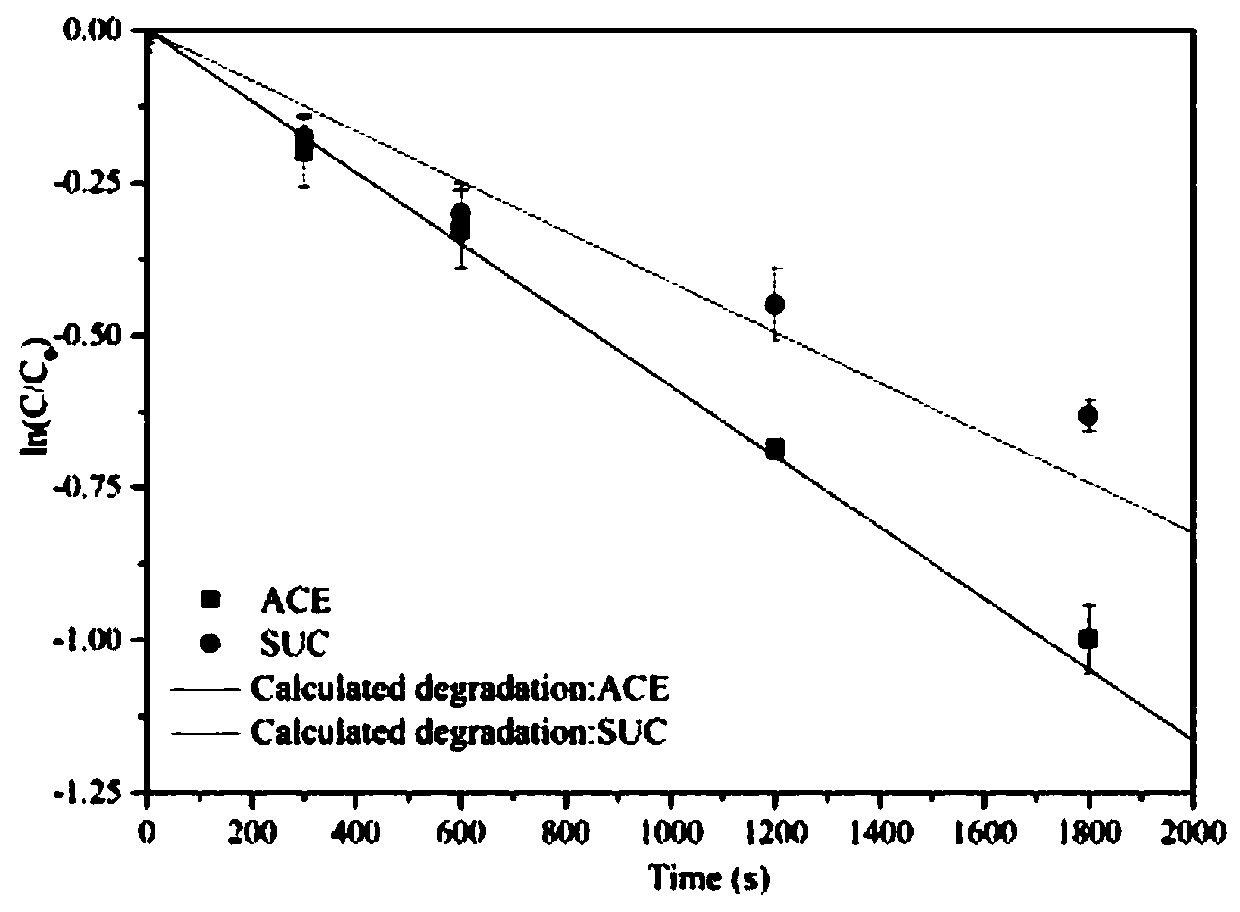
Degradation prediction method for removing micro-pollutants in secondary effluent by ultraviolet/sodium persulfate
ActiveCN109650487AAchieve the correct fitAchieve forecastWater/sewage treatment by irradiationWater contaminantsUltravioletChlorobenzoic Acids
The invention discloses a degradation prediction method for removing micro-pollutants in secondary effluent by ultraviolet / sodium persulfate. The method includes the following steps: S1, selecting p-chlorobenzoic acid and naproxen as reference materials, and establishing a reaction kinetics model of target pollutants, p-chlorobenzoic acid, naproxen and free redicals under ultraviolet / sodium persulfate; S2, adding the target pollutants, p-chlorobenzoic acid and naproxen into actual water, and oxidative degradation is carried out by using ultraviolet / sodium persulfate advanced oxidation technology; and S3, calculating the free radical content according to a degradation effect of the p-chlorobenzoic acid and naproxen by using the model, and predicting degradation conditions of the target pollutants. The model is established by measuring the degradation of the two reference materials to fit the degradation of the multiple pollutants in the secondary effluent under the ultraviolet / sodium persulfate, thereby evaluating the feasibility of removing the target pollutants in the secondary effluent by the ultraviolet / sodium persulfate and effectively reducing the cost of small tests.
Owner:NANJING UNIV
Calcium carbonate-based fluorescent material and preparation method of material
ActiveCN107603597AThe method is simpleSuitable for large-scale industrial productionLuminescent compositionsRare-earth elementLuminous intensity
The invention provides a calcium carbonate-based fluorescent material. The calcium carbonate-based fluorescent material takes solid calcium carbonate powder as a matrix, and a 2-chlorobenzoic acid terbium complex is attached to and / or is combined with the surface of the matrix. The fluorescence intensity is large; the fluorescence lifetime is long; and under the conditions of a same luminous intensity and luminous life, the use of rare earth elements is greatly reduced, and resources are saved. The invention also provides a method for preparing the calcium carbonate-based fluorescent material.The method is easy to operate, the loss of the rare earth elements can be effectively avoided, and the yield of the calcium carbonate-based fluorescent material is high.
Owner:河南索顿新材料有限公司
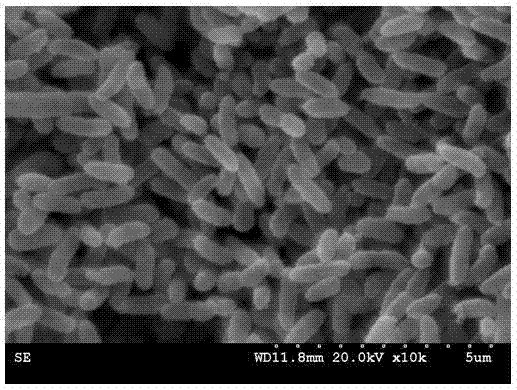
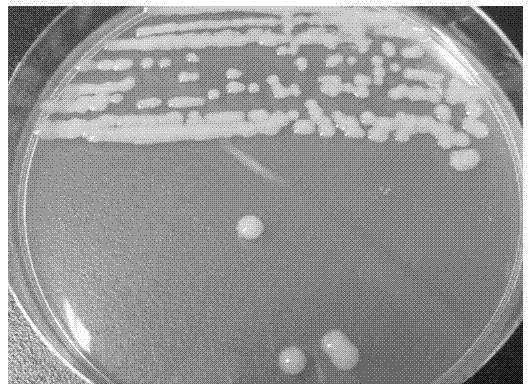
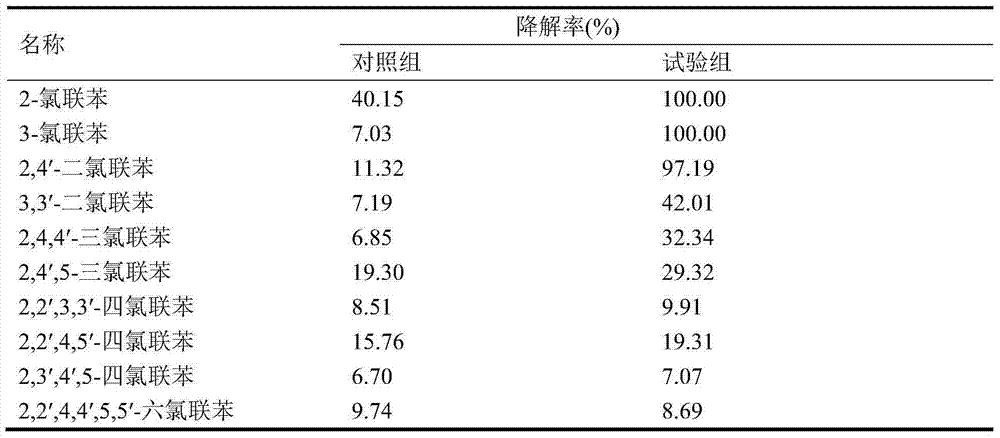
Method for screening polychlorinated biphenyl degrading bacterium and polychlorinated biphenyl degrading bacterium
ActiveCN103789209APromote degradationEfficient degradationBacteriaMicroorganism based processesBenzoic acidBioremediation
The invention discloses a method for screening a polychlorinated biphenyl degrading bacterium and the polychlorinated biphenyl degrading bacterium. The method is applied to separation of polychlorinated biphenyl aerobic degradation bacteria from polychlorinated biphenyl contaminated soil. The polychlorinated biphenyl degrading bacterium disclosed by the invention is identified to be Sphingobium fuliginis HC3. The bacterium is capable of effectively degrading chlorodiphenyl, dichlorodiphenyl and trichlorodiphenyl, and can also degrade benzoic acid, 3-chlorobenzoic acid and 4-chlorobenzoic acid. After the strain HC3 is inoculated to the polychlorinated biphenyl contaminated soil, the degradation of chlorodiphenyl...tetrachlorophenyl can be remarkably prompted, and the total degradation rate of polychlorinated biphenyl can be 44.91% within 10 days; and the degradation rate is one time higher than that of a control group. The strain HC3 can degrade low-chloro multi-chlorophenyl and low-chloro benzoic acid under the aerobic condition, so that the strain can be applied to bioremediation engineering of polychlorinated biphenyl contaminated soil so as to prompt thorough degradation of multi-chlorophenyl and improve the remediation efficiency.
Owner:ZHEJIANG UNIV
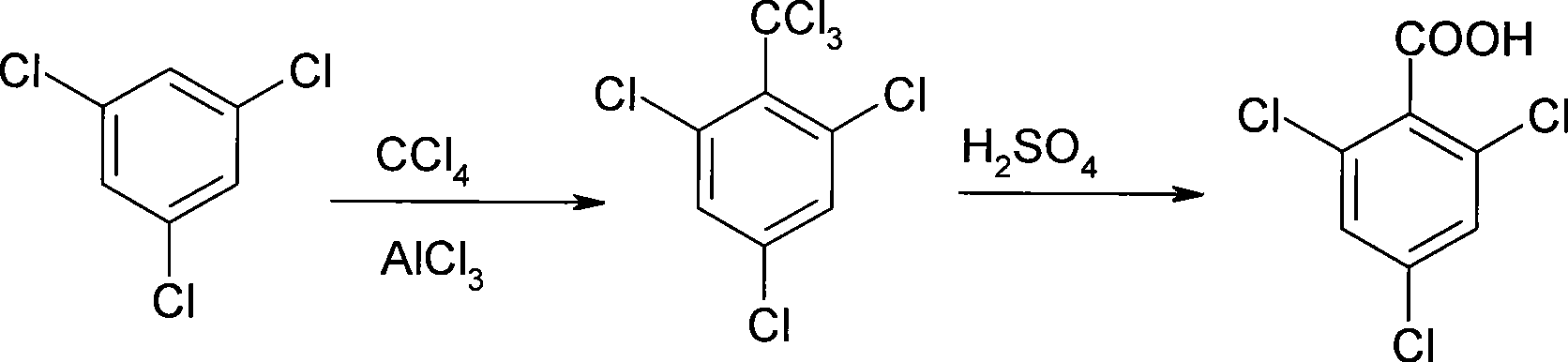
Process for producing 2, 4, 6-trichlorobenzoic acid
InactiveCN101429117ASimple preparation stepsReaction conditions are easy to controlOrganic compound preparationCarboxylic compound preparationBenzenePhenylacetic acid
The invention relates to a method for preparing 2, 4, 6-trichloro-phenylacetic acid. Specifically, 1,3,5-trichloro-benzene and carbon tetrachloride react to generate 2,4,6-trichloro-benzotrichloride; the 2,4,6-trichloro-benzotrichloride and concentrated sulfuric acid react to prepare the 2,4,6-trichloro-phenylacetic acid; and finally, the 2,4,6-trichloro-phenylacetic acid is formed by refining and crystallization. The preparation method has the advantages of low cost, simple reaction steps, easy control of reaction conditions and the like.
Owner:BEIJING ODYSSEY CHEM
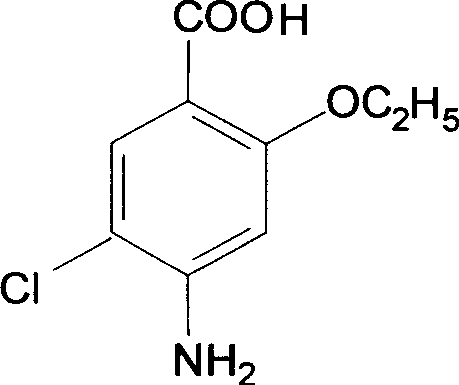
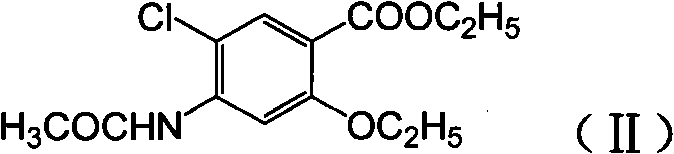
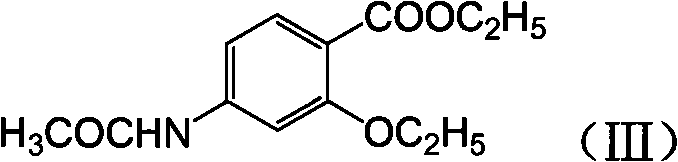
Novel synthesis method and intermediate for 2-ethoxy-4-amino-5-chlorobenzoic acid
The invention provides a preparation method for synthesizing an important mosapride intermediate 2-ethoxy-4-amino-5-chlorobenzoic acid. The method comprises the following steps: carrying out the four-step reaction of acetylation, biethylation, chlorination and hydrolysis for aminosalicylic acid or corresponding sodium salt and kali salt compounds thereof, and obtaining a product. The method has cheap and easily obtained raw materials, short synthesis steps, moderate reaction conditions, easy control, simple operation, lower pollution, high total yield, low cost and good and stable product quality, and is easily used for the industrialized production.
Owner:四川弘远药业有限公司
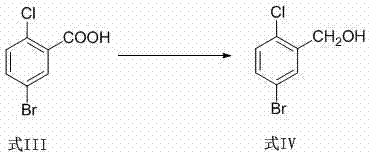
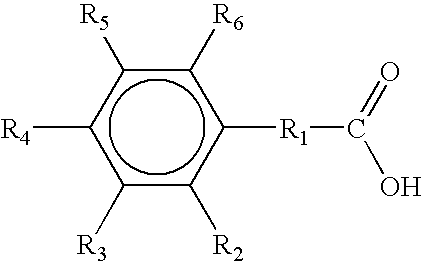
Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative
The present invention relates to the structure, preparation process and application of N-[2-(acryloxy)ethyl]-2-(arylthio) benzyl amine derivative with depression resisting activity, and belongs to the field of medicine chemical technology. The structure is shown. The preparation process includes the reaction between aryl phenol and 1, 2-dibromo ethane to produce 2-aryloxy bromoethane, the reaction of 2-aryloxy bromoethane with methanol solution of ammonia to produce 2-aryloxy ethylamine; the Ullmann sulfidation reaction between one-methoxyphenyl thiophenol and one-chlorobenzoic acid to produce sulfide; the reduction and chlorination of sulfide to produce 2-(2-arylphenylthio) benzyl chloride; and final N-alkylation with 2-aryloxy ethylamine to produce the destination product. The compound in the form of free alkali or salt is used in preparing medicine for depression.
Owner:TIANJIN UNIV
Process for synthesizing antithrombin inhibitor of non-asymmetric non-peptide kind
InactiveCN100509799CHigh yieldEasy to operate and separateOrganic chemistryChemical synthesisReagent
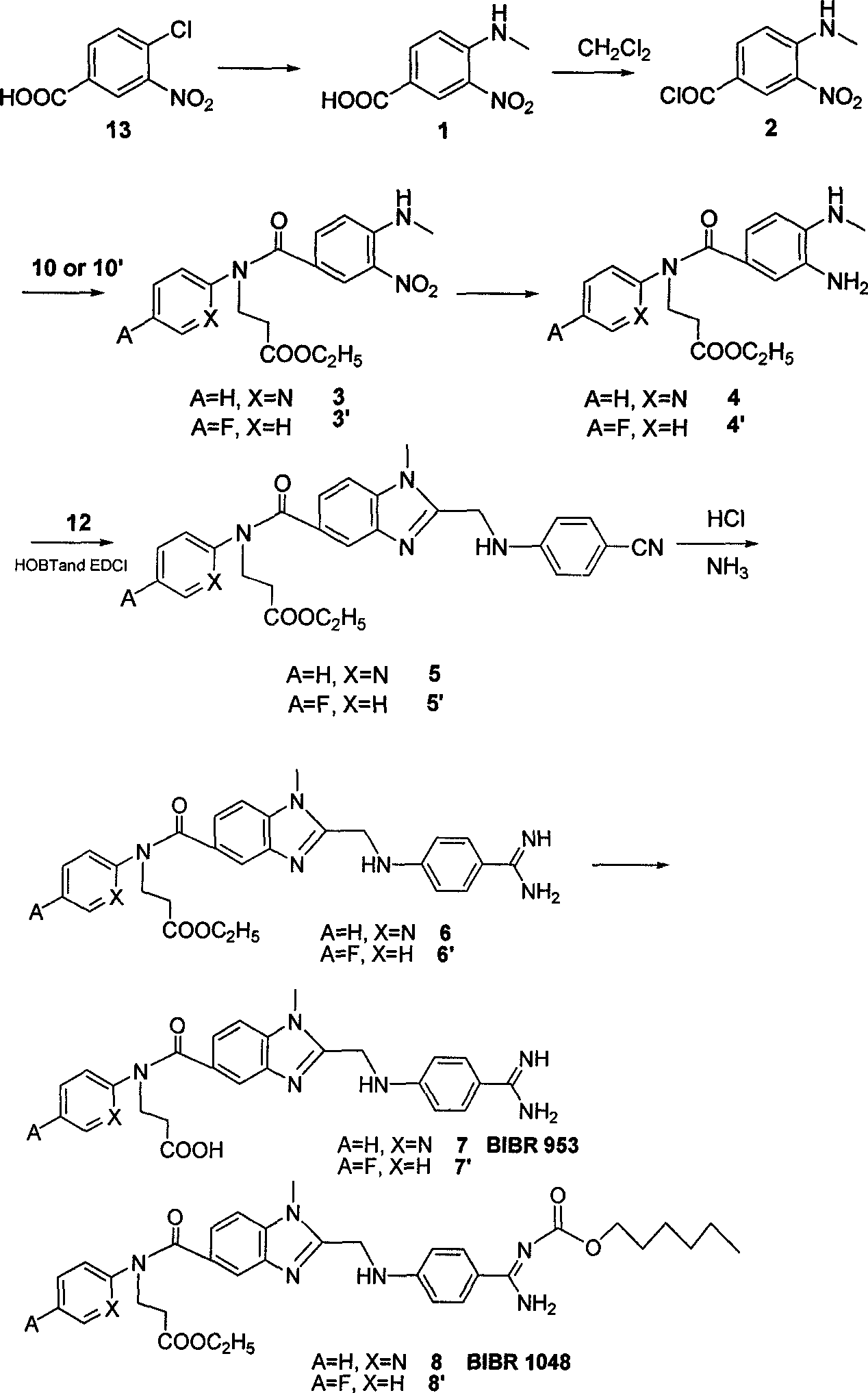
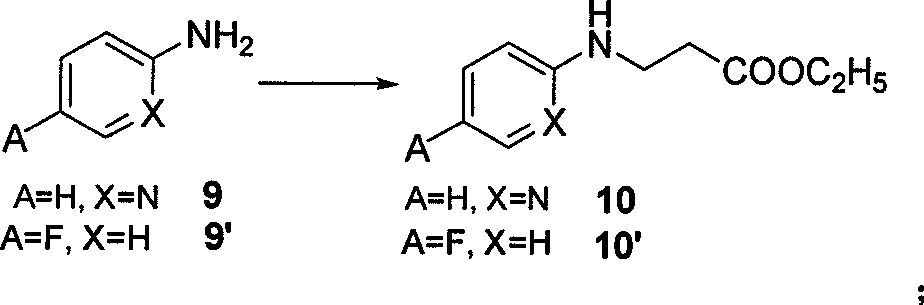
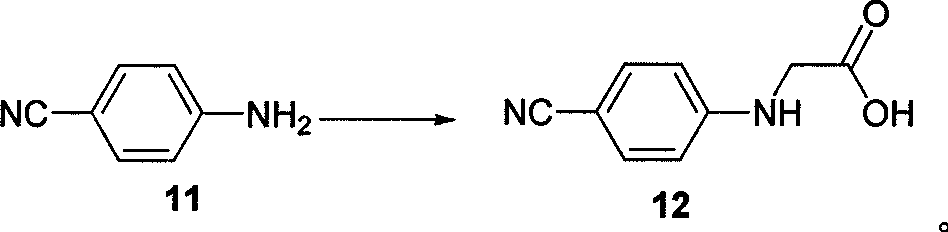
The invention belongs to the field of chemical synthesis, and relates to a synthesis method of an inhibitor containing a heterocyclic compound, especially an achiral, a Synthesis of peptide antithrombin inhibitors. The present invention uses cheap and easy-to-obtain 3-nitro-4-chlorobenzoic acid as a starting material, a highly efficient and high-yield synthetic raw material compound and a condensing agent with better effect to finally synthesize the target compound antithrombin inhibitor BIBR- 953 and BIBR-1048 and its analogs. The method of the invention has simple step operation and separation, high yield of each step, cheap and easy-to-obtain reagents, short route and high total yield of the target compound, which is nearly 50%.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
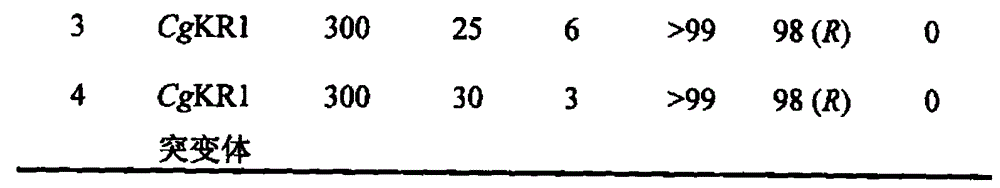


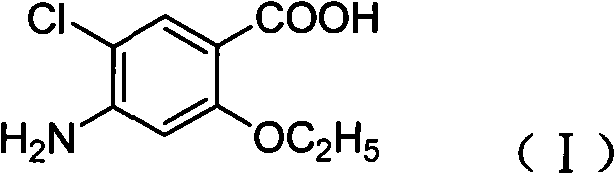
![Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative](https://images-eureka.patsnap.com/patent_img/1c1a0d5a-301b-4ea6-b72c-a61f05a5cf10/A2005100135550002C1.PNG)
![Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative](https://images-eureka.patsnap.com/patent_img/1c1a0d5a-301b-4ea6-b72c-a61f05a5cf10/A2005100135550003C1.PNG)
![Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative Prepn and application of N-[2-(aryloxy)ethyl]-2-(arylthio) benzyl amine derivative](https://images-eureka.patsnap.com/patent_img/1c1a0d5a-301b-4ea6-b72c-a61f05a5cf10/A20051001355500051.PNG)