5-bromine-2-chlorobenzaldehyde preparation method
A technology of chlorobenzaldehyde and chlorobenzoic acid is applied in the preparation of organic compounds, carboxylates, carbon-based compounds, etc., and can solve the problems of mixed solvent recrystallization, EHS risks, and toxic sulfides. Large cost advantage, avoid heavy metal pollution, improve selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
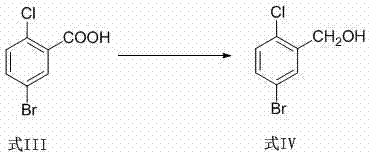
[0005] Example 1 Preparation of 5-bromo-2-chlorobenzoic acid (formula III)
[0006] Add 94 g of 2-chlorobenzoic acid (formula II) and 1120 ml of sulfuric acid into the reaction flask in sequence, stir to dissolve, and add 110 g of NBS. Heat to 40-50°C and stir until the reaction is complete. The reaction solution was added dropwise to ice water, stirred for 30 minutes, and filtered to obtain a white crude product. After the crude product was dried, it was recrystallized with toluene to obtain 114 g of a white solid with a purity of 99.2% and a yield of 80.8%.
Embodiment 2
[0007] Example 2 Preparation of 5-bromo-2-chlorobenzyl alcohol (formula IV)
[0008] Add 114 g of 5-bromo-2-chlorobenzoic acid into the reaction flask, then add THF 1140 ml, and stir to dissolve. Cool to 10-15°C, add 45.7g NaBH 4 , and stirred for 30 minutes. 71.1 g of sulfuric acid was added dropwise, and the temperature was controlled at 10-20°C. After the dropwise addition, raise the temperature to 50-60°C and stir until the reaction is complete. Cool to 10-20°C, and add 500 ml of 1N hydrochloric acid dropwise. After the dropwise addition, the layers were left to stand, and the aqueous phase was mixed with CH 2 Cl 2 After extraction, the organic phases were combined, washed with saturated brine, and the organic phase was separated. The organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 102 g of a white solid with a purity of 99% and a yield of 95%.
Embodiment 3
[0009] Example 3 Preparation of 5-bromo-2-chlorobenzaldehyde (Formula I)
[0010] 685g of 10% NaClO was adjusted to PH=7.5 with a buffer solution composed of 5% sodium dihydrogen phosphate and 5% disodium hydrogen phosphate, and set aside.
[0011] 5-bromo-2-chlorobenzyl alcohol (formula IV) 102g and 1000ml CH 2 Cl 2 Add to the reaction flask and cool to 0-5°C. Add 3.6g of TEMPO, 3.6g of tetrabutylammonium bromide, and 4.73g of NaBr in turn, and stir for 10 minutes. Add the configured NaClO solution dropwise, and add dropwise under control of 0-5°C. After the dropwise addition, keep stirring until the reaction is complete. Add 15 g of solid sodium sulfite, and stir for 20 minutes. Separated, aqueous phase with CH 2 Cl 2 Extract, combine the organic layers, and wash with saturated brine. The organic phase was separated, dried over anhydrous sodium sulfate, and filtered. Concentrate the filtrate to obtain an off-white solid, recrystallize the off-white solid with n-he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com