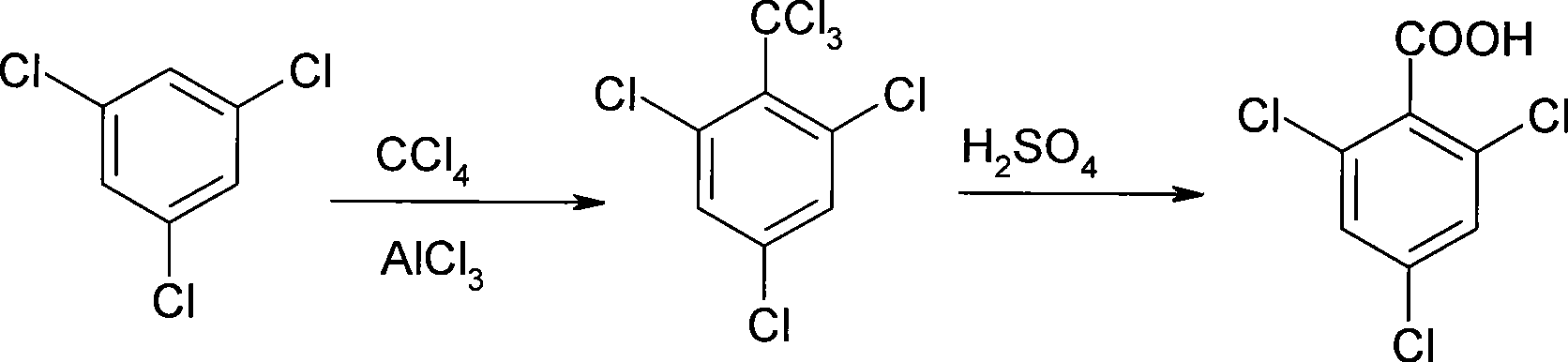
Process for producing 2, 4, 6-trichlorobenzoic acid
A technology of trichlorobenzoic acid and trichlorobenzene, applied in 2 fields, can solve the problems of no price advantage and high price of acylating reagent, and achieve the effects of easy control of reaction conditions, strong price advantage and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Prepare 2,4,6-trichlorobenzotrichloride by reacting 1,3,5-trichlorobenzene and carbon tetrachloride:
[0033] In a 1000L enamel kettle, put 100kg 1,3,5-trichlorobenzene and 280kg carbon tetrachloride in sequence, stir for 10 minutes, add 100kg anhydrous aluminum trichloride, heat and reflux at 77°C for 8 hours, and release a large amount of hydrochloric acid gas Absorb with water.
[0034] Cool down to 30°C, slowly pump the reaction materials into another 2000L reactor with 300kg of water, stir for 1.5 hours, separate the organic matter in the lower layer, wash the organic matter twice with 2 times the volume of water; then wash the organic layer with 0.3 Steam under MPa pressure was heated for 25 minutes until carbon tetrachloride did not appear. For this reason, the crude product of 2,4,6-trichlorotrichlorotoluene was obtained after recovering carbon tetrachloride.
[0035] 2. Prepare 2,4,6-trichlorobenzoic acid by reacting 2,4,6-trichlorobenzotrichloride with sul...
Embodiment 2
[0042] 1. Prepare 2,4,6-trichlorobenzotrichloride by reacting 1,3,5-trichlorobenzene and carbon tetrachloride:
[0043] In a 1000L enamel kettle, put 274kg1,3,5-trichlorobenzene and 810kg carbon tetrachloride in sequence, stir for 10 minutes, add 300kg anhydrous aluminum trichloride, heat and reflux at 80°C for 10 hours, release a large amount of hydrochloric acid gas and water absorb. Cool down to 40°C, slowly pump the reaction materials into another 2000L reactor with 1000kg of water, stir for half an hour, separate the organic matter in the lower layer, wash the organic matter twice with 2 times the volume of water; then wash the organic layer with 0.3 Steam under MPa pressure was heated for 45 minutes until carbon tetrachloride did not appear. For this reason, 2,4,6-trichlorotrichlorotoluene crude product 250kg was obtained after recovering carbon tetrachloride.
[0044] 2. Prepare 2,4,6-trichlorobenzoic acid by reacting 2,4,6-trichlorobenzotrichloride with sulfuric acid: ...
Embodiment 3
[0051] 1. Add 1,3,5-trichlorobenzene and carbon tetrachloride into the reaction kettle, add anhydrous aluminum trichloride after mixing, reflux at 75°C for 12 hours, 1,3,5-trichlorobenzene The weight ratio of benzene, carbon tetrachloride and anhydrous aluminum trichloride is 1:3.0:1.1; cool down to 45°C, add water 5 times the weight of 1,3,5-trichlorobenzene, stir After 1.5 hours, separate the organic matter in the lower layer; heat the organic matter with steam at a pressure of 0.3 MPa for 45 minutes, and recover carbon tetrachloride to obtain crude 2,4,6-trichlorotrichlorotoluene. The carbon tetrachloride in this step can be reused after being recovered, and the hydrochloric acid gas produced in the reaction process is taken out by an acid-resistant pump and absorbed by water.
[0052] 2. Add water to the reaction kettle, then add concentrated sulfuric acid, heat up to 130°C, add the crude product of 2,4,6-trichlorotrichlorotoluene prepared in step 1 at a rate of 1.6 liters...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com