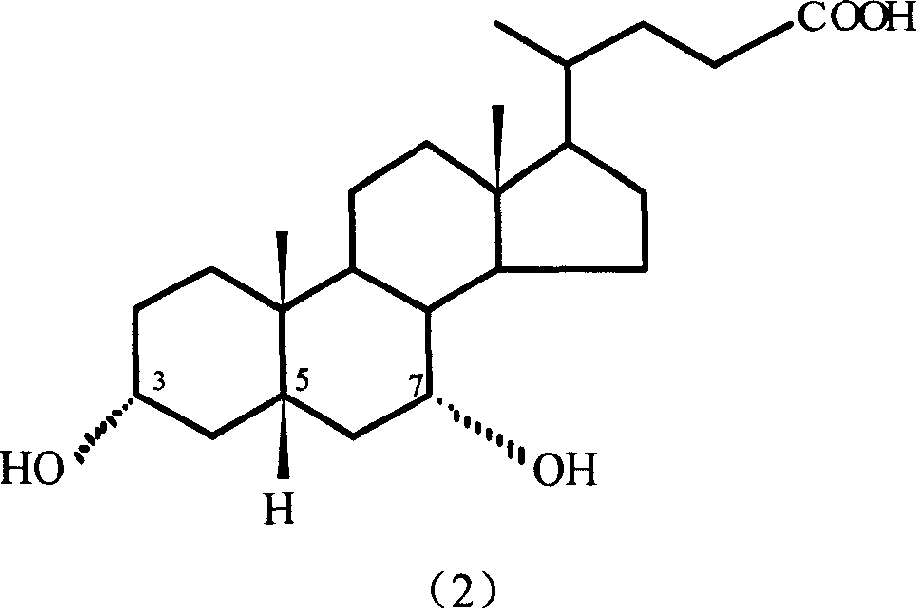
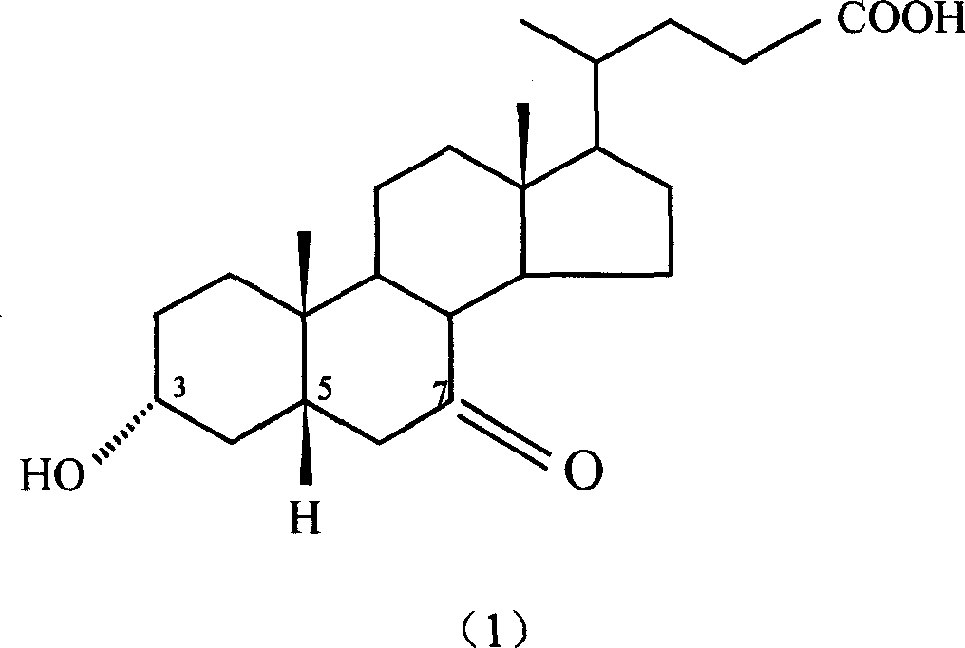
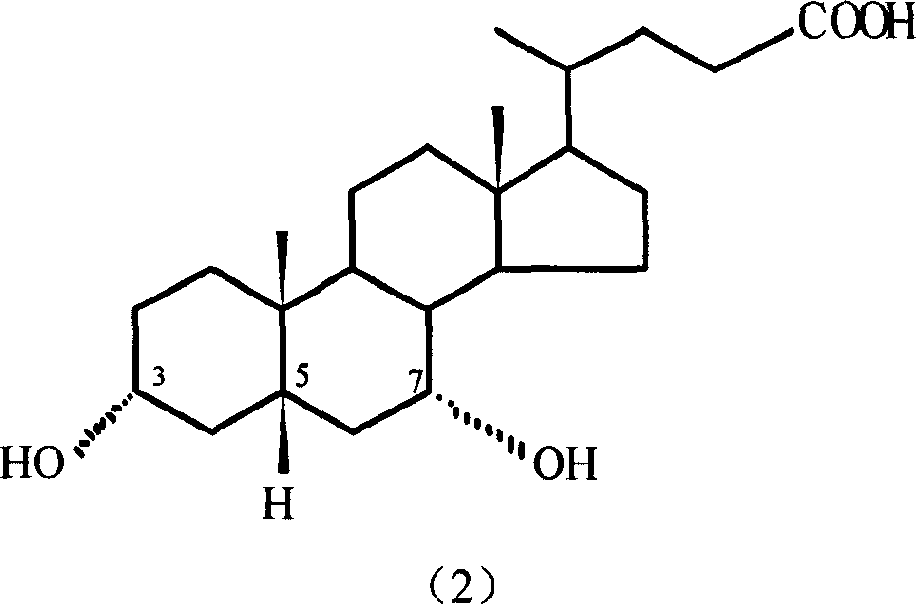
Preparation method of 7-keto lithocholic acid
A kind of ketolithocholic acid and said technology are applied in the field of preparation of 7-ketolithocholic acid, can solve the problems of harsh operating conditions, long preparation steps, difficult product separation and the like, and achieve the effect of mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 0.4g KBr in 13ml secondary deionized water, weigh 2.0g CDCA and dissolve it in 50ml acetonitrile, mix the two solutions evenly and put them into the anode compartment of the H-type diaphragm electrolyzer as the anolyte. Use 63ml of 20% dilute sulfuric acid as catholyte. HF-101 strong acid type cation exchange membrane is the diaphragm. with PbO 2 The / Ti mesh electrode is the anode, and the stainless steel plate is the cathode. The current density used is 95.2A / m 2, as detected by TLC, when CDCA basically reacted, the electrolysis was stopped, and the power-on time was 2.5h. 1.697 g of crude 7K-LCA was obtained, and the HPLC content was 90.4%. The calculated yield and current efficiency were 77.1% and 84.2%, respectively. After the product was purified by column chromatography and recrystallization, the melting point was measured: m.p. 200-203°C (literature value: 201-203°C). 1H NMR analysis results: δ0.5~1.8, 2.1, 2.2 (33H, m, -CH 2 -, -CH 2 CH 3 ,-CH...
Embodiment 2
[0031] Dissolve 0.4g KBr in 13ml secondary deionized water, weigh 2.0g CDCA and dissolve it in 50ml ethanol, mix the two solutions evenly and put them into the anode chamber of the H-type diaphragm electrolyzer as the anolyte. Use 63ml of 20% dilute sulfuric acid as catholyte. HF-101 strong acid type cation exchange membrane is the diaphragm. with PbO 2 The / Ti mesh electrode is the anode, and the stainless steel plate is the cathode. The current density used is 95.2A / m 2 , as detected by TLC, when CDCA basically reacted, stop the electrolysis, and the power-on time was about 4.5h. 1.652 g of crude 7K-LCA was obtained, with an HPLC content of 89.5%. The calculated yield and current efficiency were 74.3% and 45.5%, respectively. After the product is purified, the melting point is measured: m.p.200-203°C (literature value: 201-203°C).
Embodiment 3
[0033] Dissolve 0.4g KBr in 13ml secondary deionized water, weigh 2.0g CDCA and dissolve it in 50ml acetonitrile, mix the two solutions evenly and put them into the anode chamber of the H-type diaphragm electrolyzer as the anolyte. Use 63ml of 20% dilute sulfuric acid as catholyte. HF-101 strong acid type cation exchange membrane is the diaphragm. with PbO 2 The / Ti mesh electrode is the anode, and the stainless steel plate is the cathode. The current density used is 190.4A / m 2 , as detected by TLC, when CDCA basically reacted, the electrolysis was stopped, and the power-on time was 2.25h. 1.423 g of crude 7K-LCA was obtained, with an HPLC content of 87.9%. The calculated product yield and current efficiency were 62.8% and 38.1%, respectively. After the product is purified, the melting point is measured: m.p.200-203°C (literature value: 201-203°C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com