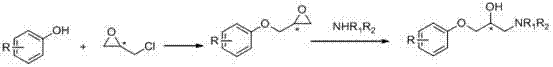
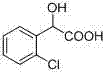
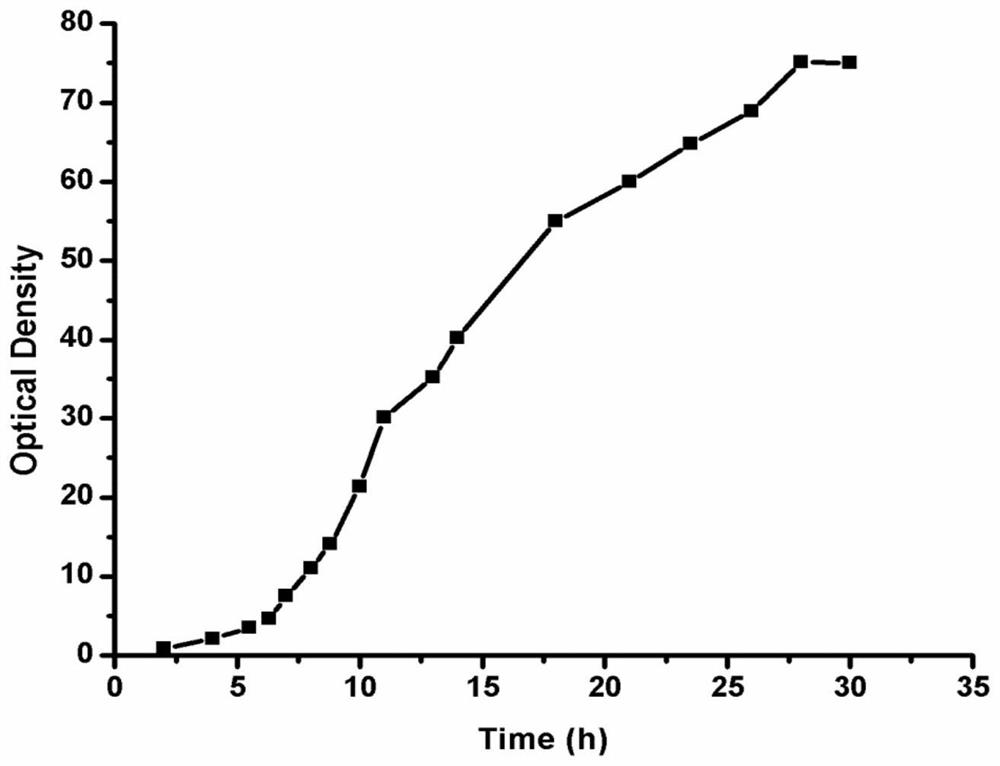
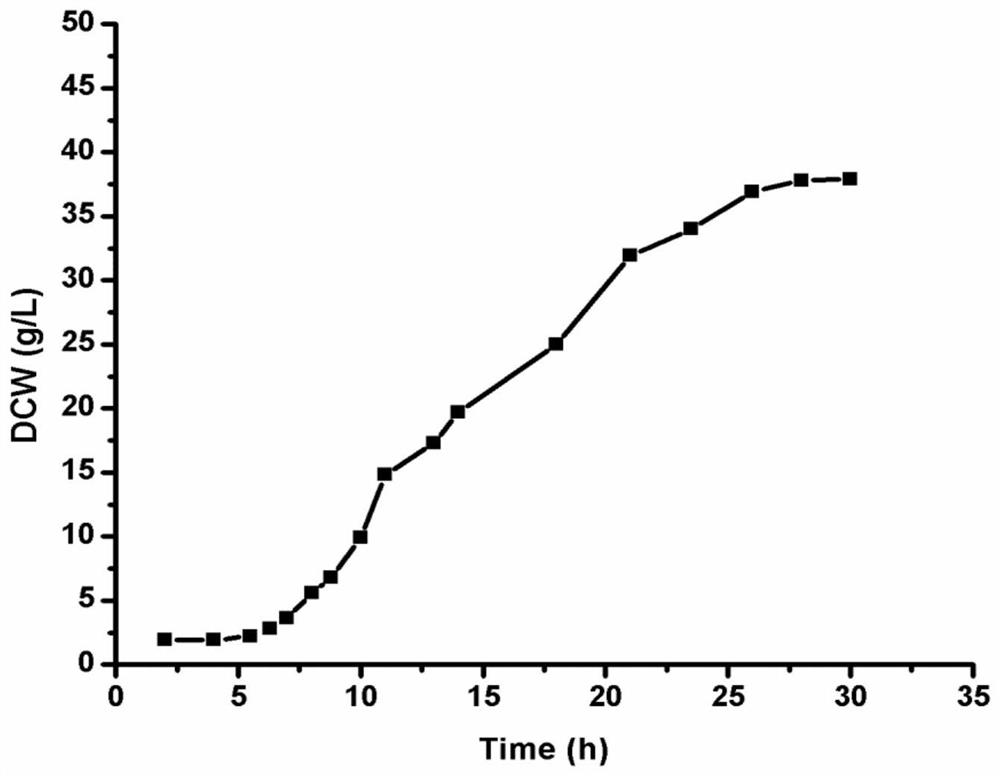
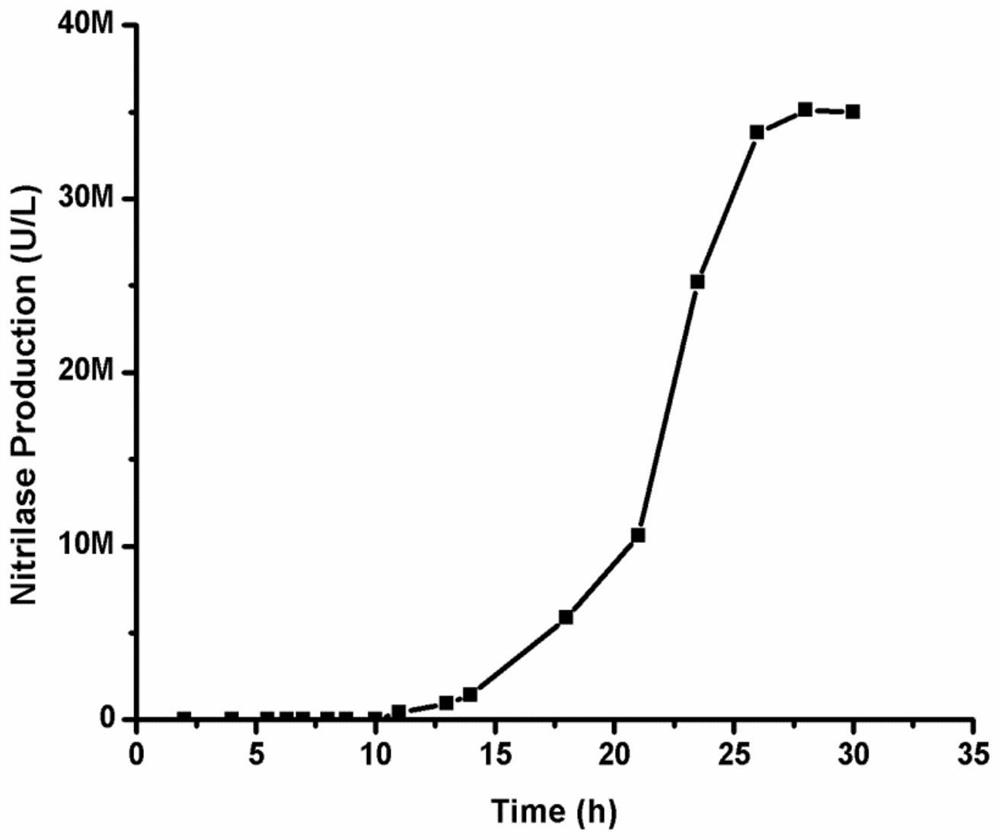
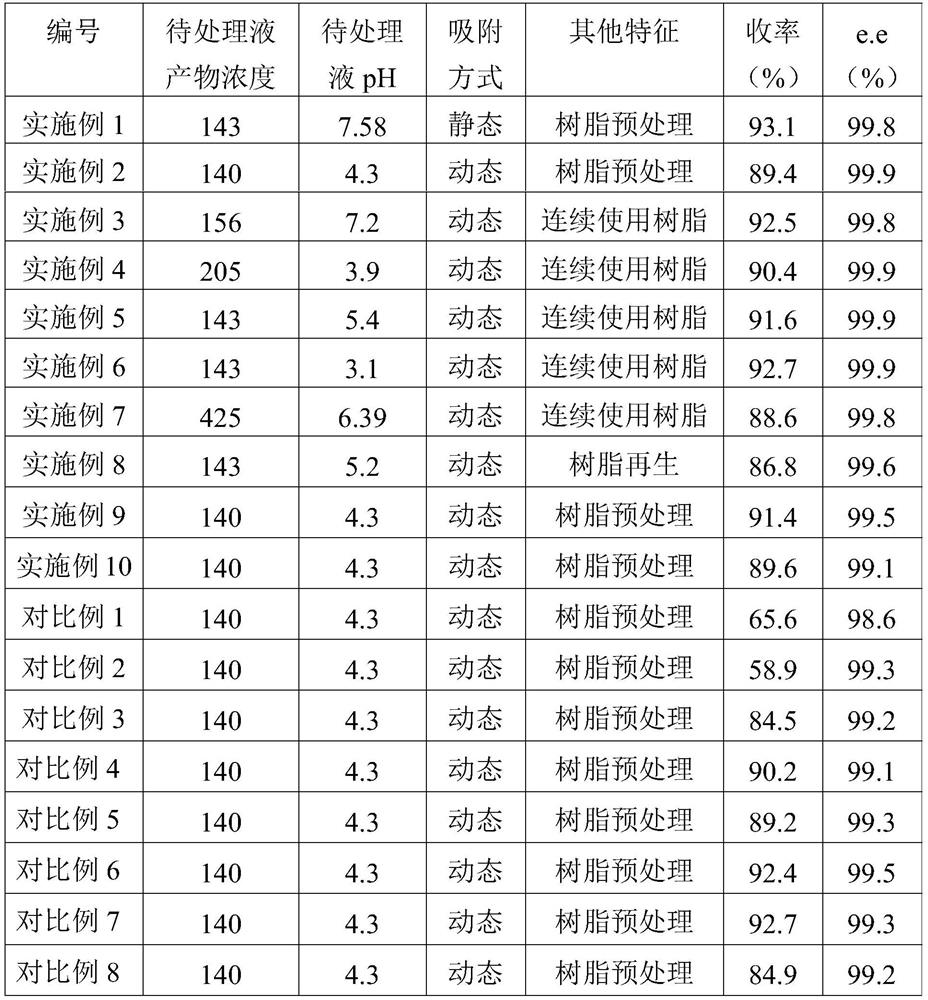
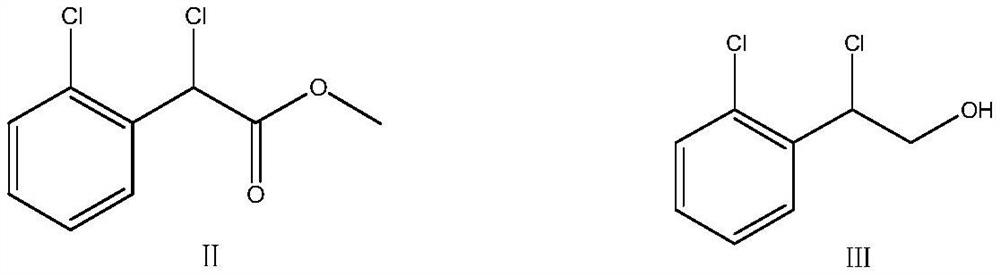
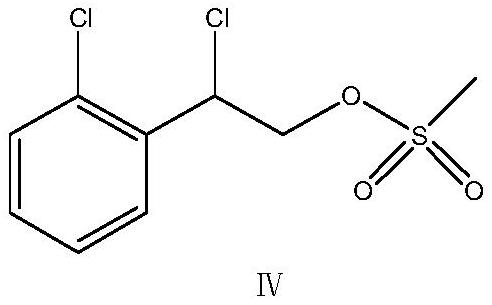
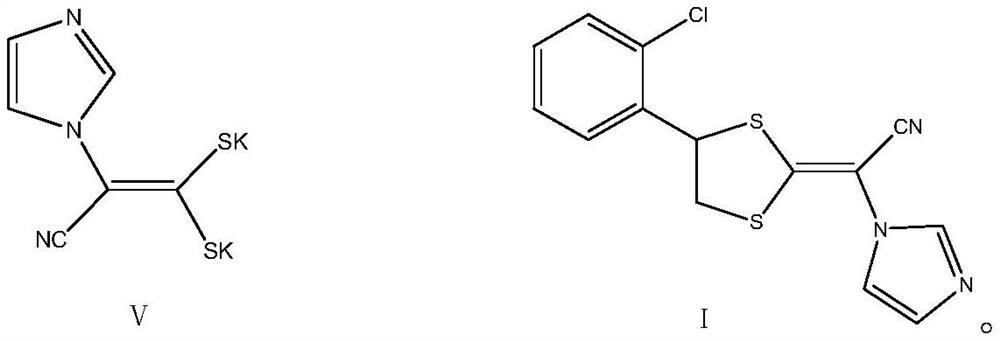
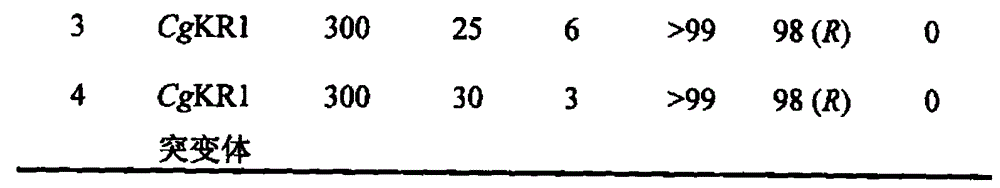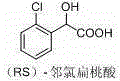Patents
Literature
35 results about "Chloromandelic Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
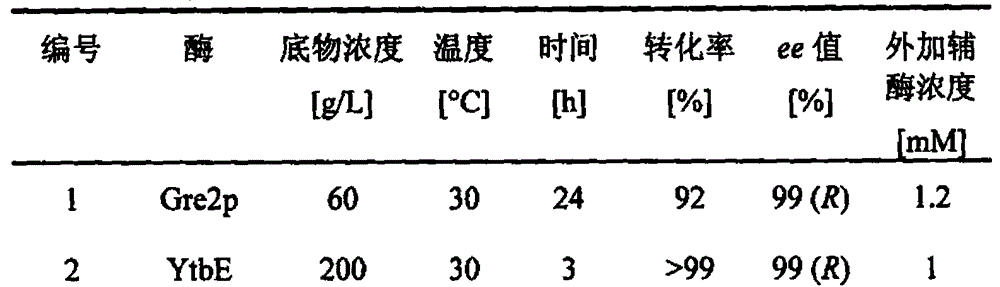
Carbonyl reductase, gene and mutant and application thereof to asymmetrical reduced carbonyl compound
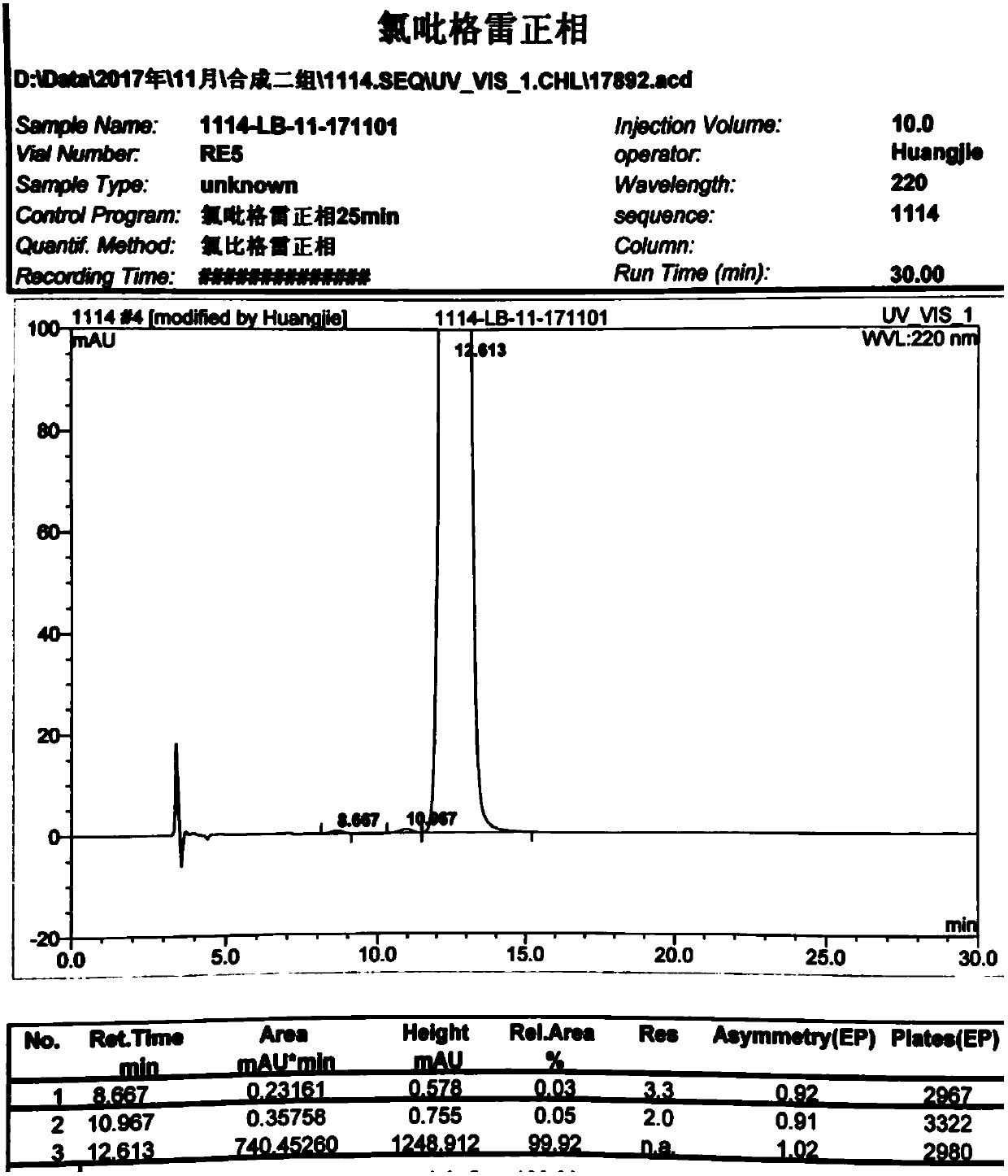
ActiveCN102618513AHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationMethyl o-chloromandelate
The invention discloses a novel carbonyl reductase, a gene, a mutant thereof, a recombinant expression vector containing the gene and the mutant, a recombinant expression transformant, a recombinase preparation method, and applications of the carbonyl reductase and recombinase to preparation of active chiral alcohols with a chiral carbonyl compound before asymmetrical reduction. The carbonyl reductase is derived from candida glabrata, is applied to preparation of a plurality of optically-active chiral alcohols such as (R)-chloromandelic acid methyl ester, (R)-2-hydroxy-4-phenyl ethyl butyrate, (R)-4-chlorin-3-phenyl ethyl butyrate and the like. Compared with other preparation methods, a product prepared through the method has high concentration, does not require additionally or slightly adding any expensive coenzyme, has high optical purity, and has the advantages of mild reaction conditions, easiness and convenience for operating, easiness for amplifying and the like, and has a good industrial application prospect in the production of clopidogrel, L-carnitine and perindopril antihypertensive medicinal intermediates.
Owner:EAST CHINA UNIV OF SCI & TECH
Carbonyl reductase mutant as well as gene and application thereof
InactiveCN104099305AImprove thermal stabilityIncreased reductase activityBacteriaOxidoreductasesMethyl o-chloromandelateMandelic acid
The invention relates to a carbonyl reductase CgKR1 mutant, a coding gene of the mutant, a recombinant expression vector containing the gene of the carbonyl reductase mutant, a recombinant expression transformant, a recombinase, a preparation method of the recombinase, and an application of the carbonyl reductase mutant to asymmetric reduction of ketonic ester for preparation of optically pure chiral hydroxyl ester, such as catalysis of o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare (R)-o-chloro mandelic acid methyl ester. Compared with wild enzymes, the carbonyl reductase mutant has the advantages that the thermal stability is substantially improved, and the catalytic activity of part of the mutant to the o-chlorobenzoic acid formyl methyl ester is also obviously improved. The multiple mutants can be applied to catalysis of the ketonic ester for asymmetric reduction to prepare the optical purely-chiral hydroxyl ester, such as catalysis of the o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare the optically pure (R)-o-chloro mandelic acid methyl ester. The carbonyl reductase mutants have the very good industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
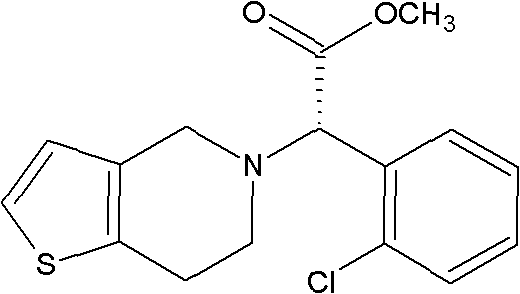
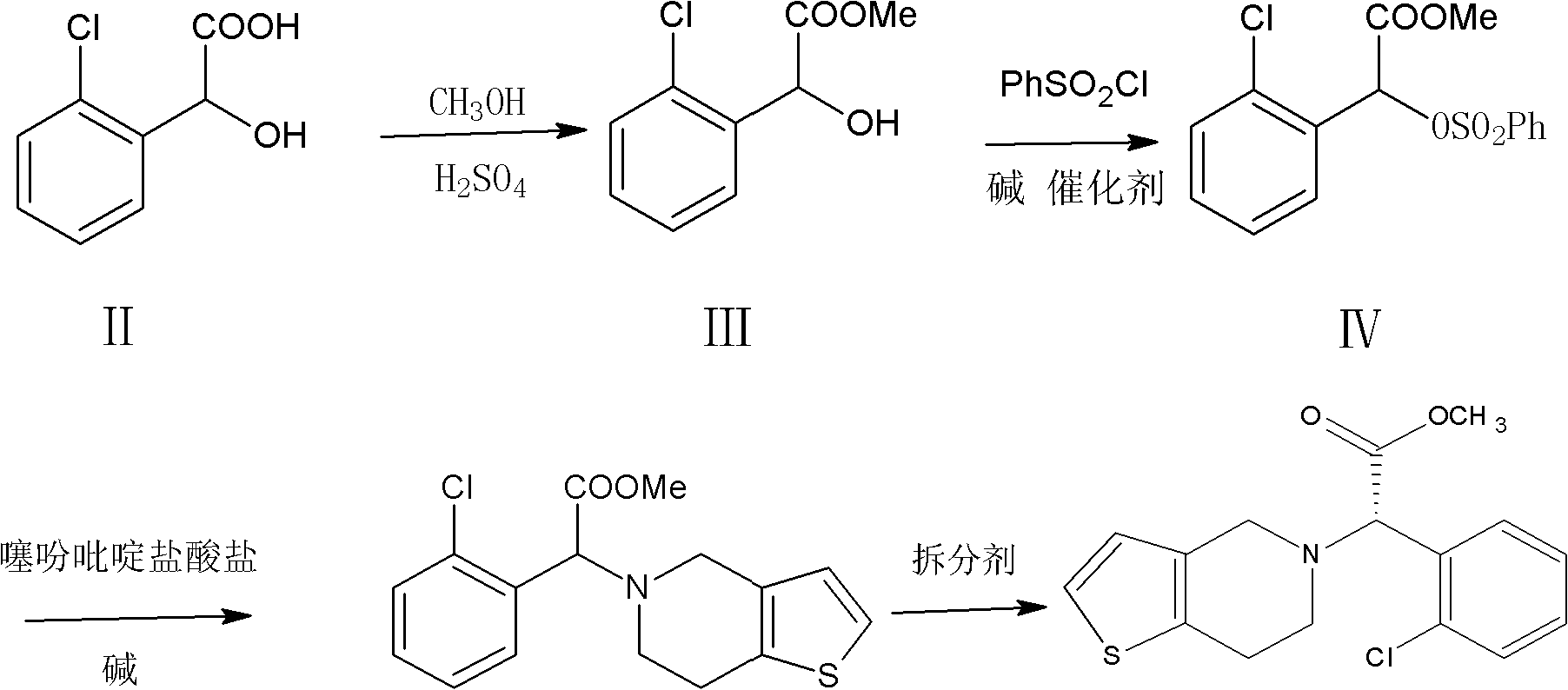
Method for preparing clopidogrel
InactiveCN101845050APromote environmental protectionEliminate the splitting stepOrganic chemistrySulfonyl chlorideMethyl o-chloromandelate
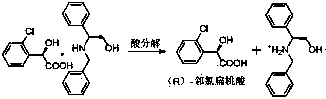
The invention relates to a method for preparing clopidogrel. The conventional synthetic methods have the disadvantages of poor environmental protection, disadvantageous industrial production, low optical purity of final products and high cost. The technical scheme adopted by the invention comprises the following steps of: performing a reaction on a compound, namely, R,S-o-chloromandelic acid and methanol to produce R,S-chloromandelic acid methyl ester; performing the reaction on the R,S-chloromandelic acid methyl ester and benzene sulfonyl chloride under the action of an alkaline catalyst to produce 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate; performing an SN2 substitution reaction on the 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate and 4,5,6,7-tetrahydro-thiophene pyridine hydrochloride under an alkaline condition to produce R,S-clopidogrel free alkali; resolving the R,S-clopidogrel free alkali in resolving solvent by using a resolving agent; and dissociating the resolved R,S-clopidogrel free alkali to prepare the clopidogrel. In a synthetic route of the invention, reaction conditions are temperate, used reaction substrates are environmentally friendly, reaction yield in each step is high, the optical purity of a final product is up to over 99.5 percent, and pollution-free production can be realized.
Owner:SHANGYU JINGXIN PHARMA
Method for producing R-mandelic acid and derivates thereof by biocatalysis
ActiveCN101701243AReduce dosageReduce outputMicroorganism based processesFermentationMandelonitrileWastewater
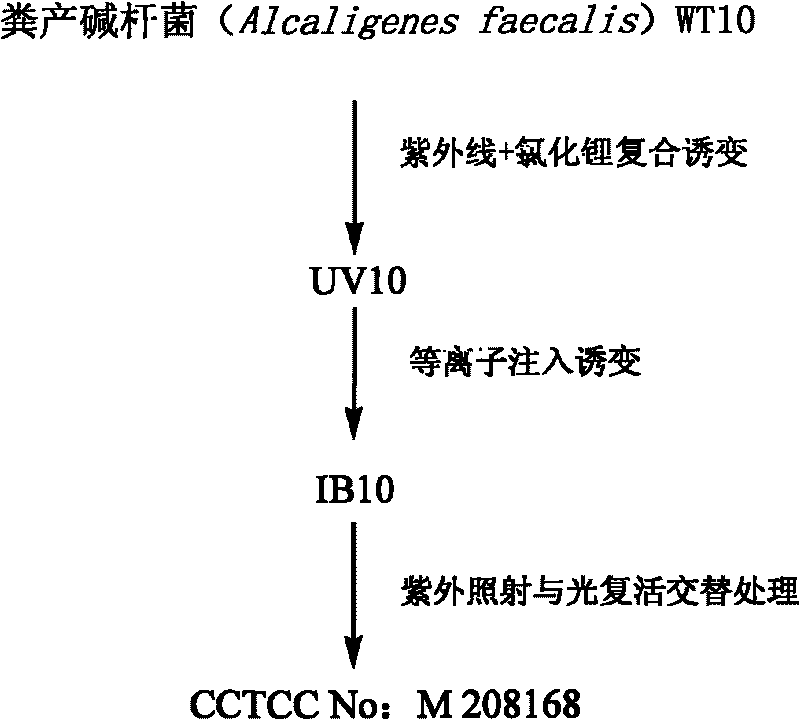
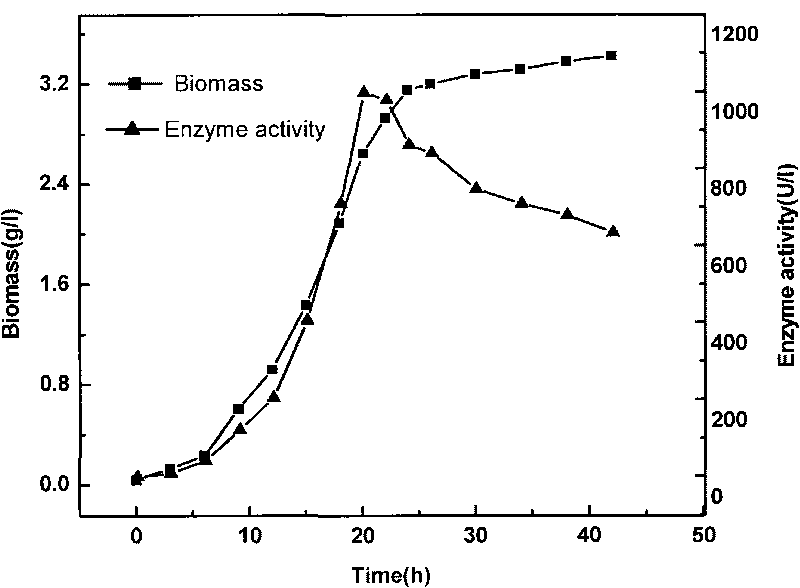
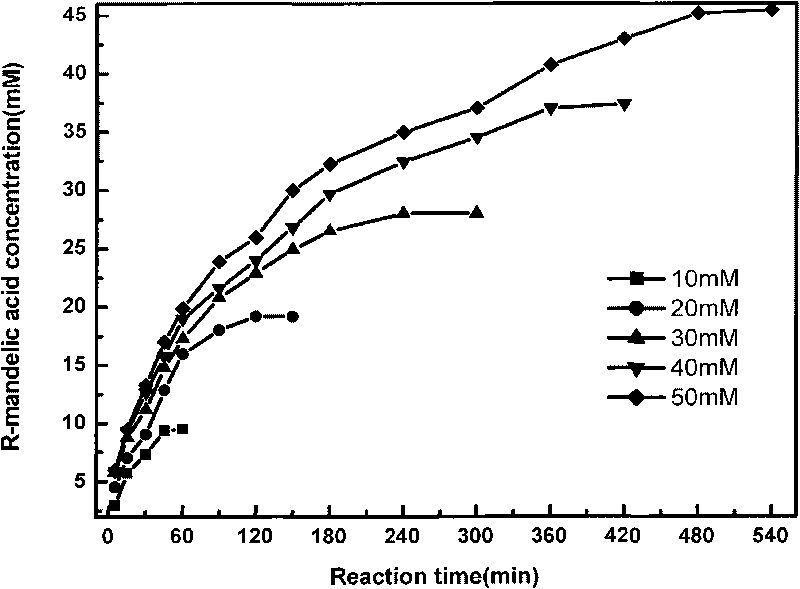
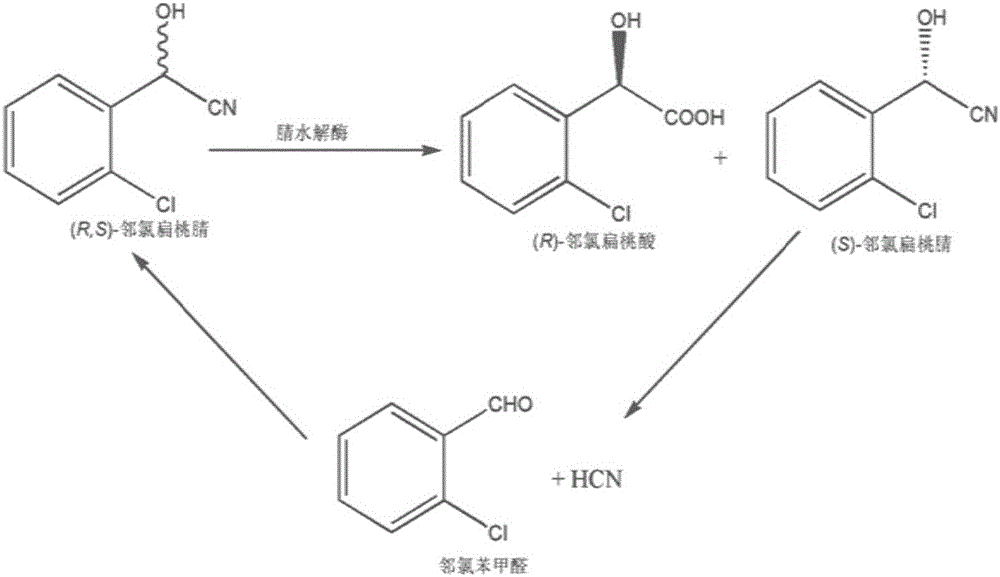
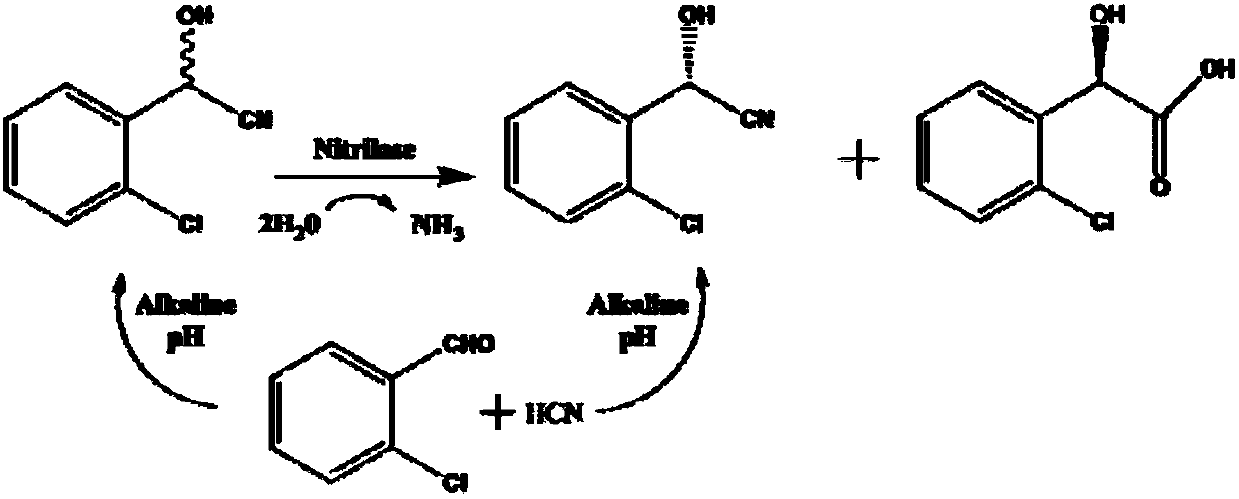
The invention provides a method for producing R-mandelic acid and derivates thereof by biocatalysis. The method comprises performing hydrolysis reaction in a reaction system which takes racemization mandelonitrile compound shown in formula (I) as the zymolyte, and nitrilase obtained by culturing bacillus foecalis alkaligenes (CCTCC No: M 208168) as the catalyst at 20-60 DEG C with pH being 8.0-8.5 to obtain chiral R-mandelic acid and derivates thereof shown in corresponding formula (II). The invention has the beneficial effects that the activity of strain bacillus foecalis alkaligenes is high, the dosage of thallus is little, the produced waste water in the reaction process is little, and pollution is less; the reaction conditions are mild and energy consumption is low; the cost is low, conversion rate is high and yield is high, and the invention is favorable for industrialized production of chiral R-mandelic acid and R-chloro mandelic acid.
Owner:ZHEJIANG UNIV OF TECH
Keto acid reductase, gene, engineering bacterium and application of keto acid reductase in synthesis of chiral aromatic 2-hydroxy acid
ActiveCN108410831AHigh yieldImprove use valueBacteriaMicroorganism based processesEnzyme GeneLeuconostoc lactis
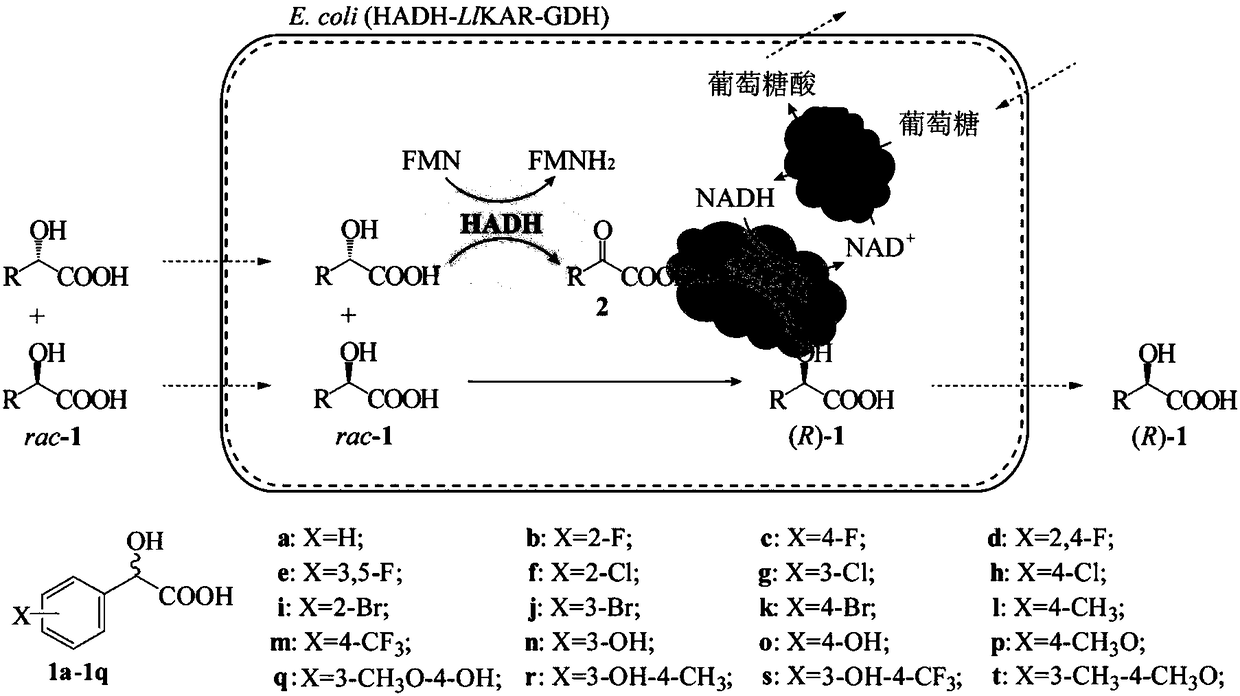
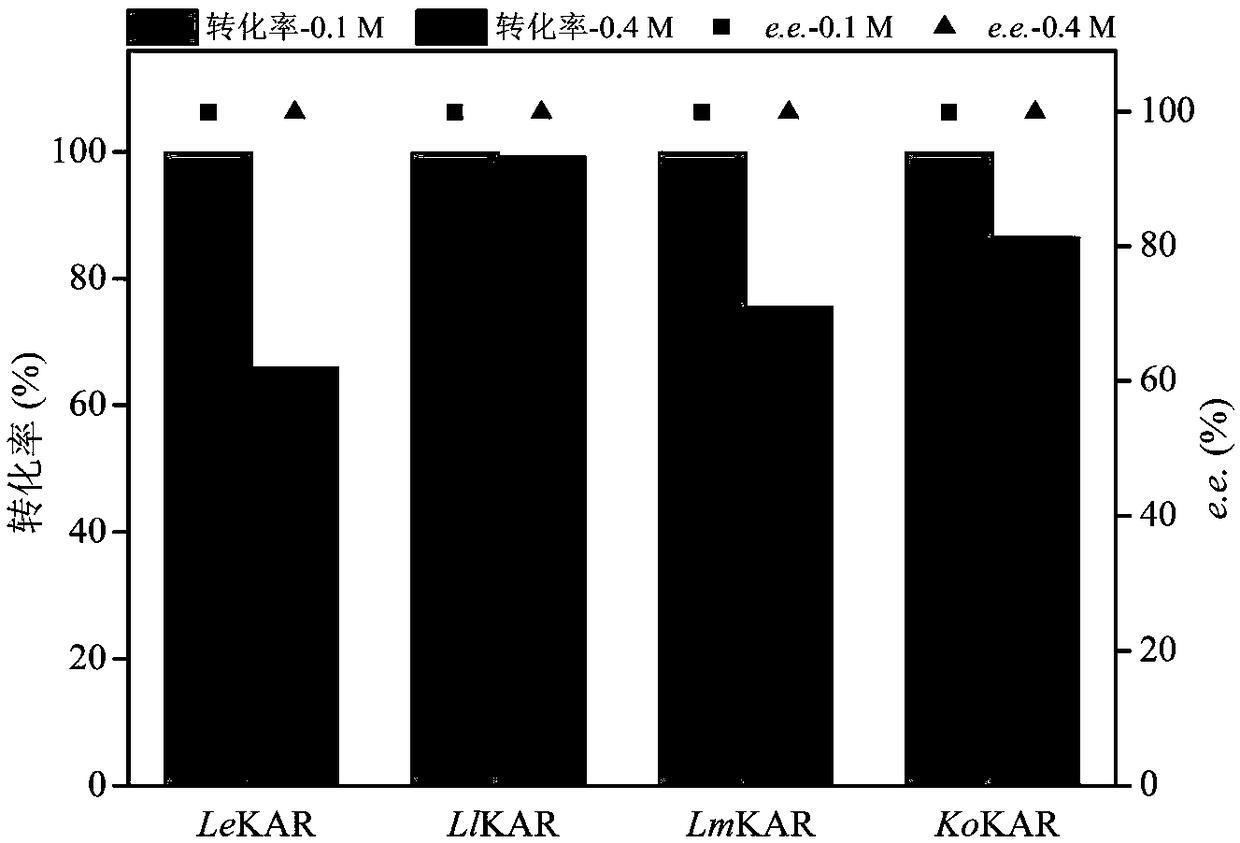
The invention discloses keto acid reductase, a gene, an engineering bacterium and application of the keto acid reductase in the synthesis of chiral aromatic 2-hydroxy acid. The keto acid reductase hasan amino acid sequence shown as in one of SEQ ID NO. 4, SEQ ID NO. 8 and SEQ ID NO. 10. The invention provides efficient keto acid reductase from Leuconostoc lactis; the efficient keto acid reductaseis capable of catalyzing broad-spectrum aromatic 2-keto acid, substrate loading capacity of a substrate of benzoylformic acid is increased from 100 mM to 400 mM. A single-bacterium dual-plasmid triple-enzyme series redox cascade system is established with the keto acid reductase, 2-hydroxy acid dehydrogenase and glucose dehydrogenase and is capable of catalyzing most racemic aromatic 2-hydroxy acid subjected to efficient deracemization to obtain chiral aromatic (R)-2-hydroxy acid, and both yield and e. e. value are greater than 99%; the system is finally applied to prepare optically pure (R)-2-chloromandelic acid by deracemization of 300 mM 2-chloromandelic acid, and the yield is up to 83.8 g / (L d).
Owner:ZHEJIANG UNIV OF TECH
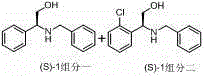
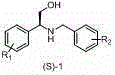
Preparation method of novel chiral resolving agent and (R)-chloromandelic acid
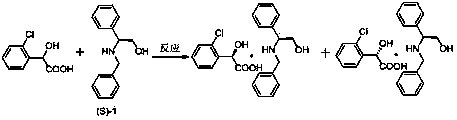
ActiveCN103804179AHigh split efficiencyChemically stableOrganic compound preparationCarboxylic compound preparationOrganosolvDiastereomer
The invention discloses a novel chiral resolving agent S-(1) which is used for chemically resolving (RS)-chloromandelic acid to prepare the (R)-chloromandelic acid. The invention further discloses a preparation method of the (R)-chloromandelic acid. The preparation method comprises the following steps: making the resolving agent S-(1) react with the (RS)-chloromandelic acid to obtain a (R)-chloromandelic acid and resolving agent diastereomer salt, decomposing the (R)-chloromandelic acid and resolving agent diastereomer salt to obtain the (R)-chloromandelic acid, and optionally recovering the S-(1). The preparation method has the beneficial effects that (1), the chiral resolving agent (S)-1 is high in resolving efficiency, stable in chemical property, and easy to separate and recycle, and the recycling purity is more than 99 percent; (2), an industrial line of the preparation method is developed, the process condition is mild, and industrialized production is facilitated; (3), the content of the prepared (R)-chloromandelic acid is more than 99 percent, and the ee value is more than 99 percent; (4), the used organic solvent can be recycled, and no special and toxic reagents are used.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
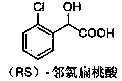
Method for preparing ortho-chloromandelic acid
InactiveCN1686998AReduce usageReduce manufacturing costOrganic compound preparationCarboxylic compound preparationEthyl acetateMandelic acid
The present invention relates to an improved preparation method of ortho-chloro-mandelic acid. Said method uses ortho-chlorobenzaldehyde and chloroform as raw material, and adopts the following steps: under the action of concentrated alkali and phase-transfer catalyst making the raw materials fully react, using ethyl acetate to make extraction, then removing ethyl acetate, adding alkali and water, using toluene to extract and remove impurities from aqueous solution of sodium ortho-chloromandelate, making acidification, using ethyl acetate to make extraction and using toluene to make crystallization, so that adopting one-kettle process to prepare ortho-chloromandelic acid.
Owner:EAST CHINA UNIV OF SCI & TECH
Separation extraction method for (R)-2-chloromandelic acid
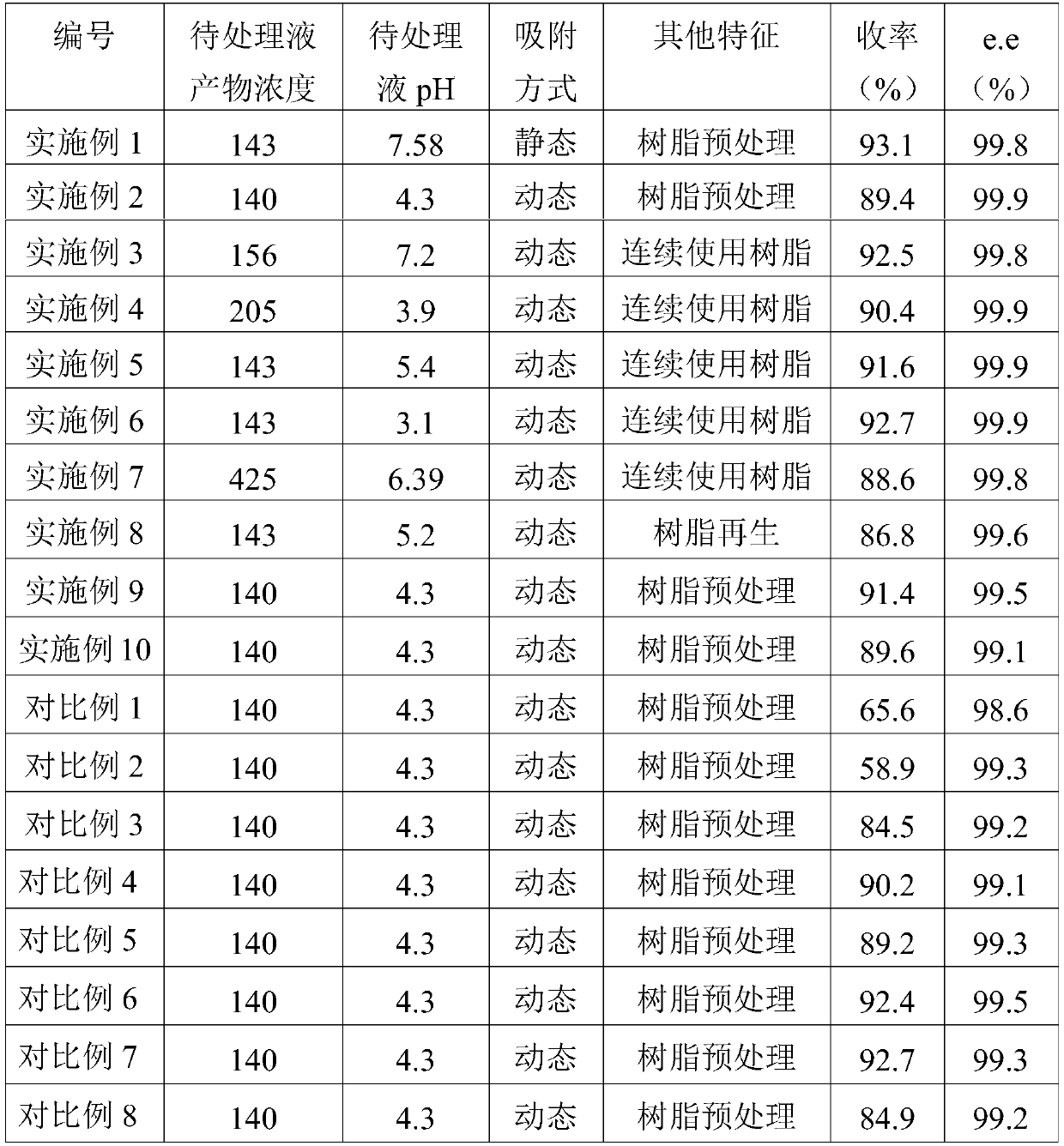
ActiveCN110172021AEasy to separateExtended service lifeCarboxylic compound separation/purificationAcid washingWastewater
The invention relates to an extraction method for a chiral medical intermediate, in particular to a separation extraction method for (R)-2-chloromandelic acid. The method at least comprises the following steps that strongly-alkaline anion resin is pretreated, wherein pretreatment comprises the steps of alkaline washing and acid washing in sequence, and quaternary ammonium salt strongly-alkaline anion resin is preferably selected as the strongly-alkaline anion resin; the treated strongly-alkaline anion resin is adopted for adsorbing a to-be-treated solution of the (R)-2-chloromandelic acid; anelution agent is adopted for elution, an eluent is obtained, extracted and concentrated, and the (R)-2-chloromandelic acid is obtained. The separation extraction method has a good separation effect and is high in yield, the adopted strongly-alkaline anion resin is long in service life, the separated water-phase system can continue to provide the reaction system for the next biological catalysis, the production cost is reduced, and discharge of waste water is reduced.
Owner:WUHAN WUYAO PHARMA
A preparation method of clopidogrel hydrogen sulfate type II
The invention discloses a preparation method of clopidogrel hydrogen sulfate type II. According to the method, clopidogrel hydrogen sulfate type II is prepared by taking clopidogrel free alkali as a raw material, and a preparation method of clopidogrel free alkali comprises the following steps: (1) preparing a reaction mixed solution of R-chloromandelic acid methyl ester by the reaction of R-chloromandelic acid and methanol in an organic solvent and in the presence of a catalyst; (2) mixing the reaction mixed solution of R-chloromandelic acid methyl ester with an organic base and a catalyst, reacting in the presence of benzenesulfonyl chloride to obtain a reaction mixed solution of methyl 2-benzenesulfonyl-2-chlorophenylacetate; (3) mixing the reaction mixed solution of methyl 2-benzenesulfonyl-2-chlorophenylacetate obtained in the step (2) with 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine hydrochloride and potassium carbonate for reaction to obtain the clopidogrel free alkali. According to the method, the solvent does not need to be supplemented in the last two steps, the solvent is directly used, and the reaction liquid concentration time is saved.
Owner:WUHAN WUYAO PHARMA
Preparation method of optically pure chiral 2-chloromandelic acid
InactiveCN102336653AQuality improvementReduce pollutionOptically-active compound separationCarboxylic compound separation/purificationOrganic solventFiltration
The invention provides a preparation method of optically pure 2-chloromandelic acid, which comprises the following steps: A) adding a chiral resolving agent, 2-chloromandelic acid and metallic compound into a solvent, dissolving by heating, cooling while stirring, carrying out vacuum filtration after a solid precipitates, drying and collecting the solid, wherein the mol ratio of chiral resolving agent to 2-chloromandelic acid is 1:4-2:1, and the mol ratio of metallic ion to 2-chloromandelic acid in the metallic compound is 1:4-2:1; and B) after recrystallizing the solid obtained in the step A), regulating the pH value to 1-2 with an acid solution, carrying out vacuum filtration to recycle the undissolved substance, extracting the mother solution with an organic solvent, drying, and carrying out spin drying to obtain the optically pure 2-chloromandelic acid product. The technique is simple and convenient, the raw materials and the resolving agent are innoxious and accessible, and the resolving agent can be recycled, thereby lowering the preparation cost and being green and economical. Meanwhile, the method is beneficial to implementing industrial mass production, and can satisfy the demands of people.
Owner:广州市金匮贸易有限公司
Biological synthesis method of (R)-o-chloromandelic acid
InactiveCN106676140ASimple process routeMild reaction conditionsMicroorganism based processesFermentationSynthesis methodsCombined use
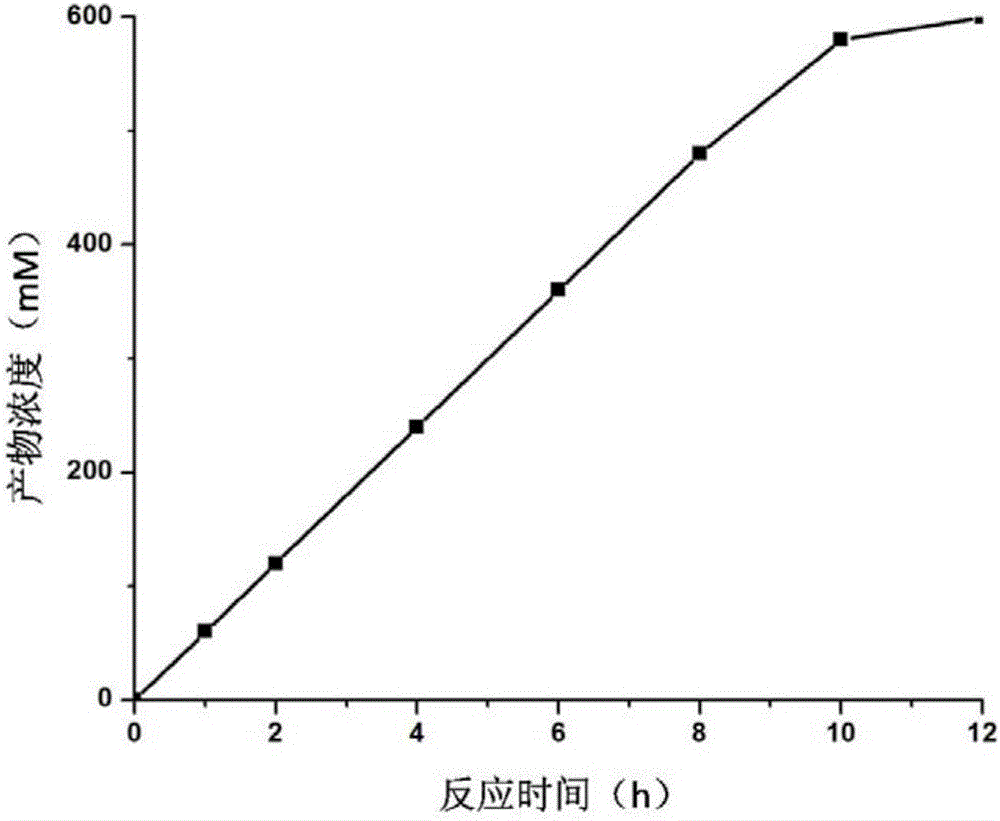
The invention provides a biological synthesis method of (R)-o-chloromandelic acid. The biological synthesis method comprises the following steps: feeding o-chloromandelic nitrile into a converting solution, conducting reaction for 8-12h under the conditions that the pH is 7.0-9.0 and the temperature is 20-40 DEG C, stopping feeding, and continuing conducting reaction for 1-4h to obtain the (R)-o-chloromandelic acid, wherein the converting solution comprises MG nitrilase-containing E.coli engineering bacteria. The method, combining use of the MG nitrilase and feeding of the o-chloromandelic nitrile, is used for preparing the (R)-o-chloromandelic acid, so that the process route is simple, reaction conditions are mild, pollution is free, the accumulated concentration of the (R)-o-chloromandelic acid during a single-batch reaction is as high as 600mM, and the ee value is greater than 98%; therefore, the biological synthesis method has a good industrial application prospect.
Owner:枣庄市杰诺生物酶有限公司 +1
Resolution method for 2-chloromandelic acid by crystalizing diastereomeric salts
InactiveCN102603518AThe synthesis method is simpleLow costOptically-active compound separationCarboxylic compound separation/purificationPharmaceutical drugCombinatorial chemistry
The invention belongs to the technical field of medicinal chemistry, and discloses a diastereomeric resolution method of 2-chloromandelic acid. According to the method, 2-chloromandelic acid with a single configuration is prepared by use of a chiral amino alcohol as a resolving agent. The ee (enantiomeric excess) of prepared 2-chloromandelic acid with the single configuration is more than 99%, the yield is not less than 75%, the synthetic method is simple, and the cost is low, so that the method is suitable for industrial production.
Owner:ZHENGZHOU UNIV
Carbonyl reductase, gene and mutant and application thereof to asymmetrical reduced carbonyl compound
ActiveCN102618513BHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationEthyl butyrate
The invention discloses a novel carbonyl reductase, a gene, a mutant thereof, a recombinant expression vector containing the gene and the mutant, a recombinant expression transformant, a recombinase preparation method, and applications of the carbonyl reductase and recombinase to preparation of active chiral alcohols with a chiral carbonyl compound before asymmetrical reduction. The carbonyl reductase is derived from candida glabrata, is applied to preparation of a plurality of optically-active chiral alcohols such as (R)-chloromandelic acid methyl ester, (R)-2-hydroxy-4-phenyl ethyl butyrate, (R)-4-chlorin-3-phenyl ethyl butyrate and the like. Compared with other preparation methods, a product prepared through the method has high concentration, does not require additionally or slightly adding any expensive coenzyme, has high optical purity, and has the advantages of mild reaction conditions, easiness and convenience for operating, easiness for amplifying and the like, and has a good industrial application prospect in the production of clopidogrel, L-carnitine and perindopril antihypertensive medicinal intermediates.
Owner:EAST CHINA UNIV OF SCI & TECH
Method used for separating and measuring 2-chloromandelic acid enantiomers via HPLC
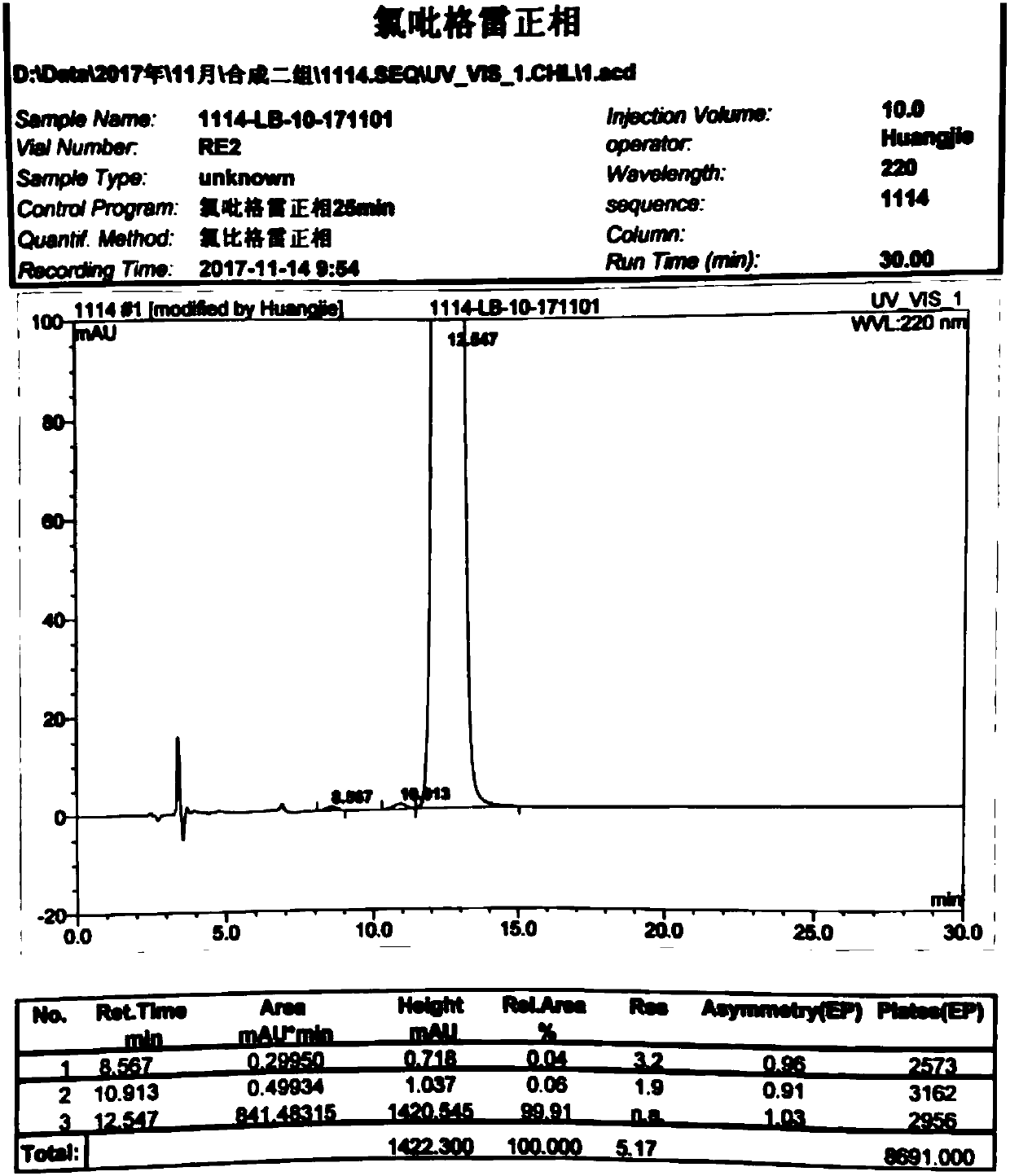
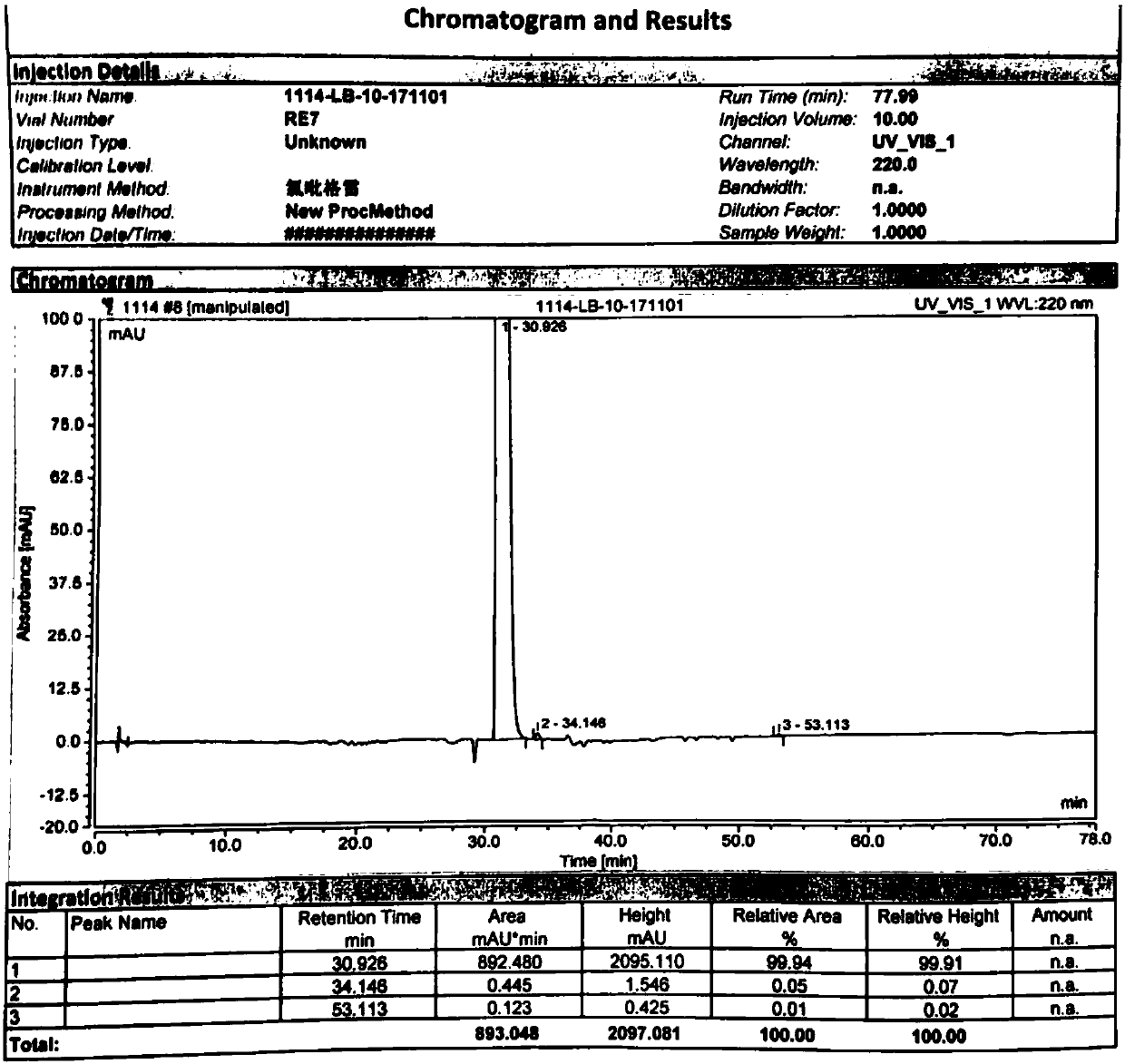
The invention discloses a method used for separating and measuring 2-chloromandelic acid enantiomers via HPLC. The method comprises following steps: R-2-chloromandelic acid samples are subjected to separating measuring via HPLC, wherein in separating measuring, cellulose-tri-4-methyl benzoate_chiral chromatographic column is taken as a stationary phase chiral chromatographic column, a mixed solution of alkanes and low alcohols is taken as a mobile phase for isocratic elution. The method is capable of realizing separating and measuring 2-chloromandelic acid enantiomers simply, rapidly, and accurately.
Owner:WUHAN WUYAO PHARMA
A kind of nitrilase and its gene and application
InactiveCN102533705BHigh optical purityHigh specific vitalityHydrolasesMicroorganism based processesMicroorganismMandelonitrile
The invention discloses a new nitrilase and gene thereof, a recombinant expression vector and a recombinant expression transformant containing the gene, a method for preparing the recombinant nitrilase or microbial cell containing the recombinant nitrilase by use of the recombinant expression transformant, and application of the microbial cell in dehydrating ortho-chlorine mandelonitrile or other analogues and producing chiral ortho-chlorine mandelonitrile or other analogues. The recombinant nitrilase disclosed by the invention comes from Labrenzia aggregate and can be used as a catalyst for dehydrating and splitting ortho-chlorine mandelonitrile or other analogues; and the recombinant nitrilase has the advantages of high catalysis efficiency, strong enantioselectivity, mild reaction conditions, environmental friendliness and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method for R-ortho-chloromandelic acid and acyl compound thereof
InactiveCN104357531AGuaranteed cheap and easy to getOvercoming the problem of low yieldFermentationKinetic resolutionHydrolysis
The invention discloses a preparation method for an R-ortho-chloromandelic acid and an acyl compound thereof. According to the preparation method, racemic chlomandelic acid is adopted as the raw material, lipase is adopted as a biological separation catalyst, acidic resin is adopted as a racemic catalyst, p-chlorobenzenethiol phenolic ester is adopted as the acyl donor to carry out the dynamic kinetic resolution to obtain the acyl compound of the R-ortho-chloromandelic acid; LiOH is adopted for hydrolysis to obtain the R-ortho-chloromandelic acid. The preparation method is moderate in reaction condition, environment-friendly, high in product yield and high in selectivity, the adopted racemic catalyst is low in cost and easy to obtain, so that the preparation method has quite high application value in industrial production.
Owner:王同俊
Preparation method of R-o-chloromandelic acid and alcohol ester thereof
InactiveCN102660625ALow costSimple processMicroorganism based processesFermentationEscherichia coliCarbonyl Reductase
The invention discloses a preparation method of R-o-chloromandelic acid and alcohol ester thereof. The method comprises the step that through inductively expressing recombinant S-mandelate dehydrogenase, carbonyl reductase and glucose dehydrogenase, using racemic o-chloromandelic acid or the alcohol ester thereof and glucose as substrates and conducting a sequential catalytic reaction, the R-o-chloromandelic acid and the alcohol ester thereof are obtained. Adopted genetic engineering bacteria are escherichia coli, bacillus subtilis, aeruginosa and saccharomyces cerevisiae. The method is an environmentally-friendly and economic preparation method of a single optcial antimer of the R-o-chloromandelic acid, has the advantages of short reaction time, low energy consumption and high yield and is easy to operate; and the yield of the R-o-chloromandelic acid (or the alcohol ester thereof) is larger than 86 percent, and the enantiomeric excess (ee) value of the R-o-chloromandelic acid (or the alcohol ester thereof) is above 95 percent. By using the preparation method, the racemic o-chloromandelic acid which has a low price and is easy to obtain is biologically catalyzed to the R-o-chloromandelic with important industrial value, so that the preparation method requires a low cost, is green and environmentally friendly, needs a simplified process and is suitable for industrial production.
Owner:ZHENGZHOU TUOXIN INST OF BIOLOGY
Method for preparing R-o-chloromandelic acid by using nitrilase engineering bacteria
ActiveCN106854673AImprove the level of research and developmentReduce manufacturing costHydrolasesGenetic engineeringHydrolase GeneNucleotide
The invention relates to the field of biotechnology and especially relates to a method for preparing R-o-chloromandelic acid by using nitrilase engineering bacteria. The method comprises carrying out centrifugation on a culture liquid obtained by fermental cultivation of engineering bacteria containing recombinant nitrilase genes, carrying out a conversion reaction process on o-chloromandelonitrile as a substrate and a buffer solution as a reaction medium in the presence of wet bacteria as catalysts, and after the reaction, carrying out separation purification on the reaction product mixed liquid to obtain R-o-chloromandelic acid, wherein the engineering bacteria containing recombinant nitrilase genes are constructed by nucleotide sequence coding genes shown in the formula SEQ ID NO. 2. The method has a fast reaction rate and a high reaction conversion rate.
Owner:枣庄市杰诺生物酶有限公司 +1
Preparation method of (R)-chloromandelic acid
PendingCN110184308AIncrease profitIncrease production levelsFermentationEscherichia coliOrganic chemistry
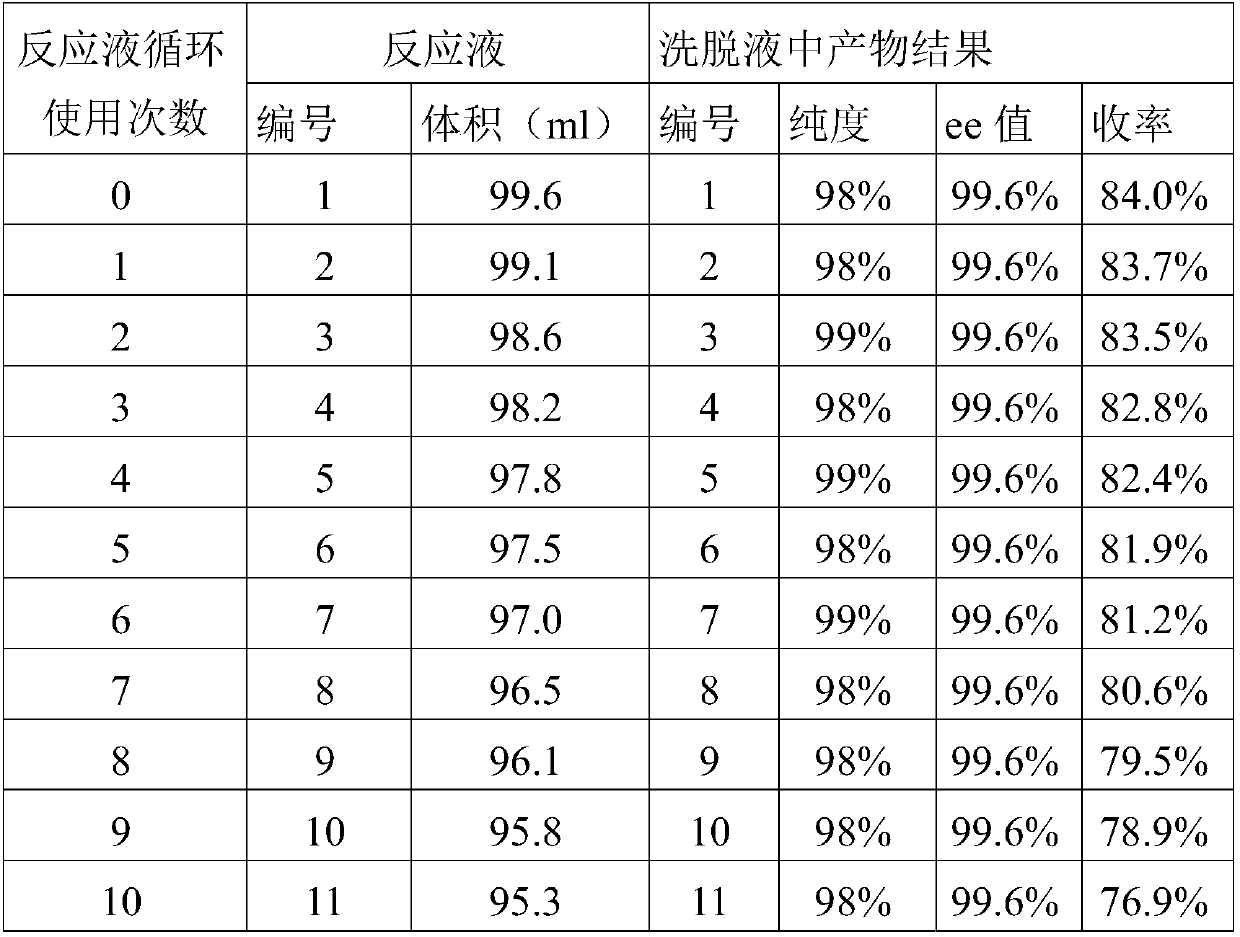
The invention belongs to the field of pharmaceutical chemistry and particularly relates to a preparation method of (R)-chloromandelic acid. The preparation method at least comprises the following steps: culturing engineered escherichia coli expressing nitrilase, taking the engineered escherichia coli as a catalyst, chloromandelonitrile as a substrate and a reaction buffer as a reaction medium forreaction, separating and collecting supernate after the reaction is finished, obtaining flow-through liquid after extracting (R)-chloromandelic acid from the supernate, and recycling the flow-throughliquid as a reaction buffer for at least one time. According to the preparation method, a utilization ratio of the reaction buffer is increased, so that the production cost of (R)-chloromandelic acidis lowered; the emission of waste liquid is significantly reduced; and green and environment-friendly production of (R)-chloromandelic acid is achieved.
Owner:WUHAN WUYAO PHARMA
Preparing method of (R)-(-)-4-chloromandelic acid
InactiveCN105085234AEasy to recycleEasy to operateOrganic compound preparationOptically-active compound separationSolubilityAlcohol
The invention discloses a preparing method of (R)-(-)-4-chloromandelic acid through resolution by taking (R)-(+)-1-(1-naphthyl)ethylamine as a chiral resolution agent. 4-chloromandelic acid and the chiral resolution agent (R)-(+)-1-(1-naphthyl)ethylamine react in a suitable alcohol solvent to generate (R)-(+)-1-(1-naphthyl)ethylamine salt of the 4-chloromandelic acid, by using different solubilities of an enantiomer salt, crystallizing and suction filtration are performed to obtain the (R)-(+)-1-(1-naphthyl)ethylamine salt of the (R)-(-)-4-chloromandelic acid, and after salt recrystallization, the (R)-(-)-4-chloromandelic acid can be obtained by acidizing; after the solution containing the (R)-(+)-1-(1-naphthyl)ethylamine is combined, alkalization treatment is performed, the resolution agent (R)-(+)-1-(1-naphthyl)ethylamine can be recycled. The preparing method has the characteristics of mild conditions, simple operation, good product yield and high optical purity. The resolution agent can be recycled for use, and is extremely suitable for industrial production of (R)-(-)-4-chloromandelic acid.
Owner:彭静
Method for resolving 2-chloromandelic acid enantiomer by enzymatic interesterification kinetics
InactiveCN109868293AImprove thermal stabilityHigh catalytic efficiencyFermentationExchange kineticsChloromandelic Acid
This patent describes a method for resolving 2-chloromandelic acid enantiomer by biocatalyst catalytic interesterification kinetics. Racemic 2-chloromandelic acid is resolved in an organic solvent medium by catalytic interesterification kinetics by utilizing high-catalytic efficiency and high-stereoselectivity lipase so as to obtain (S)-2-chloromandelic acid and (R)-acetyl-2-chloromandelic acid. The reaction system adopts an organic solvent as a reaction medium, greatly improves the thermal stability and the catalytic efficiency of the lipase, and greatly improves the substrate conversion rateand the optical activity, and the optical activity of the substrate is 98.15% or above. Compared with other resolving technologies, the method has the advantages of mild reaction conditions, simple operation and little environmental pollution, and can obtain the (S)-2-chloromandelic acid and (R)-acetyl-2-chloromandelic acid with high optical purity, and the (R)-acetyl-2-chloromandelic acid is used as a precursor of a key intermediate for synthesizing a chiral resolving agent and clopidogrel with high application prospect. The patent provides a feasible obtaining method.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
New preparation method of lanoconazole
Methanol and concentrated sulphuric acid are taken as solvent and 2-chloric mandelic acid is taken as raw material for esterification reaction, sodium hydroxide is added to be neutral after full reaction, reducing reaction is carried out with sodium borohydride, diluted hydrochloric acid is used for neutralization and water is added for dilution, distillation and extraction by 1, 2-dichloroethane are carried out to obtain 1-2-(2-chlorphenyl)-1, 2-ethylene glycol, then triethylamine and mesyl chloride are added into the 1-2-(2-chlorphenyl)-1, 2-ethylene glycol for esterification reaction, and then diluted hydrochloric acid, sodium bicarbonate and drinking water are respectively used for washing, reduced pressure distillation is carried out to remove solvent, so as to obtain sticky grease, ethyl acetate is added at the temperature below 77 DEG C for dissolution, heating is carried out until refluxing, temperature reduction, crystallization, centrifugation and drying are carried out, thus obtaining yellow solid 1-(2-chlorphenyl)-1, 2-glycol dimethyl sulphonate. Then dimethyl sulphoxide and potassium hydroxide are taken as raw materials, mixed liquid of 1-imidazoly acetonitrile, carbon disulphide and dimethyl sulphoxide is dropwise added for condensation reaction, mixed liquid of dimethyl sulphoxide and 1-(2-chlorphenyl)-1, 2-glycol dimethyl sulphonate is dropwise added for ring formation reaction, and ice analysis, extraction by ethyl acetate and reduce pressure distillation are carried out, thus obtaining E / Z-alpha-[4-(2-chlorphenyl)-1, 3-dithiolane-2-subunit]-1H-imidazolyl acetonitrile. The E / Z-alpha-[4-(2-chlorphenyl)-1, 3-dithiolane-2-subunit]-1H-imidazolyl acetonitrile is separated by silicagel column and then added with active carbon, distillation is carried out to remove most solvent, hot pressing filtering, temperature reduction, crystallization, centrifugation and drying are carried out, thus obtaining almost white or white lanoconazole powder solid.
Owner:傅军
A kind of nitrilase and application thereof
ActiveCN107245484BImprove the level of research and developmentReduce manufacturing costBacteriaHydrolasesReaction rateHydrolase
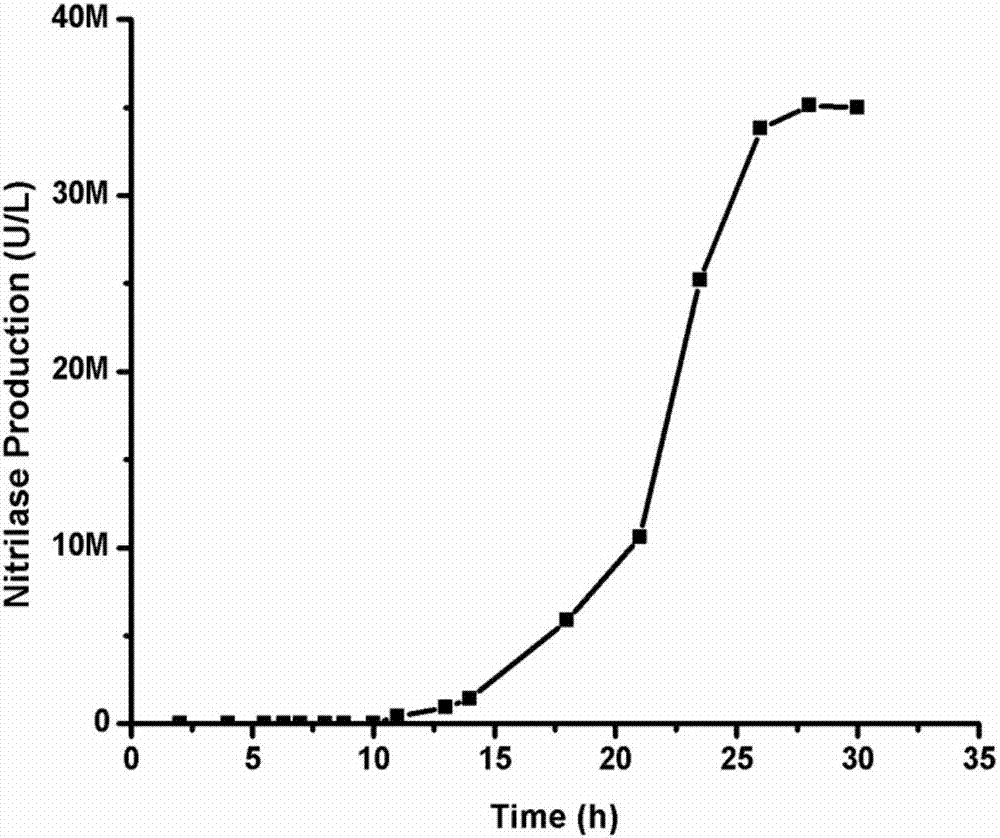
The present invention relates to the technical field of biology, particularly to a nitrilase and applications thereof, wherein the amino acid sequence of the nitrilase is represented by SEQ ID NO:1. According to the present invention, the nitrilase can completely hydrolyze 600 mM substrate within 12 h, and the 112 g / L product o-chloromandelic acid is produced due to the substrate flowing addition and the dilution effect of the pH value control, wherein the reaction conversion rate in the whole reaction process is remained at above 90%, the flowing addition is stopped after the reaction is performed for 10 h, and the substrate is completely converted after the reaction is continuously performed for 2 h; and the reaction rate is rapid, and the reaction conversion rate is high.
Owner:枣庄市杰诺生物酶有限公司
Preparation method of optically pure chiral 2-chloromandelic acid
InactiveCN102336653BQuality improvementReduce pollutionOptically-active compound separationCarboxylic compound separation/purificationOrganic solventFiltration
The invention provides a preparation method of optically pure 2-chloromandelic acid, which comprises the following steps: A) adding a chiral resolving agent, 2-chloromandelic acid and metallic compound into a solvent, dissolving by heating, cooling while stirring, carrying out vacuum filtration after a solid precipitates, drying and collecting the solid, wherein the mol ratio of chiral resolving agent to 2-chloromandelic acid is 1:4-2:1, and the mol ratio of metallic ion to 2-chloromandelic acid in the metallic compound is 1:4-2:1; and B) after recrystallizing the solid obtained in the step A), regulating the pH value to 1-2 with an acid solution, carrying out vacuum filtration to recycle the undissolved substance, extracting the mother solution with an organic solvent, drying, and carrying out spin drying to obtain the optically pure 2-chloromandelic acid product. The technique is simple and convenient, the raw materials and the resolving agent are innoxious and accessible, and the resolving agent can be recycled, thereby lowering the preparation cost and being green and economical. Meanwhile, the method is beneficial to implementing industrial mass production, and can satisfy the demands of people.
Owner:广州市金匮贸易有限公司
A method for separating and extracting (r)-o-chloromandelic acid
ActiveCN110172021BEasy to separateExtended service lifeCarboxylic compound separation/purificationPhysical chemistryOrganic chemistry
The present invention relates to a kind of extraction method of chiral medicine intermediate, relate to a kind of separation and extraction method of (R)-o-chloromandelic acid at least, comprise the following steps at least: carry out pretreatment with strong basic anion resin, pretreatment Processing comprises the step of carrying out alkali washing and pickling successively; Strong basic anion resin preferably quaternary ammonium salt strong basic anion resin; Adopt the strong basic anion resin after processing to carry out the to-be-treated solution of (R)-o-chloromandelic acid Adsorption; eluting with an eluent to obtain an eluent, which is extracted and concentrated to obtain (R)-o-chloromandelic acid. The separation and extraction method of the present invention has good separation effect and high yield, and the strong basic anion resin used has a long service life, and the separated aqueous phase system can continue to provide a reaction system for the next biocatalysis, reducing production costs and discharge of waste water.
Owner:WUHAN WUYAO PHARMA
High-efficient composite powder-shaped sewage treating agent for desizing waste water and preparation method thereof
InactiveCN106315874AGood shape distributionImprove stabilityWater contaminantsScale removal and water softeningChemical oxygen demandTherapeutic effect
The invention discloses a high-efficient composite powder-shaped sewage treating agent for desizing waste water and preparation method of the high-efficient composite powder-shaped sewage treating agent. The sewage treating agent comprises tartaric acid, ursodesoxycholic acid, 2- chloromandelic acid, calcium sulfate, magnesium carbonate, calcium carbonate, ferric trichloride, calcium oxide, sodium borate. The method is to orderly and evenly mix above matters. Compared with the prior art, the treating agent treats the desizing sewage containing 1-10 g / l PVA, wherein the COD (chemical oxygen demand) concentration of the desizing sewage reaches 5x104-10x104 mg / L, and chromaticity is 400 times; the removal rate reaches 80-85%, and recycle rate of PVA is 85-90%, and the chromaticity is 100 times below; the composite powder-shaped sewage treating agent is featured by wide adaptive temperature scale, good treatment effect, high recycle rate, and obvious decoloration.
Owner:WUHU YANGZHAN NEW MATERIAL TECH SERVICE CO LTD
Preparation method of lanoconazole
PendingCN114634492AAvoid overly lively questionsAvoid it happening againSulfonic acid esters preparationBorohydrideChloromandelic Acid
The invention relates to a preparation method of lanoconazole, and belongs to the technical field of medicine synthesis. The invention aims to solve the problems of unstable reaction and low yield in the prior art. The invention provides a preparation method of lanoconazole, which comprises the following steps: by taking 2-chloromandelic acid as a raw material, firstly carrying out esterification reaction and chlorination reaction to synthesize an intermediate compound as shown in a formula II, and further carrying out reduction reaction on the compound as shown in the formula II in the presence of hydroboron to obtain a compound as shown in a formula III; in the presence of an acid applying agent, reacting the compound shown in the formula III with methylsulfonyl chloride to obtain a compound shown in a formula IV; the preparation method comprises the following steps: adding 1-imidazolyl acetonitrile, carbon dioxide and a phase transfer catalyst into a mixed solution of an organic solvent and water, adding a strongly alkaline potassium salt for reaction to obtain a reaction solution, adding a weakly alkaline buffer system into the reaction solution, then adding an intermediate compound shown as a formula IV for cyclization reaction, and after the cyclization reaction is finished, concentrating to remove the solvent and recrystallizing to obtain the compound shown as the formula IV. The corresponding product lanoconazole is obtained. The method has the effects of mild and stable reaction and high yield.
Owner:浙江东亚药业股份有限公司
Method for synthesizing novel uric acid lowering compound Arhalofenate intermediate
InactiveCN109232237AHigh purityGood split effectOrganic compound preparationSkeletal disorderFiltrationResolution rate
The invention discloses a method for synthesizing a novel uric acid lowering compound Arhalofenate intermediate. The novel uric acid lowering compound Arhalofenate intermediate is (R)-chloromandelic acid, and is prepared by the following specific synthetic steps: taking a common organic solvent as a resolution solvent, taking racemic chloromandelic acid as a raw material, taking (R)-amine as a resolving agent, performing reflux preparation to obtain (R)-amine*(R)-chloromandelic acid, adding an acid to regulate the pH value, repeatedly extracting with ethyl acetate, merging the organic phase, adding anhydrous sodium sulfate for drying, performing suction filtration and desalting, and performing reduced pressure drying, thereby obtaining the (R)-chloromandelic acid with high optical purity.The method disclosed by the invention has the beneficial effects that the synthetic method is readily available in raw materials, excellent in separating speed and effect, high in resolution rate, lowin cost, simple and convenient in operation, low in requirements on production conditions and equipment and easy for industrial production.
Owner:TONGHUA NORMAL UNIV
Preparation method of chiral resolving agent and (r)-o-chloromandelic acid
ActiveCN103804179BHigh split efficiencyChemically stableOrganic compound preparationCarboxylic compound preparationOrganosolvCombinatorial chemistry
The invention discloses a new chiral resolving agent S-(1), which is used for chemical resolution of (RS)-o-chloromandelic acid to prepare (R)-o-chloromandelic acid. The invention also discloses a preparation method of (R)-o-chloromandelic acid, which comprises reacting the resolving agent S-(1) with (RS)-o-chloromandelic acid in a solvent to obtain (R)-o-chloromandelic acid • A resolving agent diastereomeric salt, decomposing the salt to give (R)-o-chloromandelic acid, and optionally recovering S-(1). The beneficial effects of the present invention: (1) The chiral resolving agent (S)-1 has high resolution efficiency, stable chemical properties, easy separation and recycling, and the recovery purity is greater than 99%. (2) The industrial route of the preparation method is mature, the process conditions are mild, and the industrial production is easy. (3) The percentage content of (R)-o-chloromandelic acid in the obtained product is greater than 99%, and the ee value is greater than 99%. (4) The organic solvents used can be recycled, and no special or toxic reagents are used.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
Preparation method of methyl (R)-o-chloromandelate utilizing biocatalytic asymmetric reduction
InactiveCN102206686BHigh catalytic efficiencyIncrease productivityBacteriaMicroorganism based processesChlorobenzeneMethyl o-chloromandelate
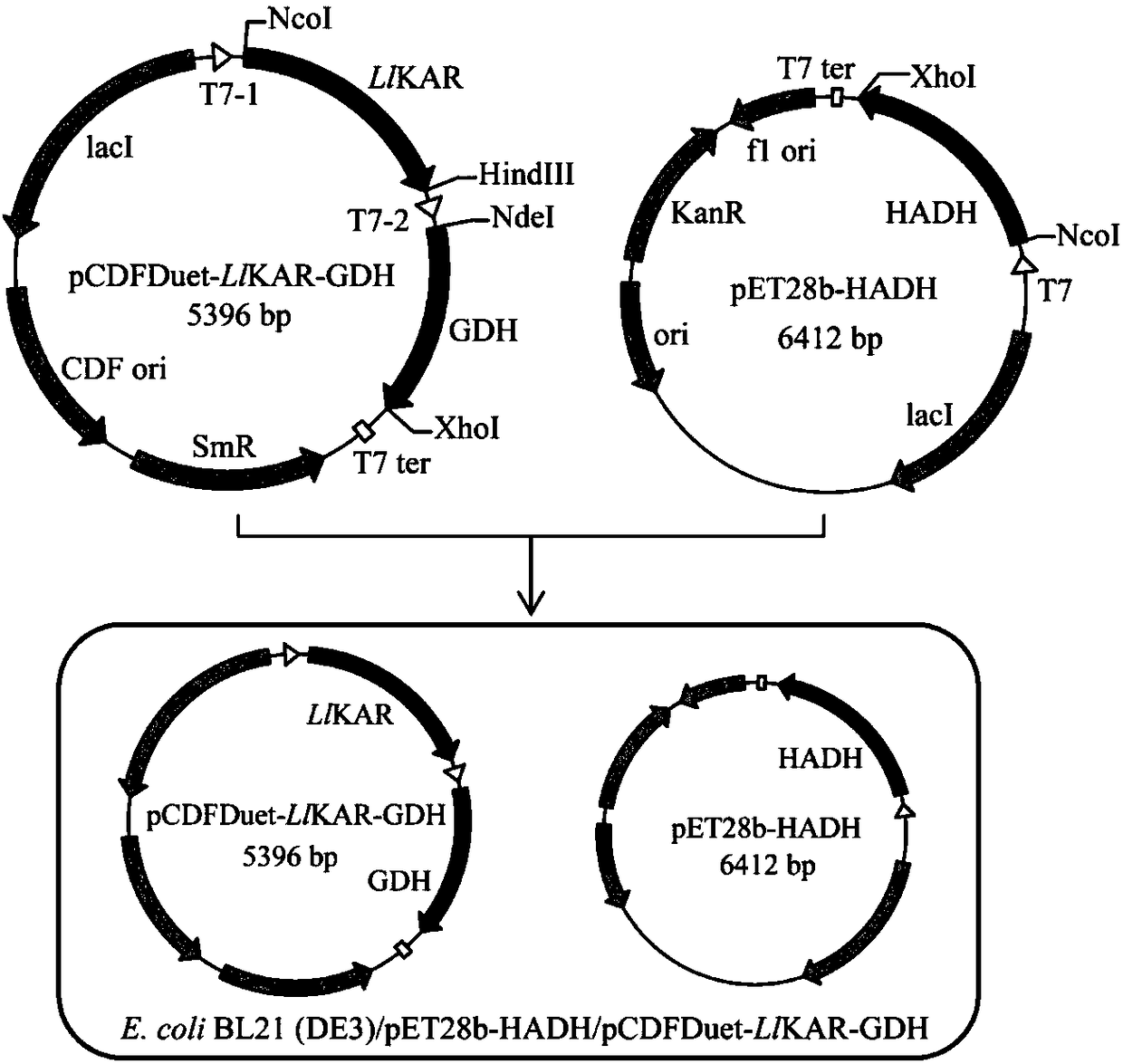
Disclosed is a method for preparing methyl(R)-o-chloromandelate, wherein a genetic engineering bacterium co-expressing a recombinant aldo-keto reductase and recombinant glucose dehydrogenase is used as a catalyst, and a methyl o-chlorobenzoylformate as a substrate to perform a biotransformation reaction in the presence of glucose. Also disclosed is a recombinant vector containing the base sequences coding the aldo-keto reductase and the glucose dehydrogenase, and the genetic engineering bacterium containing the vector and the use thereof.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com