Biological synthesis method of (R)-o-chloromandelic acid
A technology of o-chloromandelic acid and o-chloromandelonitrile, applied in the field of bioengineering, can solve the problems of high price of chiral resolving agent and low atom economy, achieve mild reaction conditions, no pollution in reaction conditions, and simple process route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
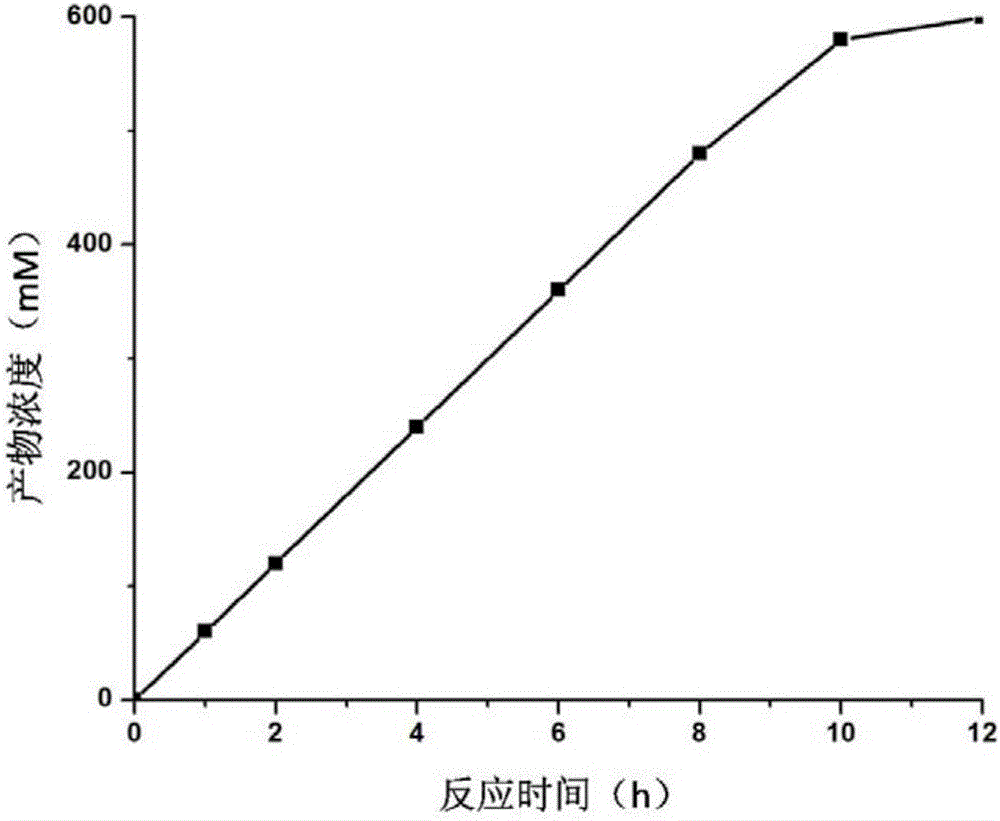
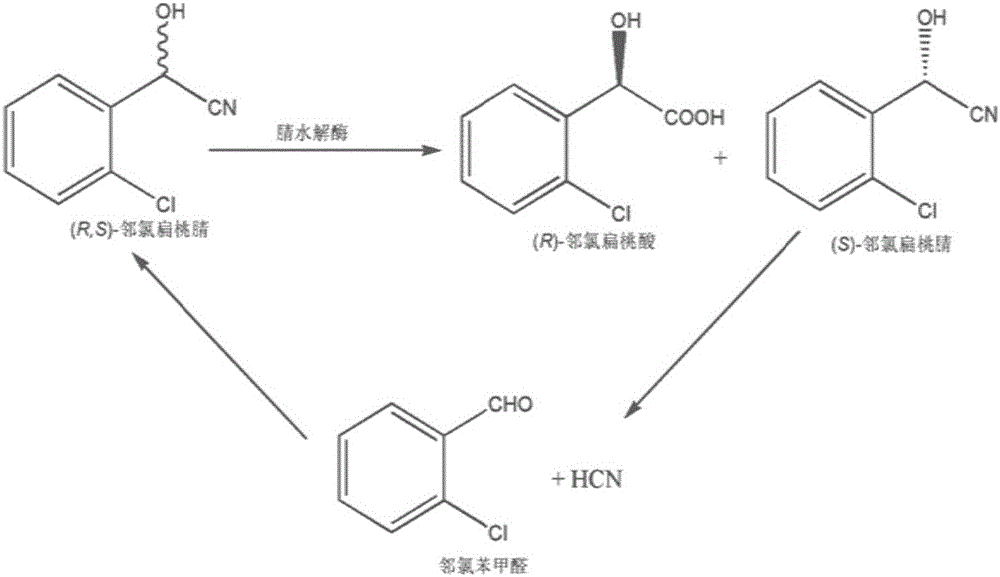
[0049] The present embodiment discloses a method for biosynthesizing (R)-o-chloromandelic acid, which comprises the following steps:
[0050] (1) Take out the E.coli engineered strains preserved at -80°C, inoculate them on LB agar plates, and then culture them overnight at 37°C;
[0051] (2), picking the monoclonal colony on the LB agar plate, and inoculating it in the LB liquid medium, then culturing overnight at 37° C. at 200 rpm;
[0052] (3) Take an appropriate amount of bacterial liquid from the LB liquid medium after overnight cultivation, inoculate it in the LB liquid medium, cultivate it at 37° C. at 200 rpm for 8 hours, then inoculate it in the fermentation medium (as shown in Table 1), and inoculate Cultured at 37℃ to OD 600 to 20, then add an appropriate amount of IPTG, lower the temperature to 30°C and continue to cultivate for 16 hours, collect the bacteria by centrifugation (take the precipitate), and use NaH with a pH of 8.0 2 PO 4 / Na 2 HPO 4 The buffer so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com