Patents
Literature
274results about "Iron cyanides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
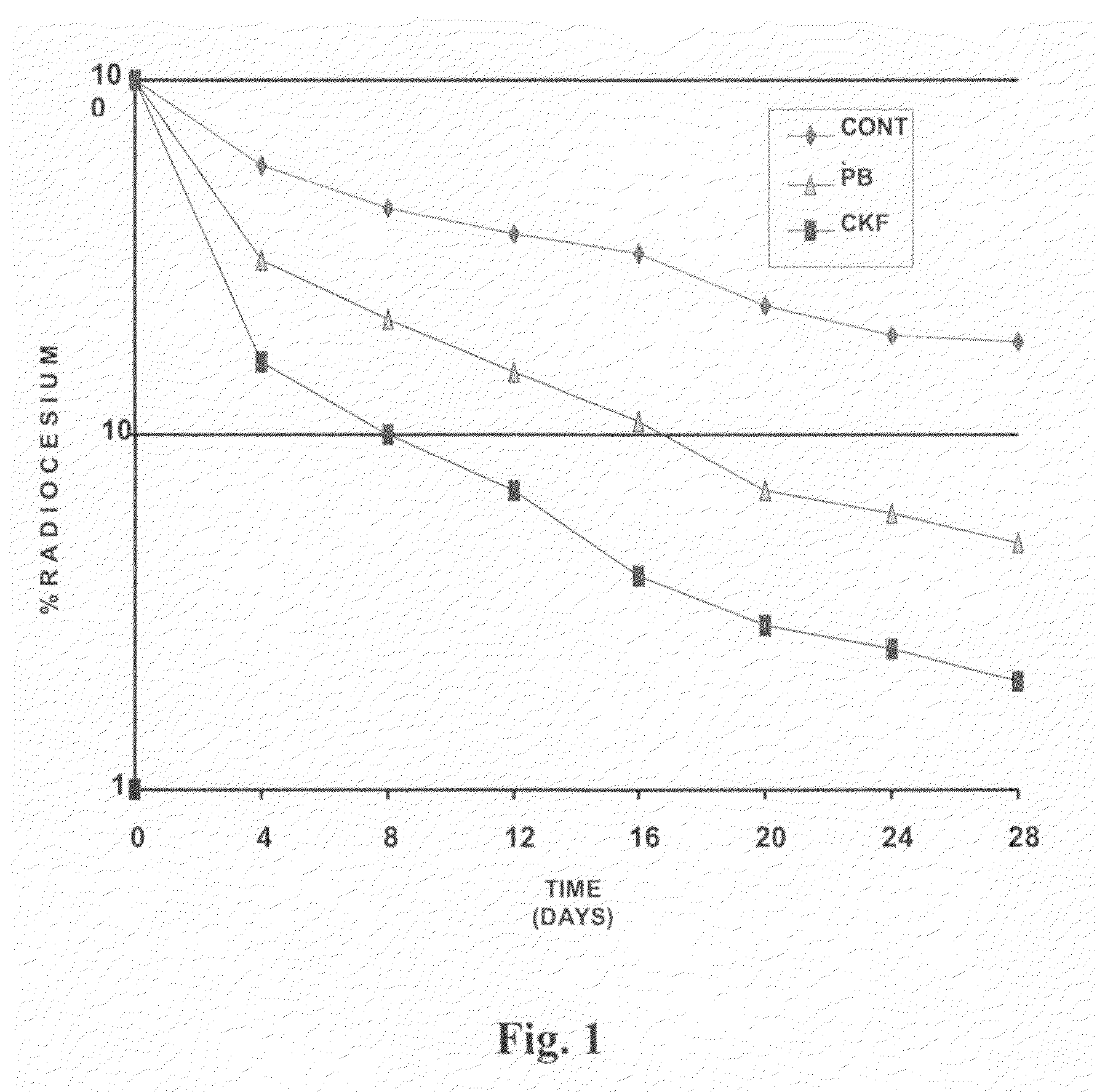
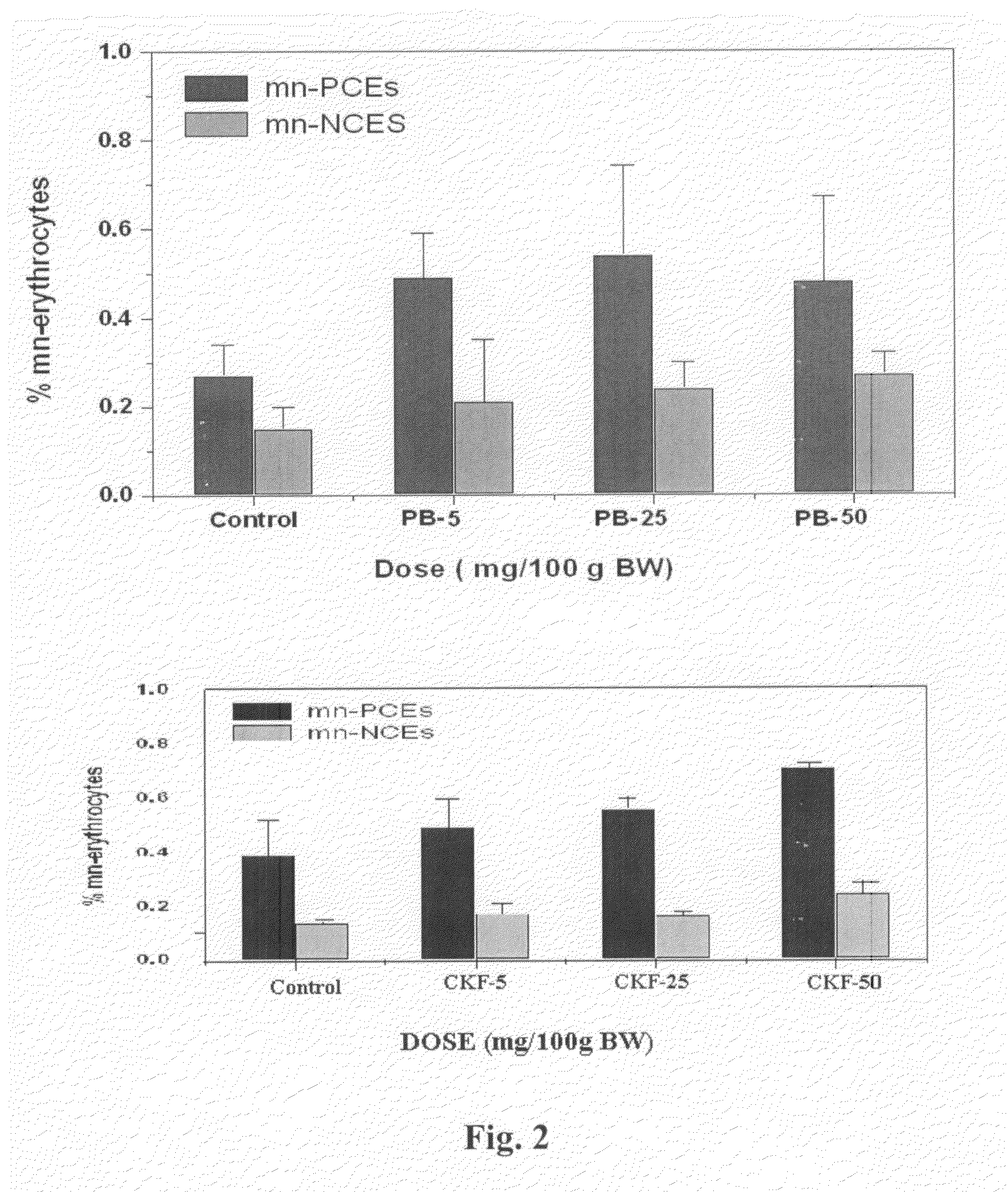
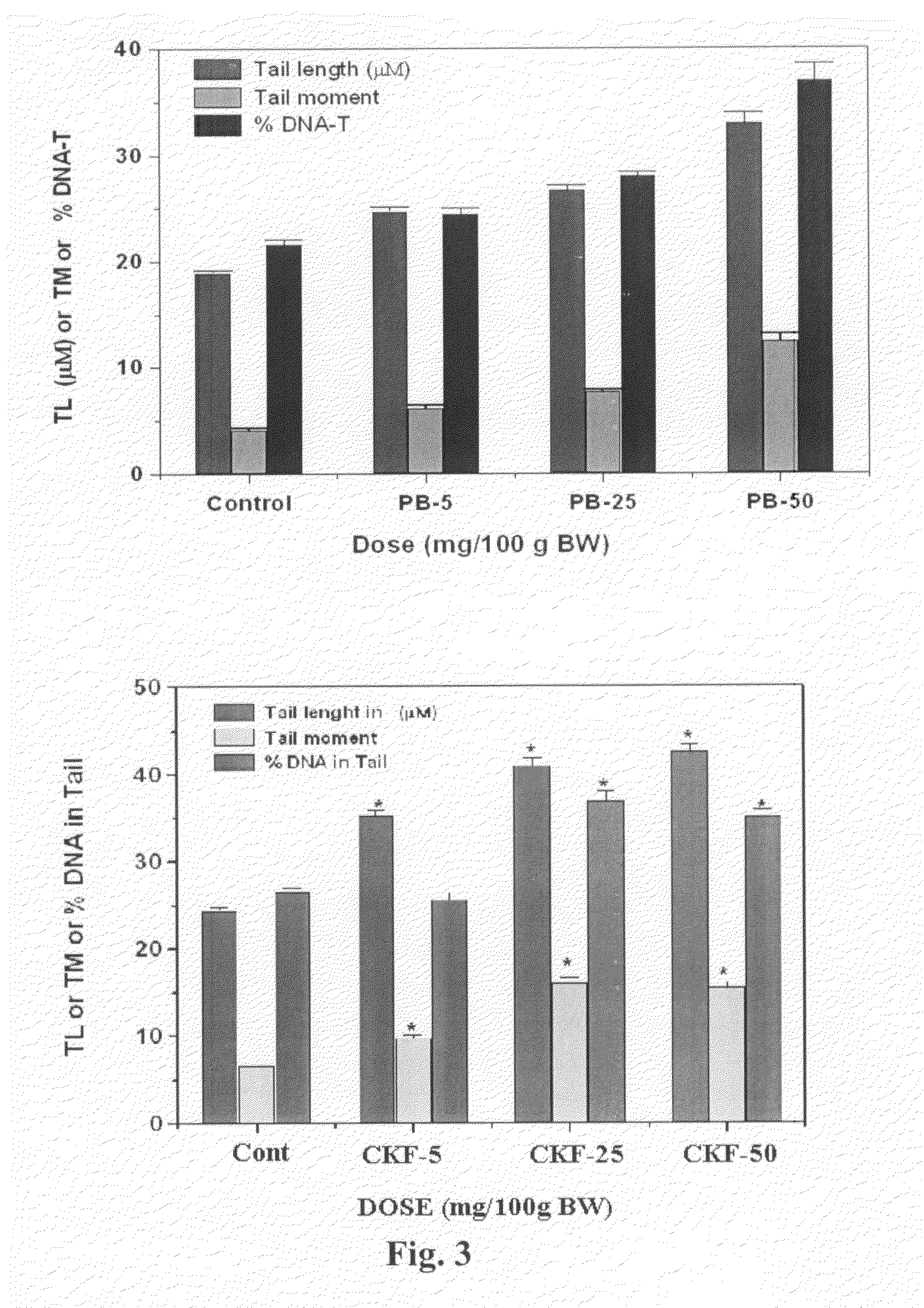
Calcium Potassium Ferrocyanide, a prophylactic mixture comprising this compound and the use thereof for decorporation of Radiocesium in subjects affected by nuclear radiation
InactiveUS7935366B2Eliminate needEasy to manageHeavy metal active ingredientsBiocideNuclear radiationMedicine
A new prophylactic mixture is prepared for the effective decorporation of *Cs, *Sr, *I from affected subjects in the event of an accidental release of the radioisotopes in the environment due to any nuclear accident. The prophylactic mixture comprising: 1) Calcium Potassium Ferrocyanide [CaK2Fe(CN)6] 2) Calcium Iodate, and 3) Calcium Carbonate, can be formulated in the form of a single tablet, capsule or suspension for easy administration to the radiation affected subjects in cases of emergency or nuclear fallout. Also claimed are the compound calcium potassium ferrocyanide (CaK2Fe(CN)6) and the use thereof for decorporation of *Cs in subjects affected by nuclear radiation.
Owner:SEC
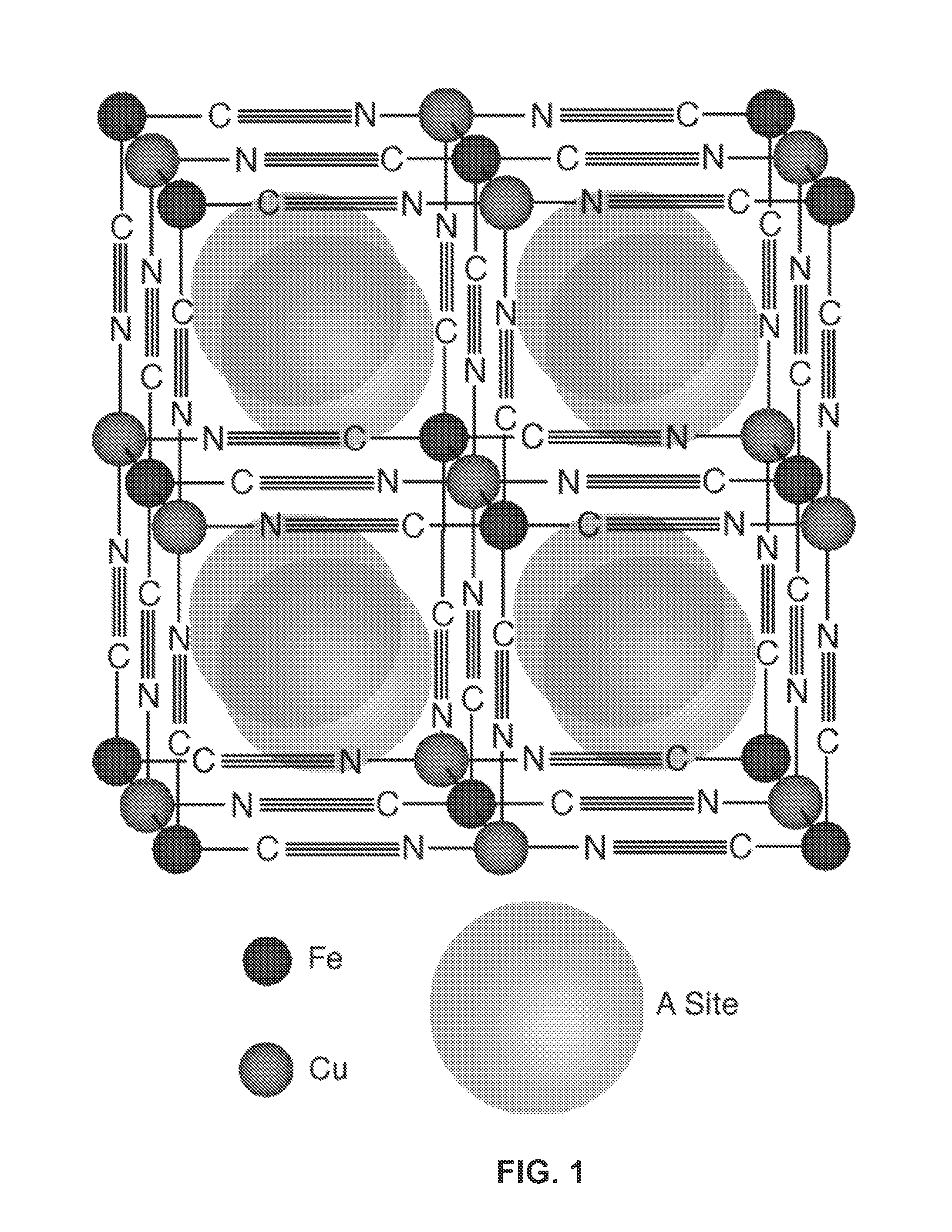
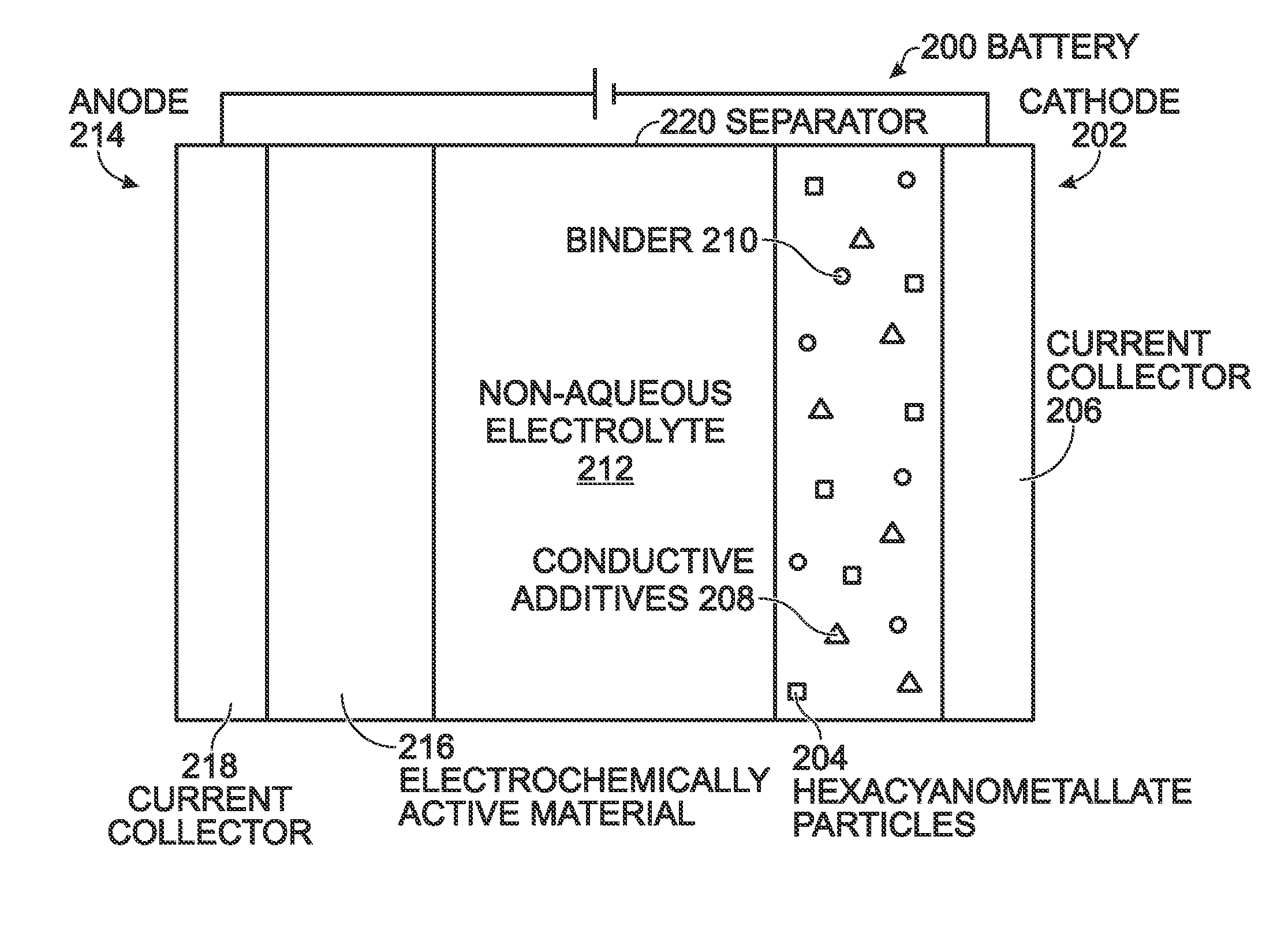
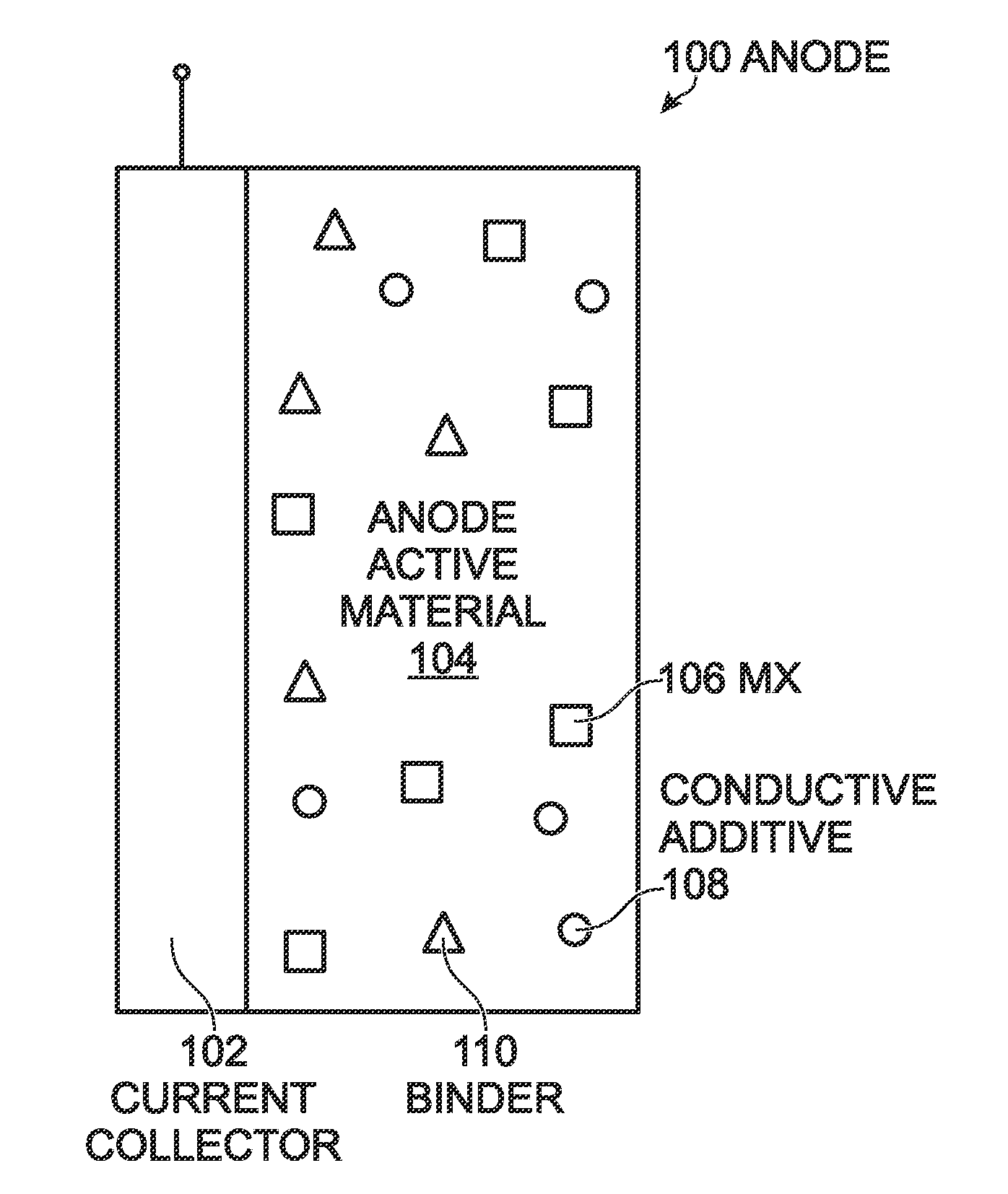
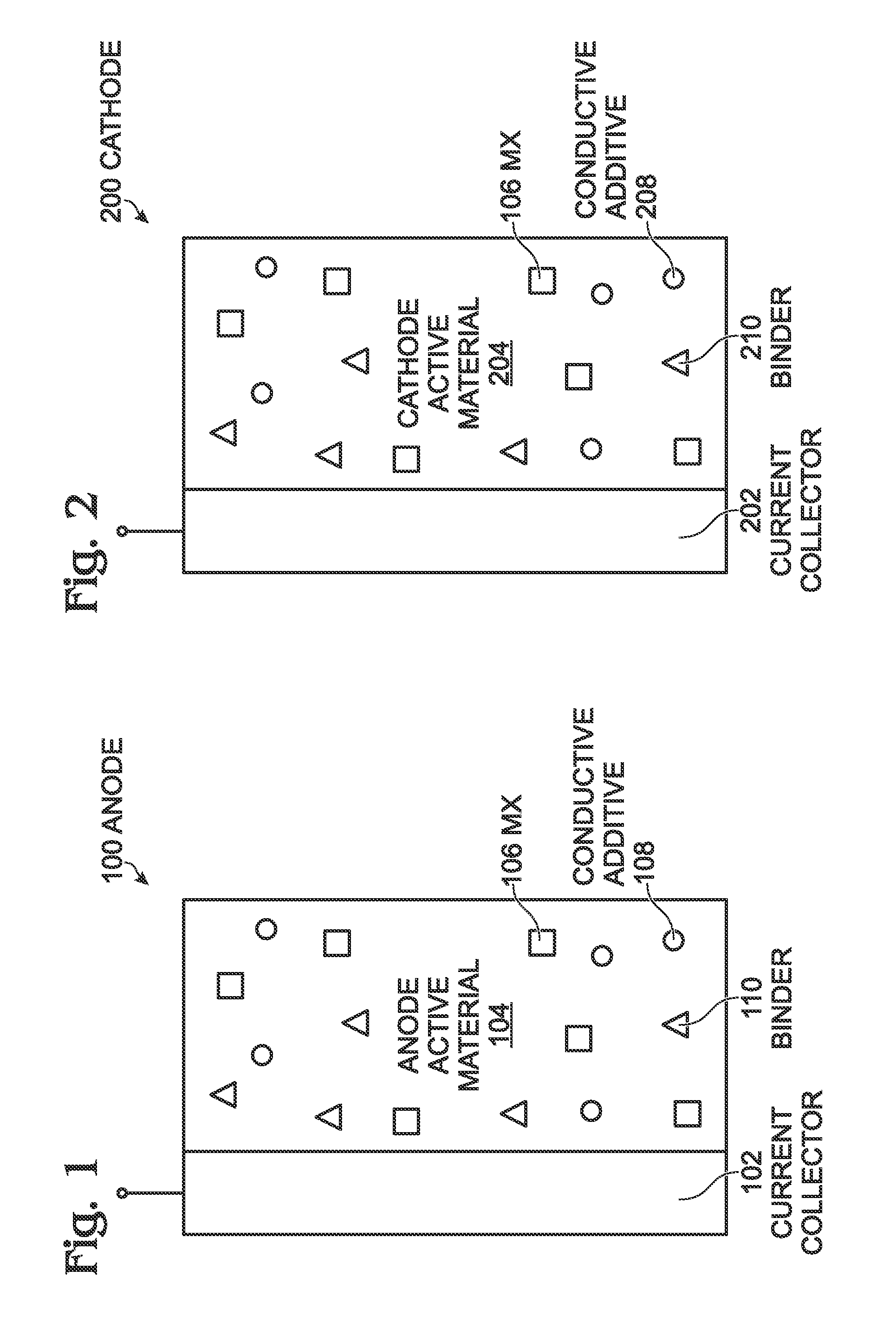
Prussian Blue Analogue Anodes for Aqueous Electrolyte Batteries
InactiveUS20140220392A1Maximizes energy storageReduces electrochemical decompositionIron cyanidesComplex cyanidesRedoxPrussian blue
A system and method producing electrodes in an aqueous electrolyte battery that maximizes energy storage, reduces electrochemical decomposition of the electrolyte, and uses Prussian Blue analogue materials for both electrodes, with an anode electrode including an electrochemically active hexacyanometalate group having two possible redox reactions of different potentials. These potentials may be tuned by substituting different electrochemically inactive components.
Owner:NATRON ENERGY INC
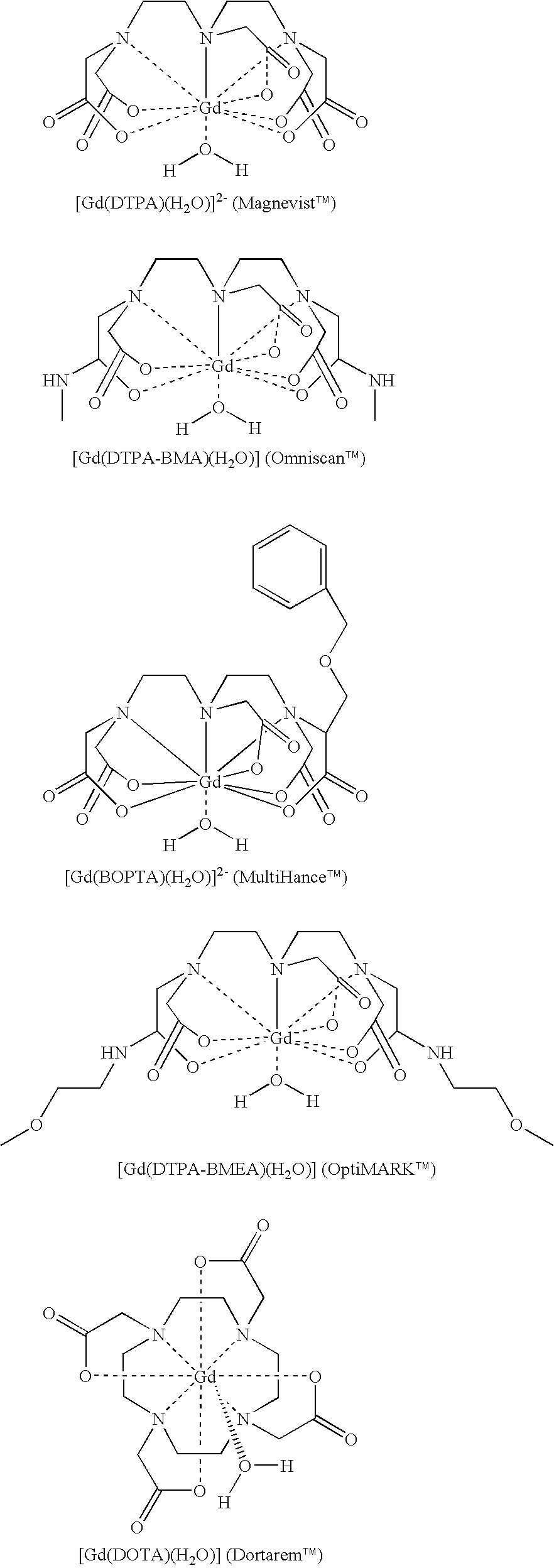
Gadolinium containing prussian blue nanoparticles as nontoxic MRI contrast agents having high relaxivity
InactiveUS20100254912A1Low leaching rateReduce solubilityMaterial nanotechnologyIn-vivo radioactive preparationsMRI contrast agentMedicine
Gadolinium+3 (Gd3+) containing (or incorporated) Prussian blue lattice contrast agents that can be used as an MRI contrast agent have unexpectedly improved r1 relaxivities of 1 or 2 magnitudes higher than the commercial Gd3+-chelates as well as exceedingly, non-toxic, low release of the Gd3+ ions into an aqueous environment at a pH of about 2 to about 7.5. The Prussian blue lattice containing Gd3+ ions therein can be used for clinical diagnosis intravenously to human beings for medical imaging. The particle sizes of the doped Prussian blue lattices are of a nanosize scale and are very stable against agglomeration.
Owner:KENT STATE UNIV
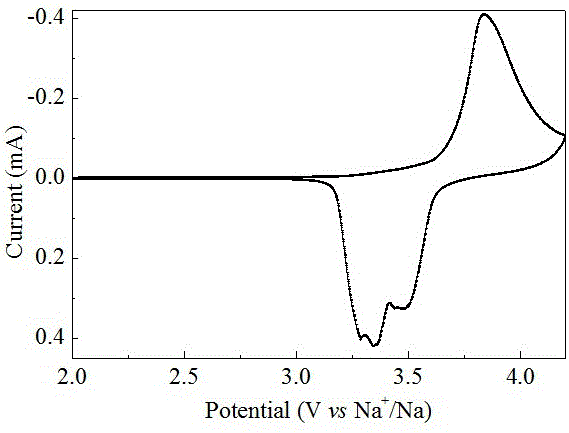
Prussian blue positive electrode material and sodium ion battery
InactiveCN107364874AImprove crystal structureFew defectsIron cyanidesCell electrodesSodium-ion batteryHigh pressure
The invention discloses a Prussian blue positive electrode material and a sodium ion battery. The preparation method comprises that the salt solution of M creeps to the mixed solution of Na4Fe(CN)6 and a sodium salt, the obtained solution is heated in a protective atmosphere to obtain a Prussian blue intermediate, the intermediate solution is transferred into a high-pressure reaction kettle, and a heating reaction is performed at a certain temperature to obtain the Prussian blue positive electrode material, or the intermediate solution is subjected to high-temperature spray drying to obtain the Prussian blue material powder. According to the present invention, through the high-temperature and normal pressure reaction and the high-pressure and high-pressure heating reaction, the prepared material has advantages of perfect crystal structure, less defect and low water content, and can improve the specific capacity and the stability of the battery in the application as the sodium ion battery positive electrode material; and the preparation method is simple, and is suitable for continuous large-scale production.
Owner:GLOBAL ENERGY INTERCONNECTION RES INST CO LTD +2
Positive electrode material for potassium ion battery and preparation method thereof, and potassium ion battery
ActiveCN107226475ARapid de-embeddingStable structureIron cyanidesCell electrodesHigh energyElectrical battery
The invention discloses a positive electrode material for a potassium ion battery and a preparation method thereof, and the potassium ion battery. The positive electrode material has a chemical formula of K<x>P[R(CN)<6>], wherein x is no less than 0 and no more than 2, P is a transition metal ion, and R is Fe<2+> or Fe<3+>. The preparation method comprises the following steps: dissolving potassium ferricyanide or potassium ferrocyanide and a transition metal salt to prepare a uniform solution; then carrying out a hydrothermal reaction; separating a precipitate produced in the hydrothermal reaction; and carrying out washing and vacuum drying so as to obtain the positive electrode material. The preparation method in the invention is simple in process, easy to operate, low in cost for required raw materials and suitable for large-scale industrial production. The prepared positive electrode material has an open three-dimensional network frame structure, and great interstitial sites allow potassium ions to shuttle and to be stored, so the potassium ion battery assembled from the positive electrode material has high discharge capacity, long cycle life, and high energy density and power density.
Owner:XI AN JIAOTONG UNIV
Potassium-sodium-manganese-iron-based Prussian-blue electrode material, and preparation method and application thereof
InactiveCN106549155AImproved magnification performanceImprove cycle stabilityMaterial nanotechnologyIron cyanidesNew energyManganese
The invention discloses a potassium-sodium-manganese-iron-based Prussian-blue electrode material, and a preparation method and application thereof, belonging to the technical field of synthesis of new-energy materials. According a technical scheme in the invention, the potassium-sodium-manganese-iron-based Prussian-blue electrode material has a general chemical formula of K<x>Na<y>MnFe(CN)<6>, wherein x and y are both more than 0 and less than 2; and the material has a cubic structure and a particle size of 10 to 200 nm. The invention also discloses the preparation method for the potassium-sodium-manganese-iron-based Prussian-blue electrode material and application of the material to the positive electrode of a sodium-ion battery. The potassium-sodium-manganese-iron-based Prussian-blue electrode material prepared in the invention has the cubic structure and a particle size of 10 to 200 nm; the nanometer three-dimensional structure of the material leads to increase in the specific surface area of the material and effective reduction in transfer distance of ions or electrons in the electrode material, so the rate performance and cycle stability of the electrode material are improved; and the electrode material has excellent electrochemical performance as a positive electrode material for the sodium-ion battery.
Owner:HENAN NORMAL UNIV
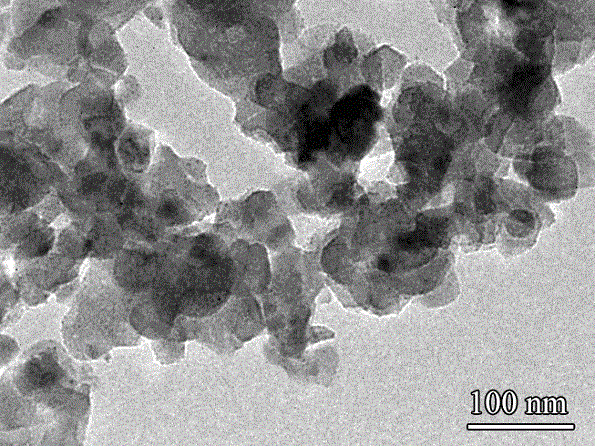
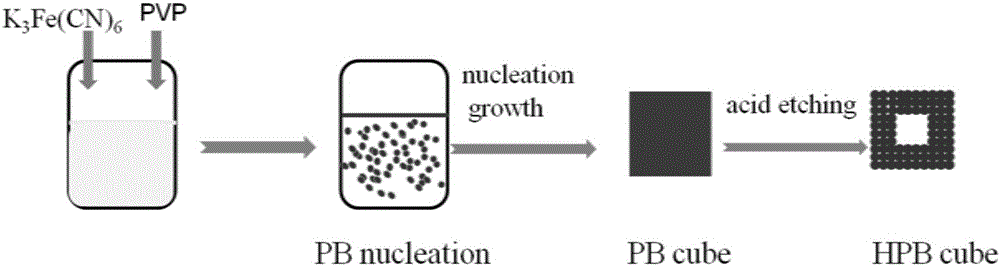
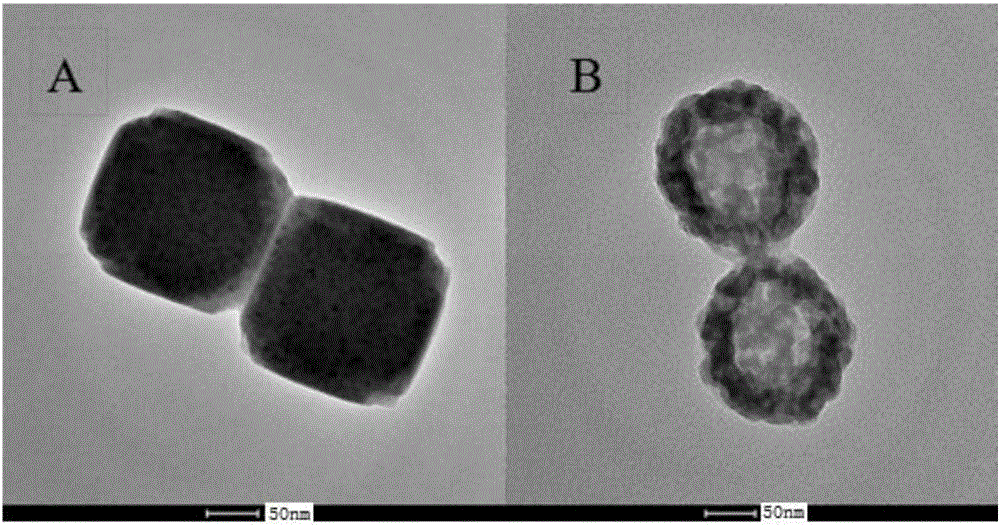
Preparation method and application of hollow Prussian-blue nanometer cube
InactiveCN105836762AHigh specific surface areaImprove catalytic performanceMaterial nanotechnologyIron cyanidesAcid etchingPotassium ferricyanide
The invention discloses a preparation method and application of a hollow Prussian-blue nanometer cube. The preparation method for the hollow Prussian-blue nanometer cube comprises the following steps: step 1, heating polyvinylpyrrolidone hydrochloric acid solution containing potassium ferricyanide and preparing a Prussian-blue nanometer cube of a solid structure by using a slow chemical aging method; and step 2, dispersing the Prussian-blue nanometer cube prepared in the step 1 and polyvinylpyrrolidone in a hydrochloric acid solution at the same time, carrying out a hydrothermal reaction and then carrying out centrifugation, washing and drying so as to obtain the hollow Prussian-blue nanometer cube. According to the invention, the uniform cubic morphology of the prepared Prussian-blue nanometer material is guaranteed by using the slow chemical aging method and controllable acid etching under hydro-thermal conditions is carried out, so Prussian-blue forms a unique hollow cubic morphology; and thus, catalytic activity of the material is improved. The prepared hollow Prussian-blue nanometer cube shows excellent electrochemical catalysis response in application to a hydrogen peroxide sensor.
Owner:NORTHWEST UNIV(CN)
Method for preparing Prussian blue positive electrode material, and sodium ion battery
InactiveCN107364875AAvoid hydrolysisHigh specific capacityIron cyanidesCell electrodesHigh sodiumSodium-ion battery
The invention discloses a method for preparing a Prussian blue positive electrode material, and a sodium ion battery, wherein the molecular formula of the Prussian blue positive electrode material is Na2-xMyFe(CN)6, x is more than o 0 and is less than 2, y is more than 0 and is less than 1, and M is a transition metal. The preparation method comprises: adding a Na4Fe(CN)6 solution into a solution 2 containing the salt solution of M, a sodium salt and a pH value adjusting agent in a dropwise manner, maintain the pH value of the mixed solution at 6-7, and heating for a certain time in a protection atmosphere to obtain the Prussian blue positive electrode material. According to the present invention, the method has characteristics of simple process, easy control and continuous and scale production, and can be used for preparing the high sodium content Prussian blue positive electrode material; and the prepared Prussian blue positive electrode material as the sodium ion battery positive electrode material can improve the specific capacity of the battery, can reduce the polarization in the battery charging and discharging process, and has wide application prospects in the field of electric energy storage in the power grid.
Owner:GLOBAL ENERGY INTERCONNECTION RES INST CO LTD +2
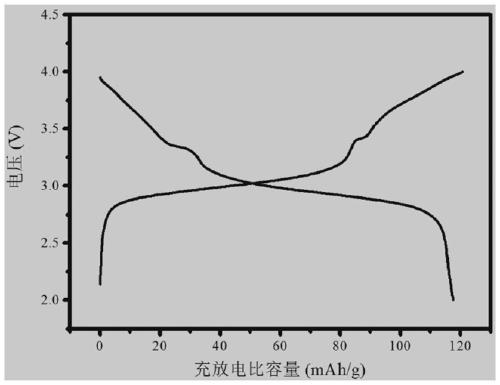
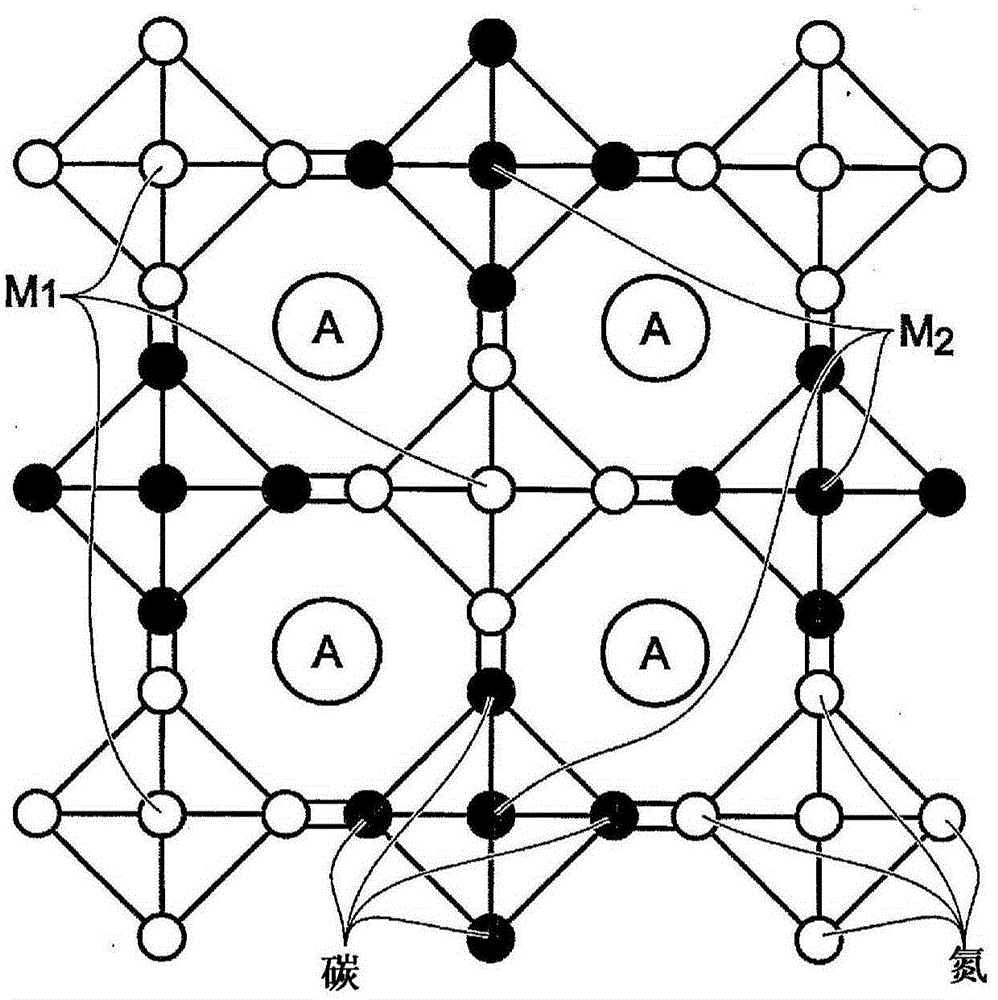
Prussian Blue Analogue Electrodes without Zeolitic Water Content
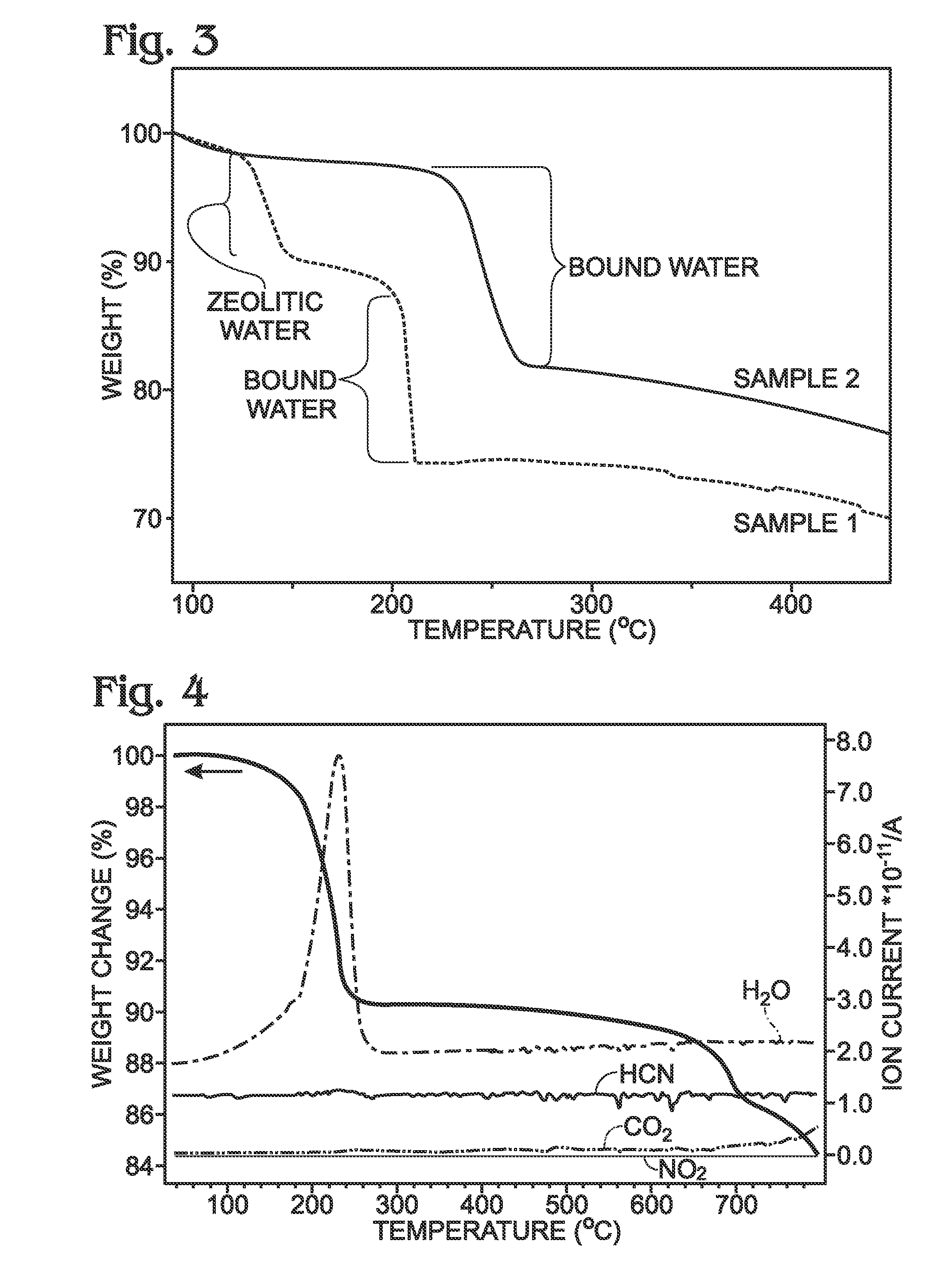
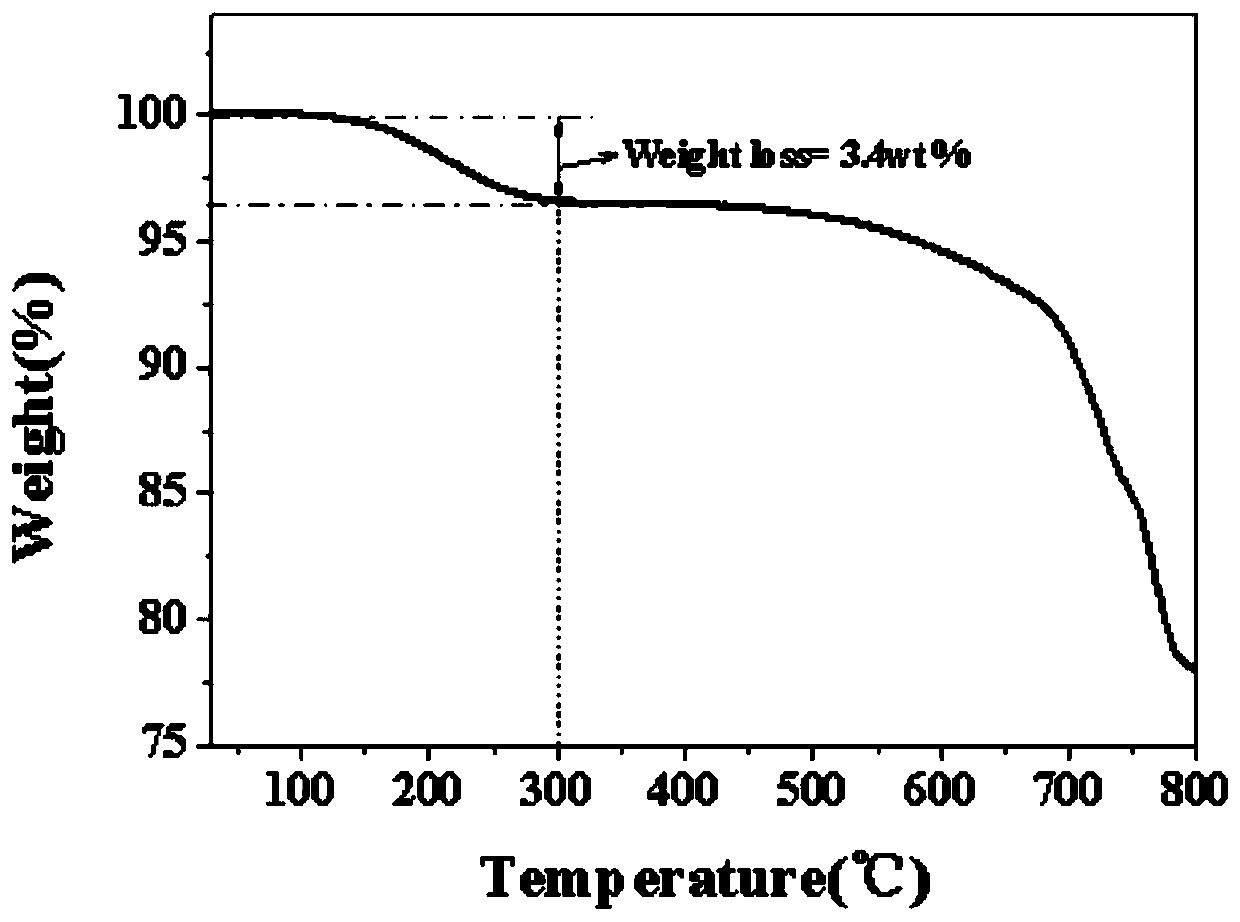
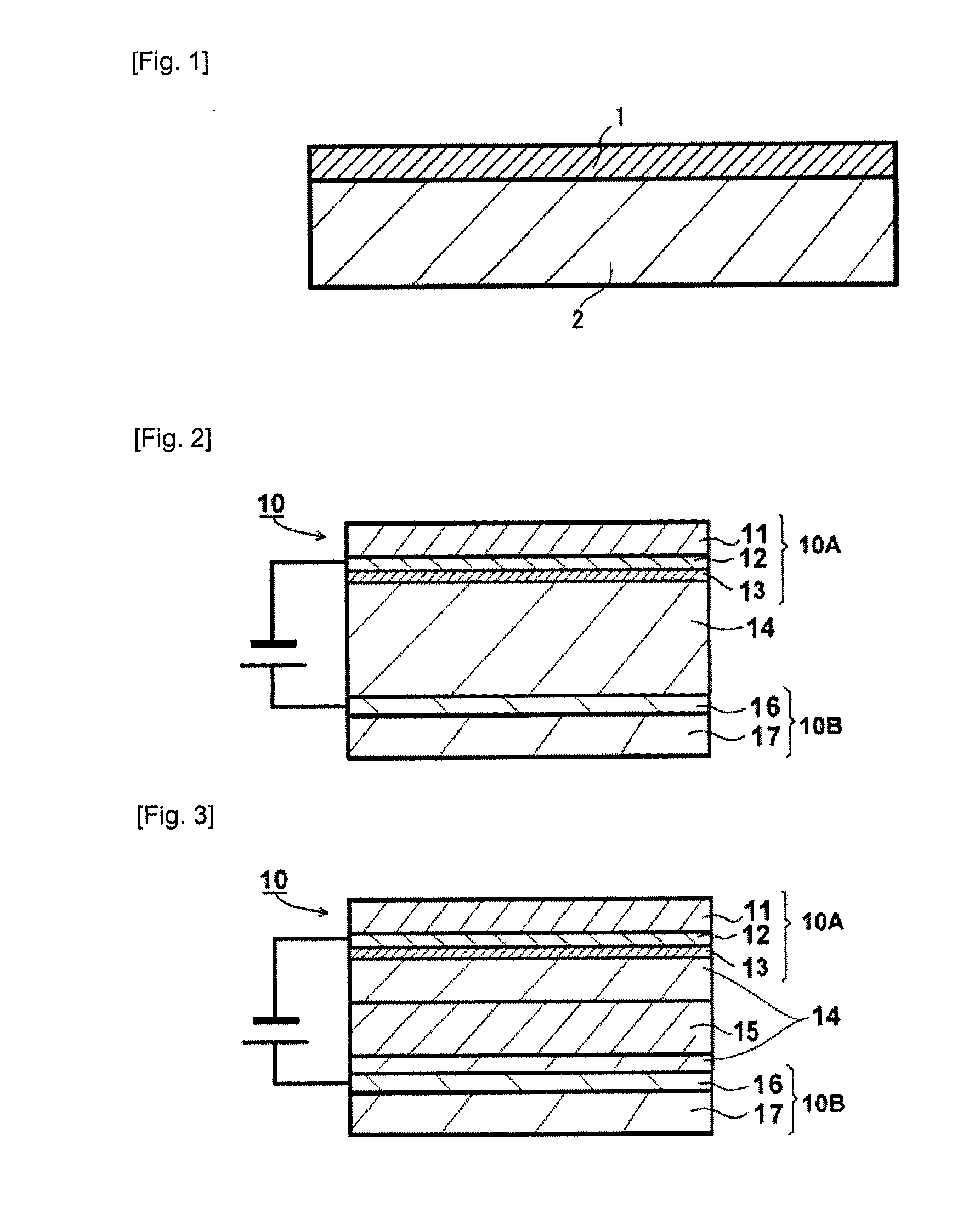
A battery is provided with a hexacyanometallate cathode. The battery cathode is made from hexacyanometallate particles overlying a current collector. The hexacyanometallate particles have the chemical formula AXM1MM2N(CN)Z.d[H2O]ZEO.e[H2O]BND. where A is a metal from Groups 1A, 2A, or 3A of the Periodic Table, where M1 and M2 are each a metal with 2+ or 3+ valance positions, where “ZEO” and “BND” indicate zeolitic and bound water, respectively, where d is 0, and e is greater than 0 and less than 8. The anode material may primarily be a material such as hard carbon, soft carbon, oxides, sulfides, nitrides, silicon, metals, or combinations thereof. The electrolyte is non-aqueous. A method is also provided for fabricating hexacyanometallate with no zeolitic water content in response to dehydration annealing at a temperature of greater than 120 degrees C. and less than 200 degrees C.
Owner:SHARP KK
Prussian white analog cathode material and preparation method and application thereof
ActiveCN110002465AImprove crystal structureReduce defectsIron cyanidesSecondary cellsNew energyAqueous solution
The invention belongs to the field of new-energy batteries, and particularly relates to a Prussian white analog cathode material and a preparation method and application thereof. The preparation method of the Prussian white analog cathode material comprises the following steps: (1), acquiring an aqueous solution of K4Fe (CN)6, which is recorded as a solution A; (2), acquiring a mixed aqueous solution of a transition metal salt of Mn and potassium citrate, which is recorded as a solution B; and (3), dropwise adding the solution A into the solution B, continuing heating and stirring after completion of dropwise addition, aging for several hours, performing solid-liquid separation, collecting and washing a precipitate, and drying to obtain the Prussian white analog cathode material. The Prussian white analog cathode material adopts an open three-dimensional network frame structure, has a large gap position, and can guarantee free deintercalation of various ions such as Li<+>, Na<+> and K<+>.
Owner:HUAZHONG UNIV OF SCI & TECH
Prussian blue cathode material with high sodium content, preparation method and applications thereof, and sodium ion battery
ActiveCN110235292AGood effectRaise the sodium contentIron cyanidesSecondary cellsHigh sodiumPrussian blue
The invention belongs to the technical field of novel energy storage materials, and relates to a Prussian blue cathode material with a high sodium content, a preparation method and applications thereof, and a sodium ion battery. The molecular formula of the Prussian blue cathode material is Na<x>MNFe(CN)<6>; wherein M and N represent a same transition metal or different transition metals andcan be Fe, Co, Mn, Ni, Cu, Zn, Cr, V, Zr, or Ti; 1.8<x<2; 0<a<1; 0<b<1; and a+b=1. The cathode material has millimeter cubic morphology and excellent electrochemical properties. The preparation method has the advantages that the crystallization is slow, the sodium content of the cathode material is high, the preparation method is simple and easy to perform, the production efficiency and the yieldare high, the raw materials are cheap, and industrial production and enlarged production are easy to realize.
Owner:LIAONING STARRY SKY SODIUM BATTERY CO LTD
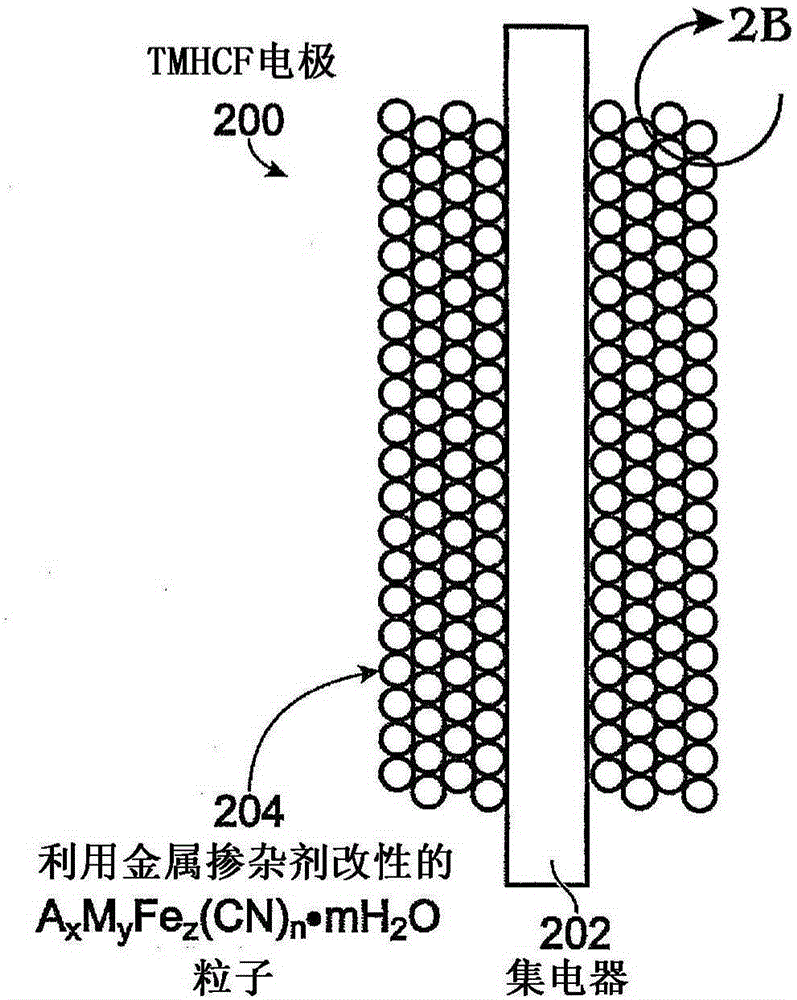
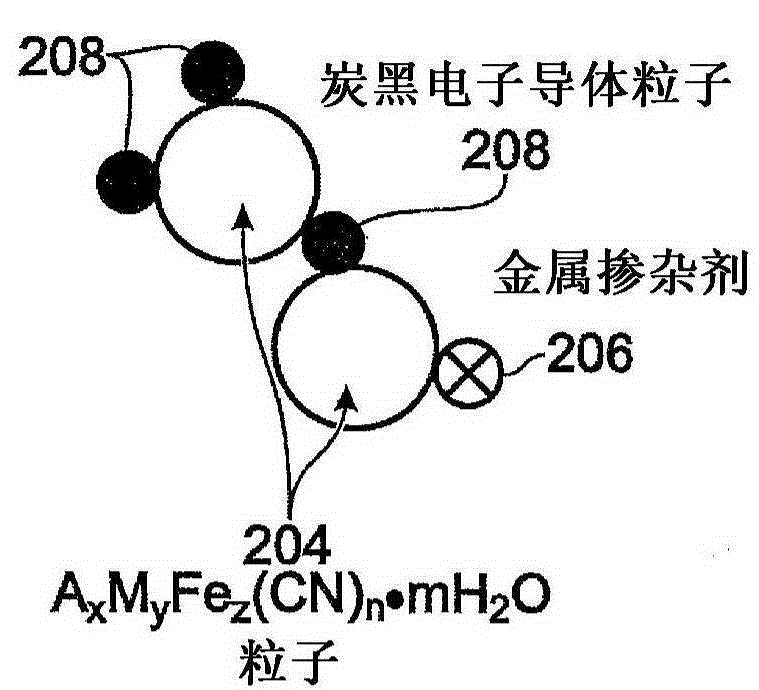
Metal-doped transition metal hexacyanoferrate (TMHCF) battery electrode
InactiveCN105190964AEasy transferFast transferIron cyanidesCell electrodesDopantAlkaline earth metal
A method is provided for synthesizing a metal-doped transition metal hexacyanoferrate (TMHCF) battery electrode. The method prepares a first solution of AxFe(CN)6 and Fe(CN)6, where A cations may be alkali or alkaline-earth cations. The method adds the first solution to a second solution containing M-ions and M'-ions. M is a transition metal, and M' is a metal dopant. Subsequent to stirring, the mixture is precipitated to form AxMcM'dFez(CN)n.mH2O particles. The AxMcM'dFez(CN)n.mH2O particles have a framework and interstitial spaces in the framework, where M and M' occupy positions in the framework. Alternatively, the method prepares AaA'bMyFez(CN)n.mH2O particles. A and A' occupy interstitial spaces in the AaA'bMyFez(CN)n.mH2O particle framework. A metal-doped TMHCF electrode is also provided.
Owner:SHARP KK
Edging cube-shaped cobalt-iron prussian blue nanometer material and preparation method thereof
InactiveCN107021510ANovel shape and structureControllableIron cyanidesPotassium ferricyanideCobalt salt
The invention relates to an edging cube-shaped cobalt-iron prussian blue nanometer material and a preparation method thereof. The preparation method comprises the following steps of firstly, stirring and dissolving a surfactant into water under the magnetic action; after dissolving, sequentially adding sodium citrate and cobalt salt to prepare a solution A; then, dissolving potassium ferricyanide solid into water to prepare a solution B; gradually dripping the solution A into the solution B while stirring; after dripping, continuing to stir for a period of time; standing and aging the reaction liquid for a period of time, naturally cooling, centrifuging, washing, and drying, so as to obtain the edging cube-shaped cobalt-iron prussian blue nanometer material. Compared with the prior art, the synthesizing method has the advantages that by changing the conditions, the edging cube-shaped cobalt-iron prussian blue nanometer material is prepared; the morphology and structure of the material are not reported in the literature; the preparation is simple, the implementing is easy, and the large-batch preparation effect is realized.
Owner:TONGJI UNIV
Prussian blue positive electrode material, sodium ion battery and preparation method and application thereof
ActiveCN111377462AControllable crystallization rateReduce crystal defectsIron cyanidesSecondary cellsSodium cyanideEthylenediaminetetraacetic acid
The invention discloses a prussian blue positive electrode material, a sodium ion battery, and a preparation method and application thereof. The molecular formula of the prussian blue positive electrode material is NaxM [Fe (CN) 6] y.nH2O, wherein M is transition metal, x is less than or equal to 2 and greater than or equal to 1.8; y is less than or equal to 1 and greater than or equal to 0.95; and n is less than or equal to 2 and greater than or equal to 0. The prussian blue positive electrode material has low lattice defect and stable performance; and the sodium ion battery prepared from thematerial is high in capacity and good in cycle performance. The preparation method comprises the following steps of: S1, adding an aqueous solution of weak acid into an aqueous solution of sodium ferrocyanide and transition metal ethylenediamine tetraacetic acid sodium salt to obtain a precipitate; and S2, drying the precipitate. The preparation method can be adopted to control the crystallization speed, and has advantages of simple process, low production cost, non-toxicity, harmlessness and short production period.
Owner:浙江钠创新能源有限公司
Method of producing prussian blue-type metal complex nanoparticles, and prussian blue-type metal complex nanoparticles obtained by the method, dispersion of the nanoparticles, method of regulating the color of the nanoparticles, and electrode and transmitted light-regulator each using the nanoparticles
ActiveUS20100133487A1Suitable for mass productionExcessive amountMaterial nanotechnologyConductive materialNanometer sizeCoordination complex
To provide a method of producing Prussian blue-type metal complex nanoparticles without necessarily requiring complicated steps and an excessive amount of raw materials, but allowing one to obtain nanometer-size fine particles having desired fine particle properties, and Prussian blue-type metal complex nanoparticles obtained by the method, a dispersion of the nanoparticles, a method of regulating the color of the nanoparticles, and an electrode and a transmitted light-regulator each using the nanoparticles; Prussian blue-type metal complex nanoparticles are produced by: mixing an aqueous solution containing a metal cyano complex anion having predetermined metal atom MA as a central metal and an aqueous solution containing a cation of predetermined metal atom MB, thereby precipitating the crystal of a Prussian blue-type metal complex having the metal atom MA and the metal atom MB; and then mixing the Prussian blue-type metal complex with an aqueous solution containing a metal cyano complex anion having the metal atom MC as a central metal and / or an aqueous solution containing a cation of the metal atom MD. The thus-obtained Prussian blue-type metal complex nanoparticles have a preferable electrochemical responsiveness, and the particles can be used for forming a thin film including them to construct a light-transmitted regulator.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Prussian blue flower-like nano-structure material as well as preparation and application thereof
ActiveCN107082438AAlleviate volume effectImproved magnification performanceIron cyanidesSecondary cellsNano structuringSodium-ion battery
The invention relates to a preparation method of a Prussian blue flower-like nano-structure electrode material. The preparation method comprises the following steps: 1) firstly, dissolving nickel chloride hexahydrate and anhydrous sodium citrate into de-ionized water; 2) dissolving sodium ferrocyanide decahydrate into de-ionized water; 3) pouring a solution of step 2) into a mixed solution obtained by step 1) and uniformly stirring to obtain a mixed solution; 4) standing the mixed solution obtained by step 3); 5) centrifuging and collecting sediment; washing the sediment for several times; drying in vacuum to obtain Prussian blue precursor powder; 6) adding the precursor powder into a sodium hydroxide solution and carrying out ultrasonic treatment; 7) centrifuging and collecting a product and washing; drying in vacuum to obtain light green powder, namely the Prussian blue flower-like nano-structure electrode material. The preparation method provided by the invention has the beneficial effects that the specific surface area is remarkably enlarged so that reaction sites of electrolyte and the electrode material are effectively increased and an ion diffusion distance is reduced; when the Prussian blue flower-like nano-structure electrode material is used as a positive electrode active material of a sodium ion battery, the material has the characteristics of high power and good cycling stability.
Owner:WUHAN UNIV OF TECH
Prussian blue sodium ion battery positive electrode material and preparation method thereof
PendingCN111943228AIncrease contentImprove stabilityMaterial nanotechnologyIron cyanidesElectrical batteryPhysical chemistry
The invention provides a preparation method of a prussian blue sodium ion battery positive electrode material, which comprises the following steps: adding a slow-release agent into a precursor solution A and a precursor solution B, slowly releasing ferrous ions and transition metal ions in the process of mixing and reacting the precursor solution A and the precursor solution B, and forming a competitive relationship with the transition metal ions by the slow-release agent and ferrocyanide radicals. The reaction kinetics is greatly reduced, and the control on the reaction rate is realized. Theslow reaction greatly enhances the crystallinity of the material, can improve the morphology of the Prussian blue crystal, and reduces the generation of vacancies and the introduction of coordinationwater and crystal water. When the sodium-ion battery positive electrode material is used as a sodium-ion battery positive electrode material, a sodium-ion battery has relatively high initial Na<+> content and good stability and rate capability.
Owner:GLOBAL ENERGY INTERCONNECTION RES INST CO LTD +2
High-performance super-capacitor electrode material Co-Fe type prussian blue nano cube as well as preparation method and application thereof
InactiveCN107253731AMild sourceRich sourcesMaterial nanotechnologyIron cyanidesCapacitancePotassium ferricyanide
The invention relates to a high-performance super-capacitor electrode material Co-Fe type prussian blue nano cube as well as a preparation method and application thereof, and belongs to the technical field of electrode material preparation. According to the preparation method, inorganic cobalt salt and potassium ferricyanide are adopted as reaction raw materials, trisodium citrate is adopted as a complexing stabilizer, a liquid-phase precipitation reaction method is implemented, reaction conditions such as the use amount and the stand aging time of potassium ferricyanide are controlled, a Co-Fe type prussian blue nano cube material with good dispersibility and large specific area is prepared, and the size of the material is about 300nm. By adopting the Co-Fe type prussian blue nano cube material provided by the invention, the capacity of an electrochemical electric container is increased, properties such as the rapid charge and discharge property and the service life of the electric container are improved, the capacitance of the electric container is as high as 447F / g in constant current charge and discharge of 5A / g in a 1mol / L sodium sulfate solution, and the good rate capability is achieved.
Owner:DALIAN NATIONALITIES UNIVERSITY
Sodium nitroprusside synthesis process
The invention relates to a sodium nitroprusside synthesis processes, wherein potassium nitroprusside and copper sulfate pentahydrate are adopted as reaction raw materials and are subjected to a reaction under a suitable reaction condition to generate copper nitroprusside, the copper nitroprusside reacts with sodium bicarbonate to generate sodium nitroprusside, and concentration crystallization, centrifugation and vacuum drying are performed after the complete reaction to obtain the product. The synthesis process has characteristics of simpleness, easy operation, high product purity, and high yield, and is suitable for industrial production.
Owner:KAMP PHARMA
Preparation method of prussian blue type energy storage material
ActiveCN107634220ASimple production processAvoid lossIron cyanidesCell electrodesAmmonium perchlorateSodium-ion battery
The invention provides a preparation method of a prussian blue type energy storage material, and specifically relates to a preparation method of a prussian blue type sodium-ion battery electrode material with high yield. The preparation method comprises the following steps: S1, dissolving divalent transition metal perchlorate and sodium ferrocyanide into water respectively to obtain a divalent transition metal perchlorate solution and a sodium ferrocyanide solution; S2, dissolving sodium perchlorate into water to obtain a sodium perchlorate solution; and S3, mixing the divalent transition metal perchlorate solution and the sodium ferrocyanide solution with the sodium perchlorate solution to obtain a mixed solution, and stirring and standing the mixed solution to obtain a sediment product;and separating and drying the sediment product to obtain the prussian blue type energy storage material. According to the preparation method, a great deal of loss of the prussian blue type material inthe cleaning process is avoided, and high-yield production can be realized. The preparation method is simple and high in yield, and is easy for industrial large-scale production.
Owner:浙江钠创新能源有限公司
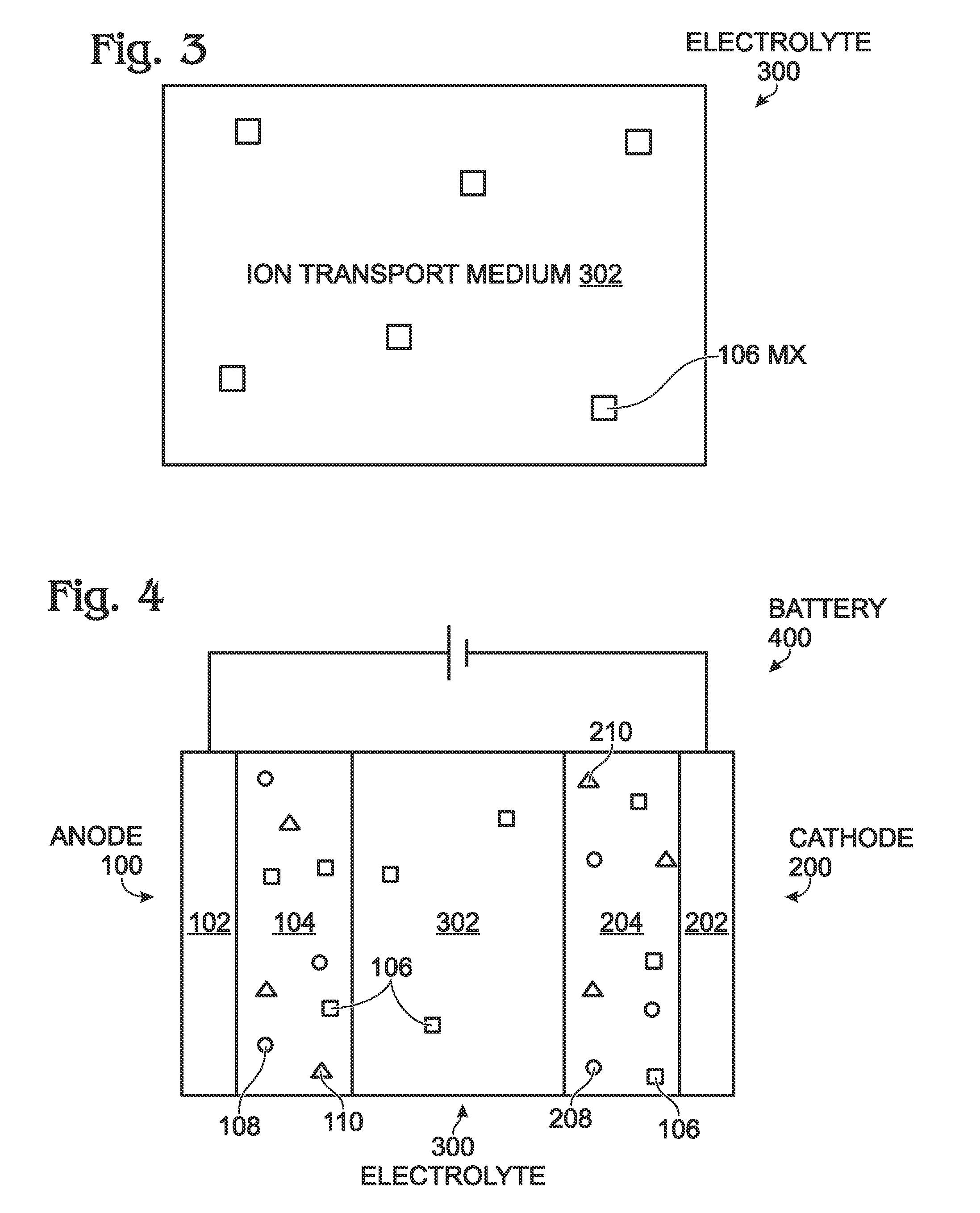
Sodium and Potassium Ion Batteries with Halogen Salts
InactiveUS20150357646A1Improve performanceGood capacity retentionElectrode thermal treatmentIron cyanidesManganeseCobalt
A sodium or potassium battery is provided, prior to an initial charge and discharge cycle, with a halogen salt additive. As is conventional, the battery is made up of the following components: an anode, a cathode, and an electrolyte. In addition, the battery includes a halogen salt (MX), where M is a metal and X is a halogen element. The halogen salt is added to the anode, the cathode, the electrolyte, or combinations thereof. The concentration MX with respect to the component(s) to which it is added is in the range of 0.01% to 10% in weight. The element X can be selected from the group of halogen elements listed in the Periodic Table. M is a material such as lithium, sodium, potassium, cesium, magnesium, calcium, barium, titanium, manganese, iron, cobalt, nickel, copper, zinc, ammonium, or combinations thereof. Advantageously, the electrolyte may be either aqueous or non-aqueous.
Owner:SHARP KK
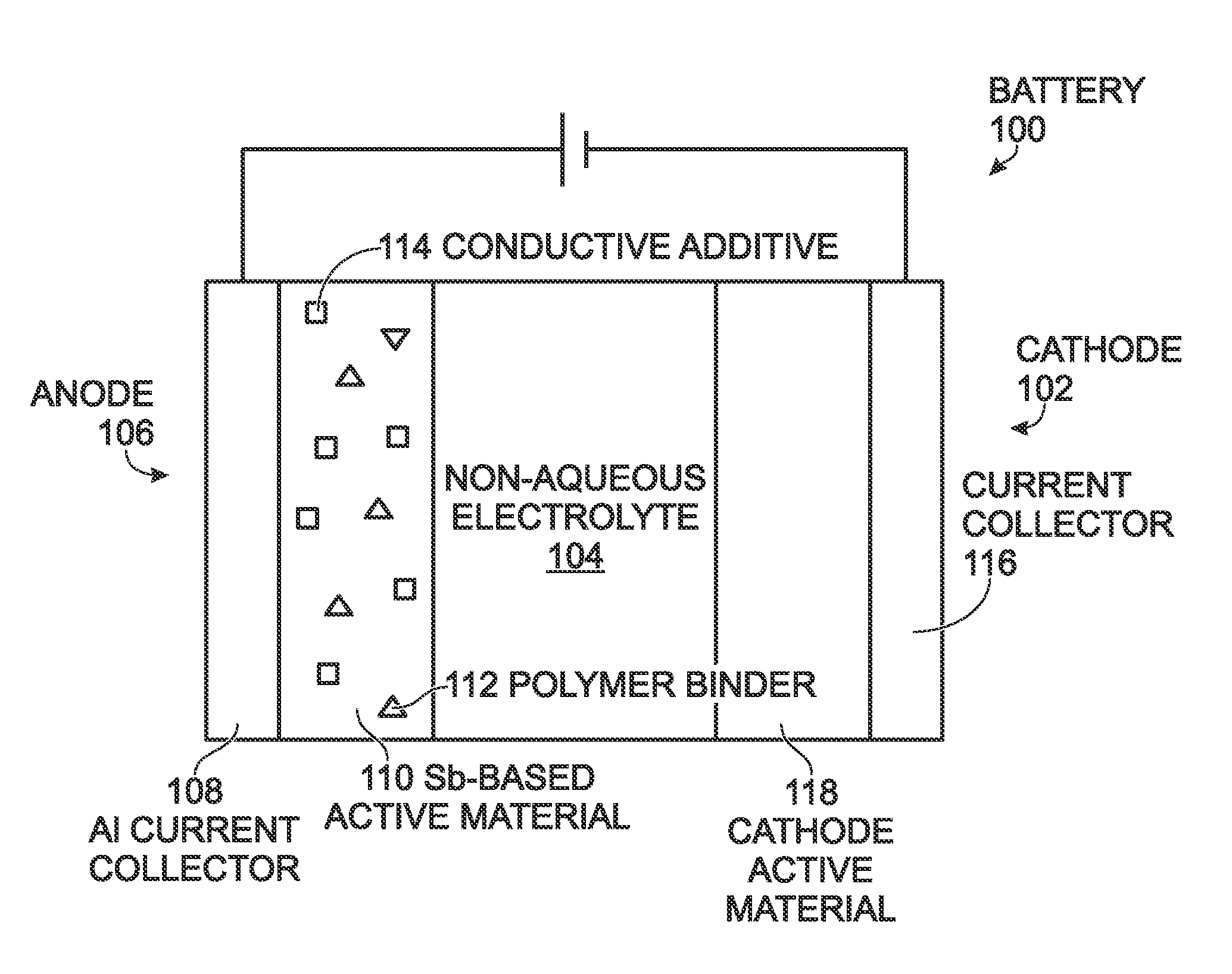
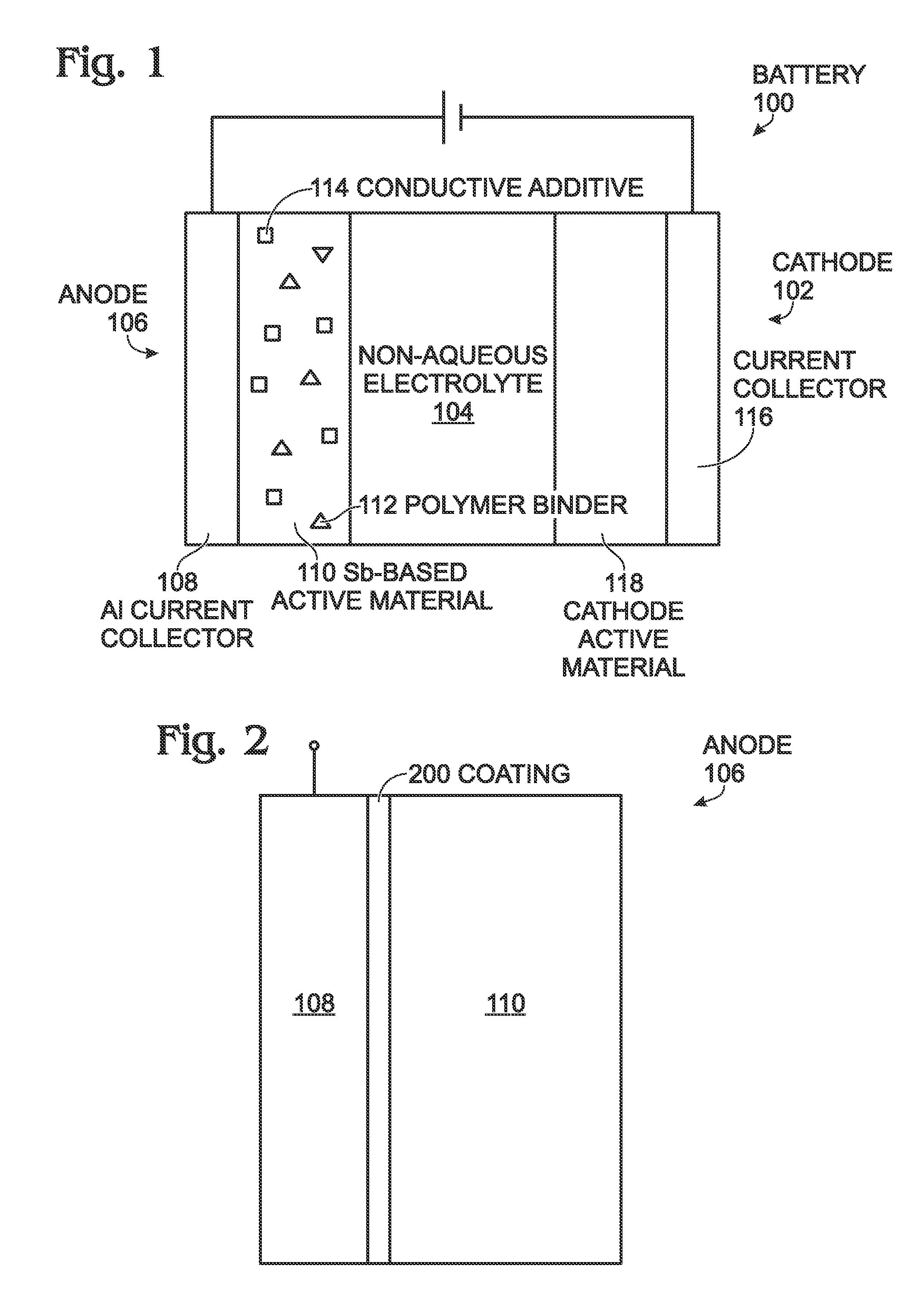
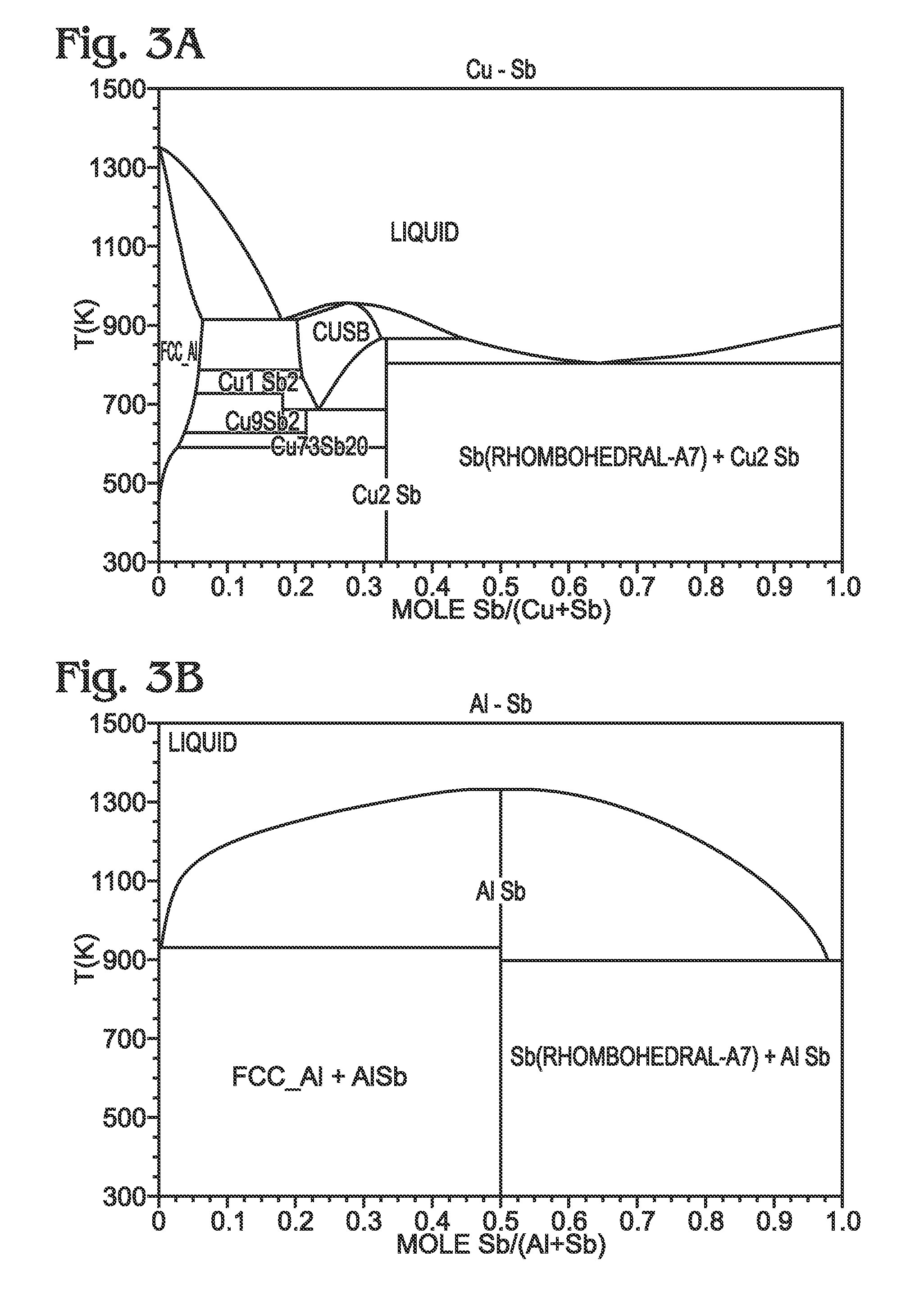
Antimony-Based Anode on Aluminum Current Collector
ActiveUS20150349338A1Improve adhesionImprove compatibilityElectrode thermal treatmentIron cyanidesGalliumAntimony
An electrochemical battery is provided with an aluminum anode current collector and an antimony (Sb)-based electrochemically active material overlying the aluminum current collector. The Sb-based electrochemically active material may be pure antimony, Sb with other metal elements, or Sb with non-metal elements. For example, the Sb-based electrochemically active material may be one of the following: Sb binary or ternary alloys of sodium, silicon, tin, germanium, bismuth, selenium, tellurium, thallium, aluminum, gold, cadmium, mercury, cesium, gallium, titanium, lead, carbon, and combinations thereof. The aluminum current collector may additionally include a material such as magnesium, iron, nickel, titanium, and combinations thereof. In one aspect, the anode further composed of a coating interposed between the aluminum current collector and the Sb-based electrochemically active material. This coating may be a non-corrodible metal or a carbonaceous material. The cathode is may be composed of a number of different active materials including sodium-based Prussian Blue analogues.
Owner:SHARP KK
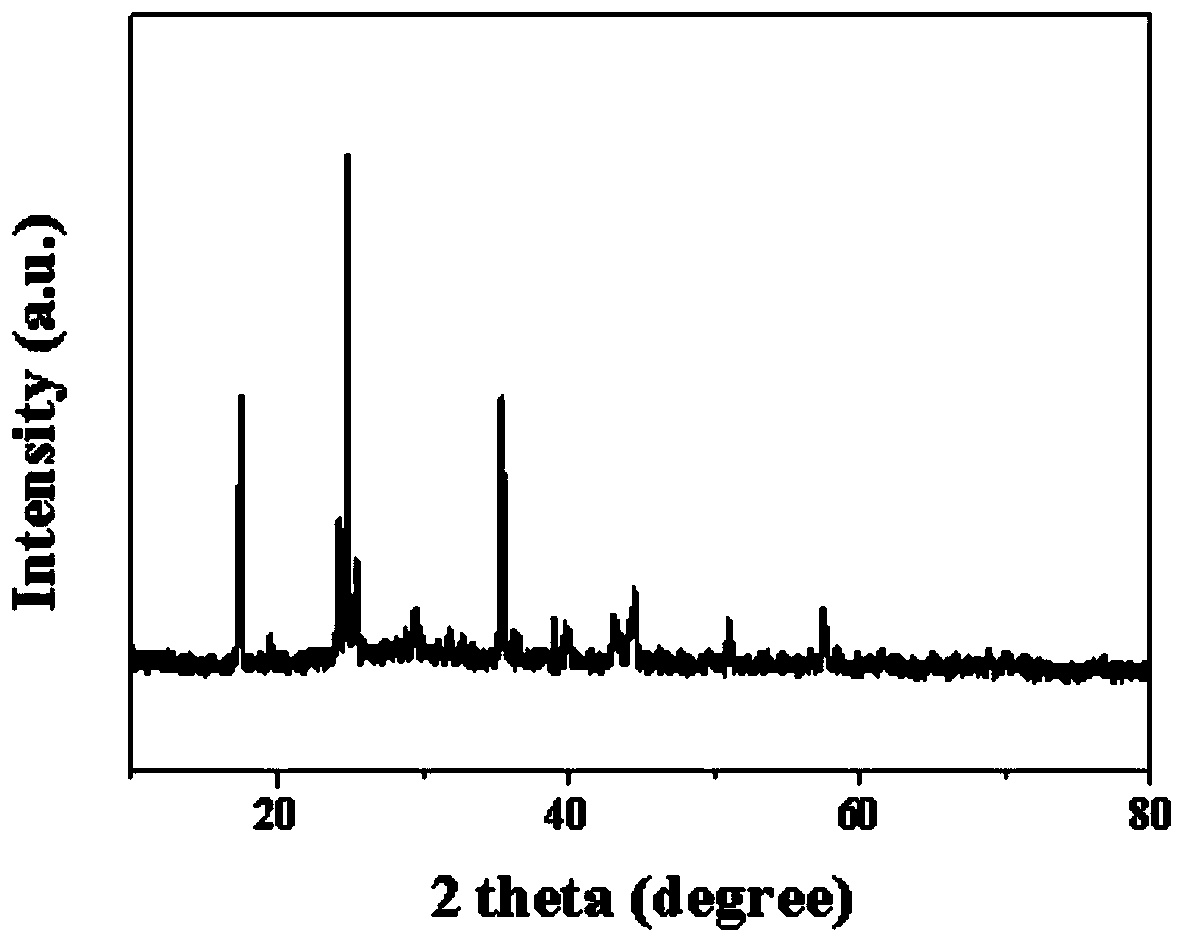
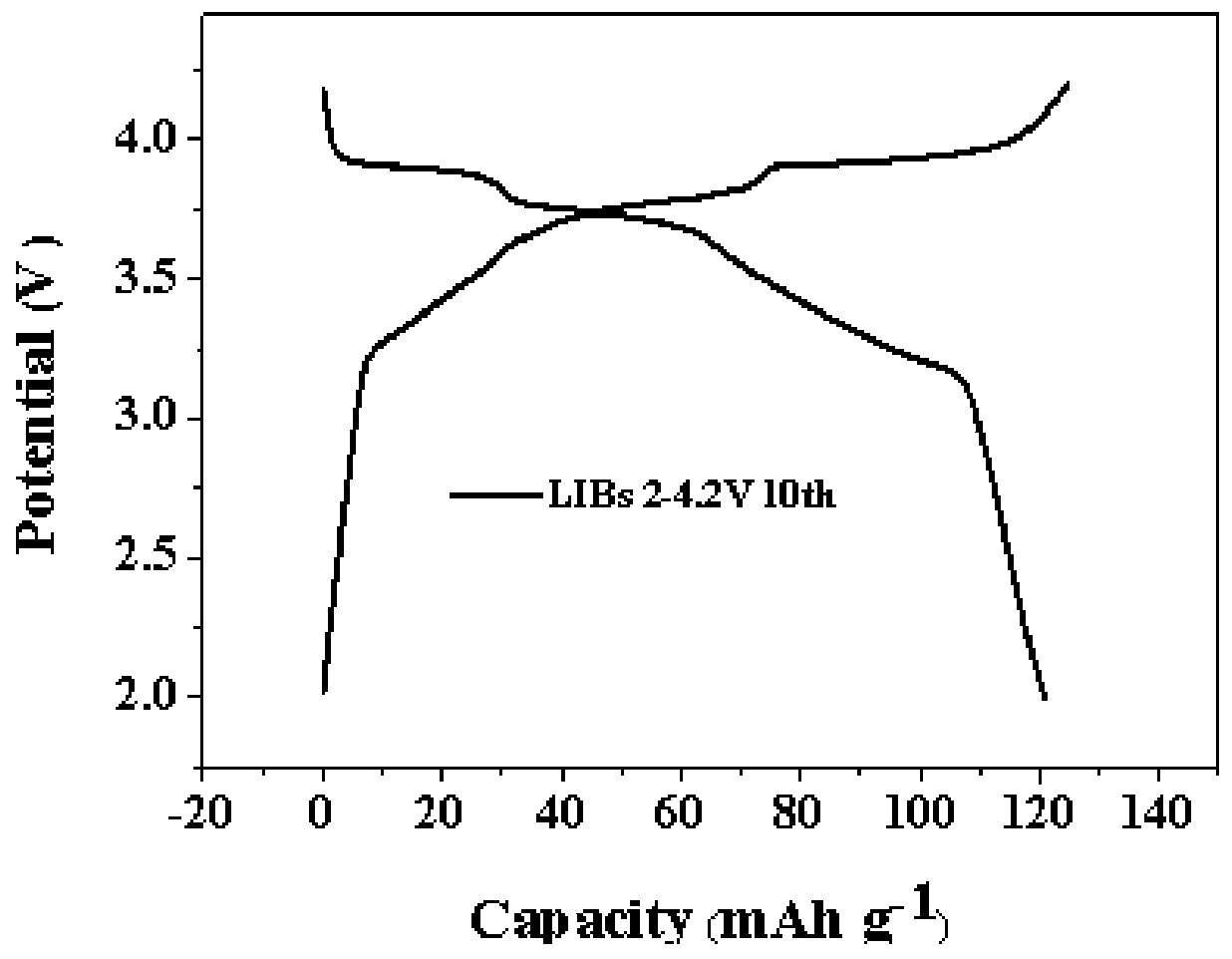
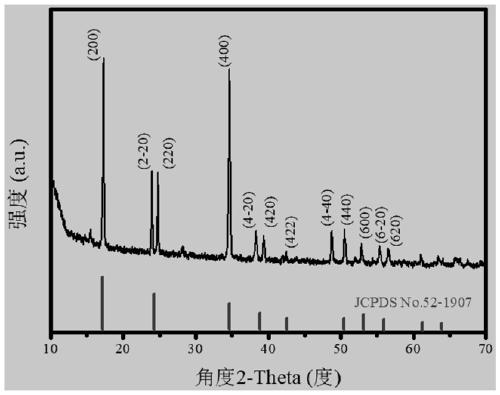
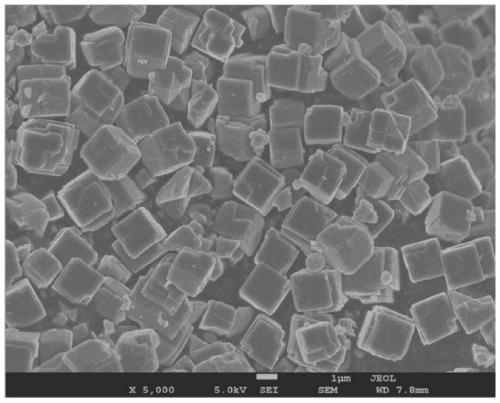
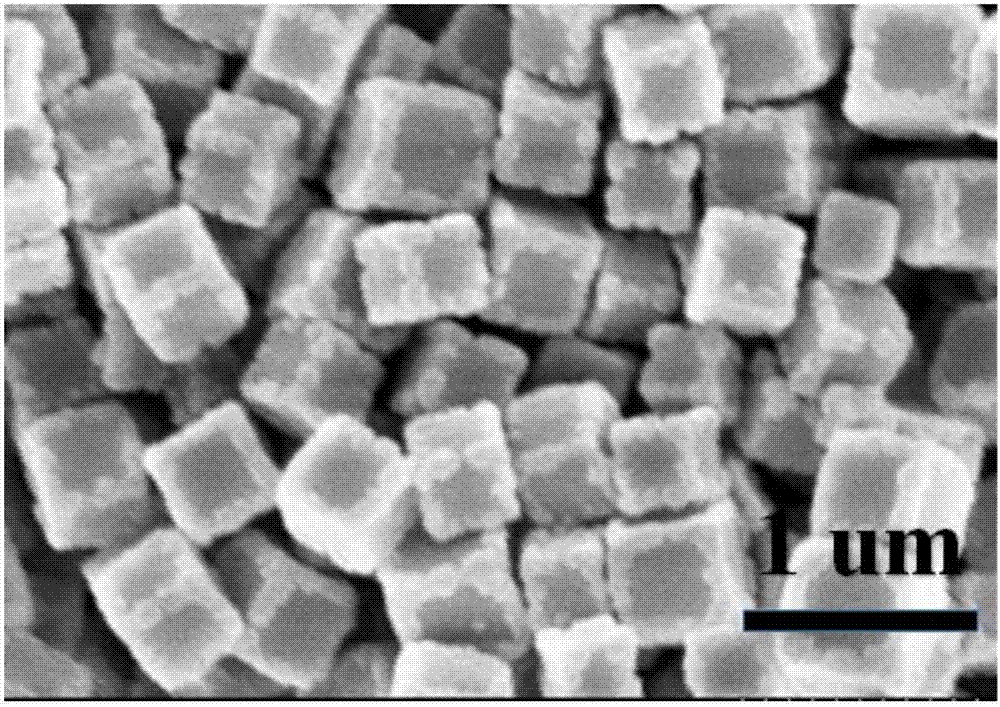
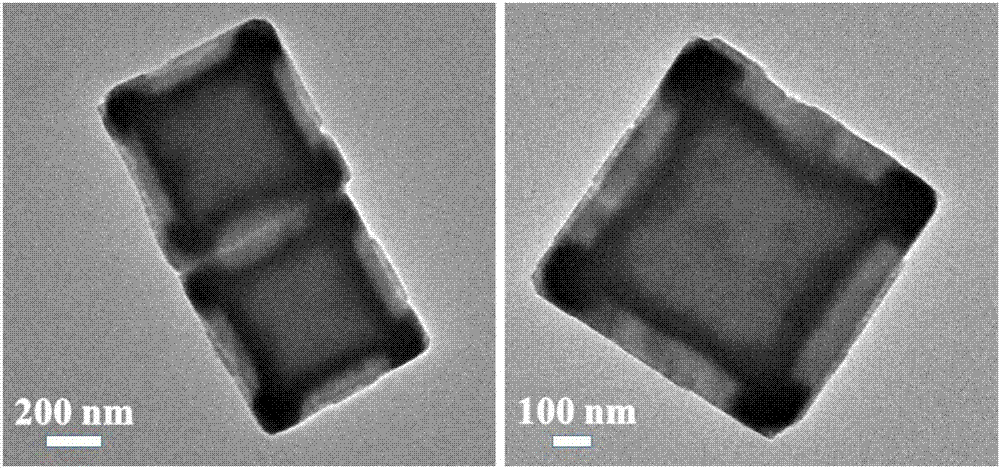
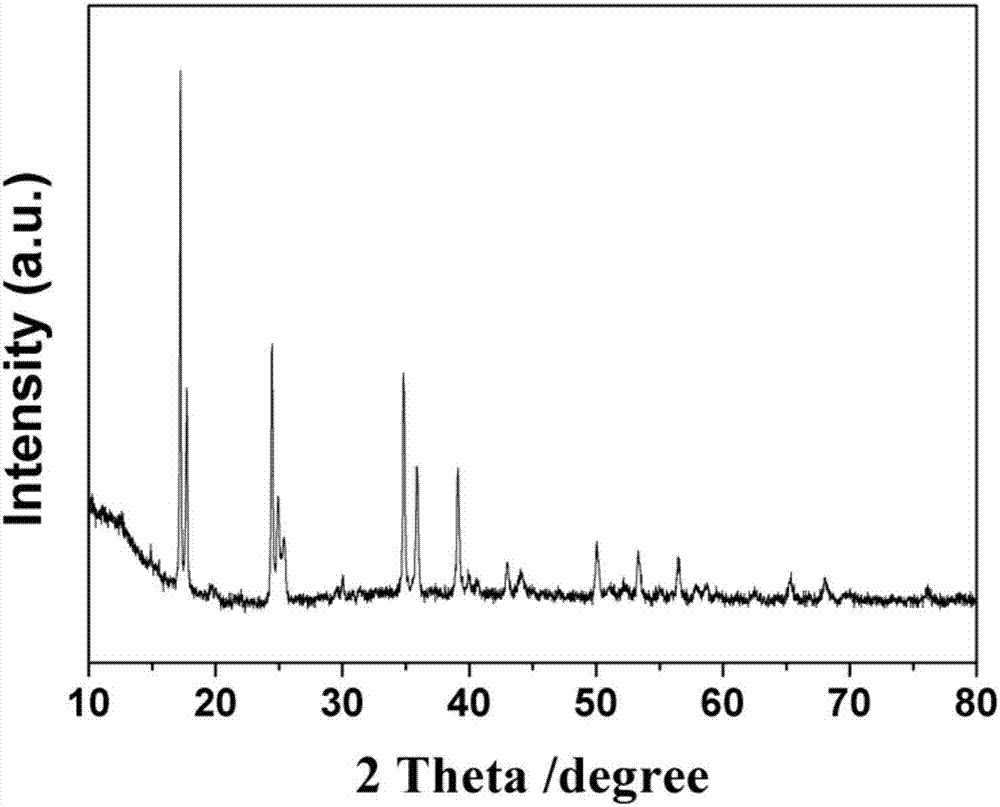
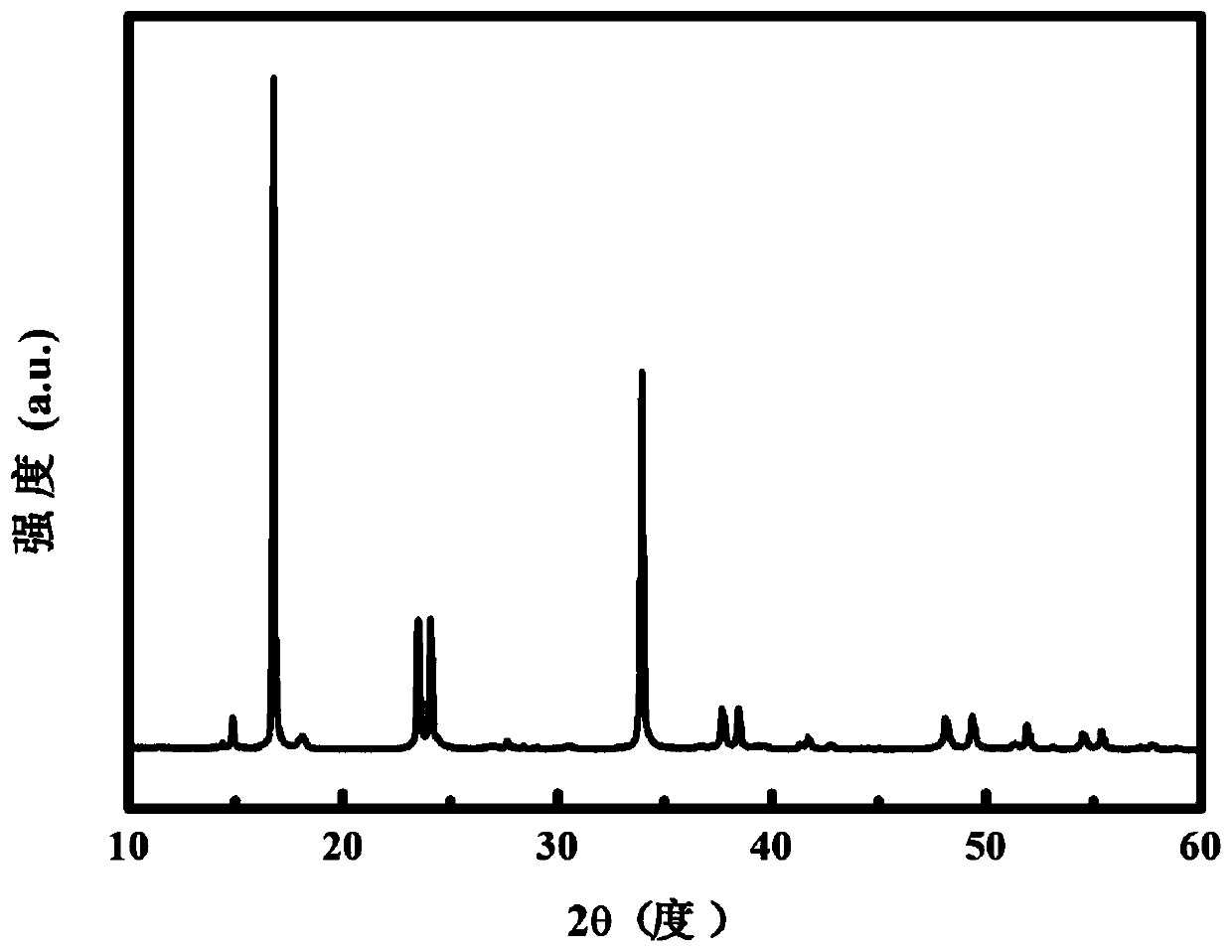
Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof
InactiveCN105271307AGood dispersionLow priceMaterial nanotechnologyIron cyanidesElectrical batteryPolyethylene glycol
The invention discloses a prussian-blue derivative Cd2[Fe(CN)6] nanorod and a preparation method thereof. The preparation method for the Cd2[Fe(CN)6] nanorod comprises: firstly adding proper amount of potassium ferrocyanide, polyethylene glycol (PEG-400), a cadmium salt and deionized water into a hydrothermal reaction kettle core, putting into a baking oven and performing 120 DEG C-180 DEG C constant-temperature reaction, and naturally cooling to room temperature after the reaction is finished; and performing centrifugation washing and drying on the prepared product, so as to prepare the Cd2[Fe(CN)6] nanorod. After the prepared Cd2[Fe(CN)6] powder is tested by an X-ray diffractometer and a scanning electron microscope, Cd2[Fe(CN)6] is a nanorod, is uniform in particle size distribution, and can be used as a material used for making electrochromic devices and a secondary battery electrode material.
Owner:SHANGHAI SECOND POLYTECHNIC UNIVERSITY
Preparation method of Prussian blue based sodium ion battery positive electrode material
InactiveCN106654263AReduce defectsSlow responseIron cyanidesCell electrodesSingle crystalSodium-ion battery
The invention discloses a preparation method of a Prussian blue based sodium ion battery positive electrode material. The preparation method takes ferrous oxalate and sodium oxalate as raw materials, and makes the raw materials react with sodium ferrocyanide; a reaction speed can be slower and the production speed of a Prussian blue mono-crystal becomes slow, so that the aim of reducing defects in a Prussian blue crystal is realized. A sodium ion battery positive electrode prepared from the Prussian blue crystal can still keep good stability after being subjected to a plurality of times of charge-discharge cycles, and the service life is long. The method is simple in preparation process, easy to implement and suitable for large-scale production.
Owner:DONGGUAN JIAQIAN NEW MATERIAL TECH CO LTD
Process to separate the vanadium contained in inorganic acid solutions
A chemical process that recovers the vanadium contained in inorganic acid solutions, precipitating it as a solid compound of vanadium and alkali metal or monovalent cation ferrocyanide, is disclosed. Separation is carried out electrochemically, depositing the compound onto a metal immersed in the acid solution that contains vanadium as well as other dissolved metals, to which a ferrocyanide salt of an alkali metal or a monovalent cation has been previously added. If the inorganic acid present in solution is different than nitric acid, the vanadium can also be separated by direct addition of a ferrocyanide salt of an alkali metal or a monovalent cation to the acid solution containing vanadium. The method described allows recovery of vanadium without modifying the initial composition of the solution, except for the concentration of the vanadium dissolved.
Owner:UNIVE SIMON BOLIVAR
Preparation method of low-defect nano-Prussian blue, and application of nano-Prussian blue
InactiveCN106745068AReduce defectsImprove cycle stabilityIron cyanidesCell electrodesSodium-ion batteryPrussian blue
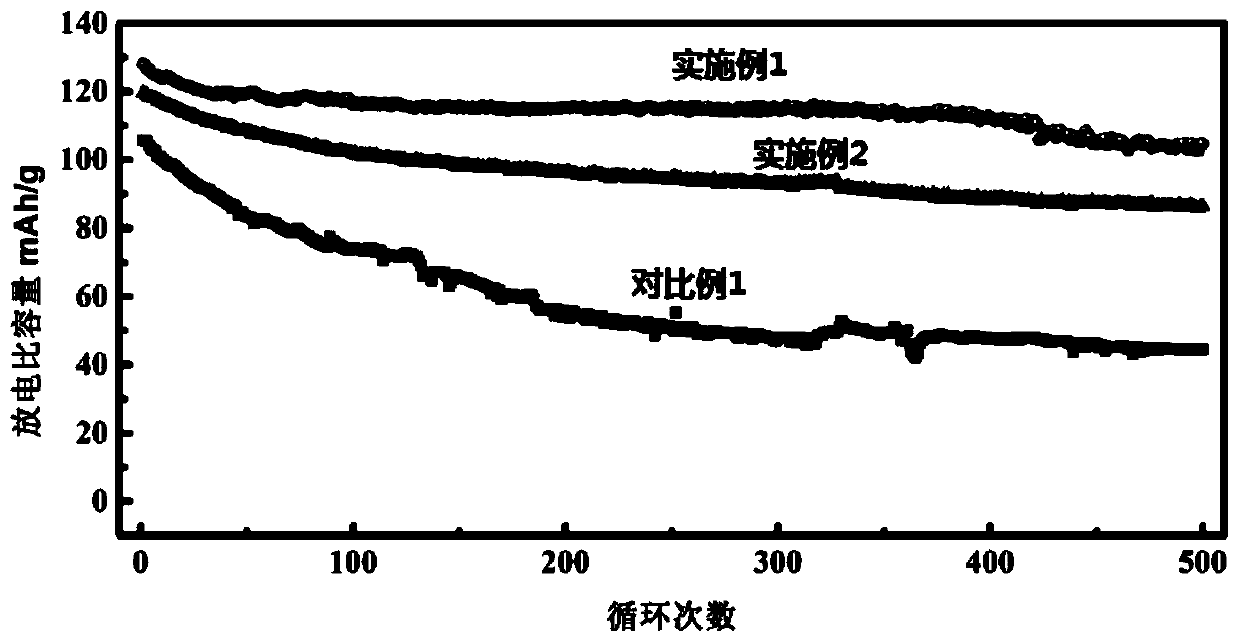
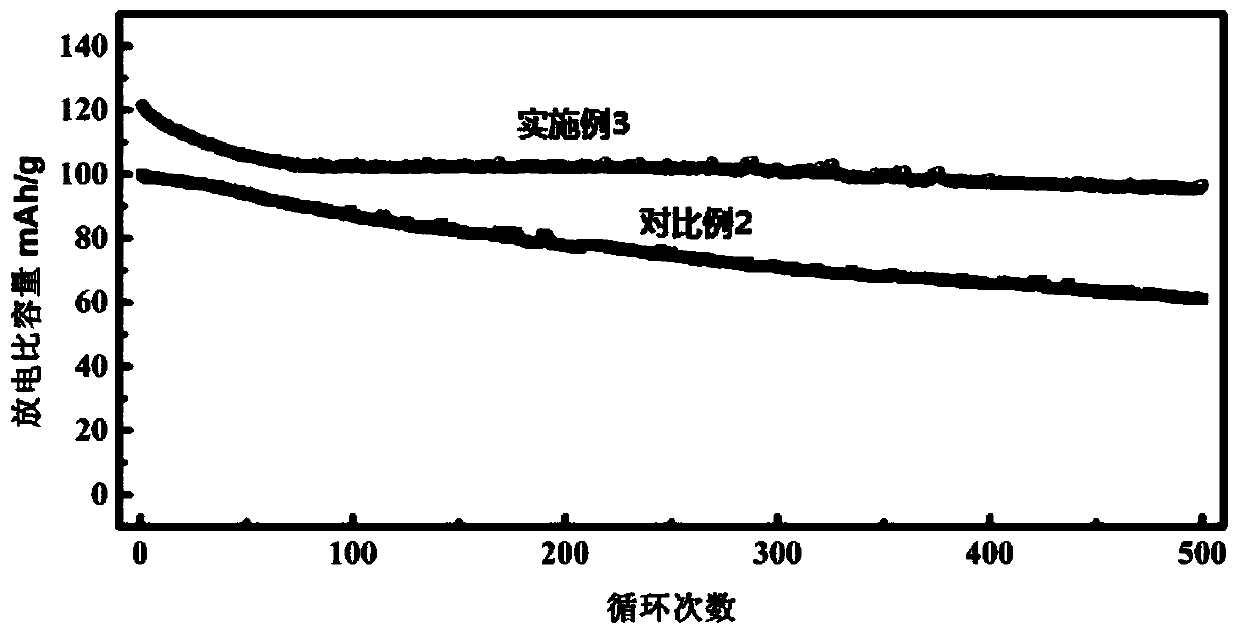
The invention relates to a preparation method of low-defect nano-Prussian blue. The method is characterized by comprising the following steps: preparing solution A by ferrous oxalate and sodium oxalate, and preparing solution B by sodium ferrocyanide; adding a surfactant into the solution B, carrying out ultrasonic agitation, and marking as solution C; carrying out ultrasonic agitation on the solution A; adding the solution C into the solution A subjected to the ultrasonic agitation, and carrying out constant-temperature stirring to obtain blue precipitates; washing the blue precipitates, and carrying out vacuum drying at the temperature of 1-130DEG C overnight after the blue precipitates are washed, thus finally obtaining the Prussian blue. The invention also discloses application of the Prussian blue as a cathode material of a sodium-ion battery. The preparation method has the beneficial effects that the defects in Prussian blue in the prior art are effectively reduced, cycling stability is good, capacities are respectively 106mAhg-1 and 98mAhg-1 after circulation is carried out for 500 times at the current density of 300mAhg-1, and a capacity retention rate is high.
Owner:HUAZHONG UNIV OF SCI & TECH
High-entropy Prussian blue material and preparation method thereof
ActiveCN113690433AHigh crystallinityImprove cycle stabilityIron cyanidesCell electrodesElectrical batterySource material
The invention discloses a high-entropy Prussian blue material, and the molecular formula of the high-entropy Prussian blue material is NaxMIN[Fe(CN)6]zwH2O, wherein M is n different transition metal elements, n is greater than or equal to 5, yn is greater than or equal to 0.01 and less than or equal to 0.90, y1 + y2 + y3 + y4 + y5+... + yn = 1, w is less than or equal to 4.0, x is greater than or equal to 1.40 and less than or equal to 1.95, and z is greater than or equal to 0.90 and less than or equal to 0.98. The high-entropy Prussian blue material is of a monoclinic phase structure, the microstructure of the high-entropy Prussian blue material is large-size crystal particles, and the crystal particles are uniform in size, single in shape and regular in polyhedral morphology. The invention also provides application of the high-entropy Prussian blue material as a positive electrode material in a sodium-ion battery and a preparation method of the high-entropy Prussian blue material. A coprecipitation method is adopted, and the high-entropy Prussian blue material with high specific capacity, good rate capability and excellent cycle performance is obtained through selection of source materials, technological process design and selection and control of technological parameters in preparation.
Owner:ZHEJIANG UNIV HANGZHOU GLOBAL SCI & TECH INNOVATION CENT
Prussian blue material, and preparation method and application thereof
ActiveCN110921681ALattice integrityReduce water of crystallizationIron cyanidesCell electrodesFerrous saltsElectrical battery
The invention discloses a Prussian blue material, and a preparation method and an application thereof. The preparation method comprises the steps: mixing sodium ferrocyanide or a hydrate of sodium ferrocyanide with deionized water to obtain a solution I; mixing soluble ferrous salt, ascorbic acid and sodium citrate with deionized water to obtain a solution II; dropwise adding the solution I and the solution II into deionized water at the same time, and carrying out a coprecipitation reaction to obtain a suspension of a Prussian blue material; and aging and post-processing the suspension to obtain the micron-sized Prussian blue material with a cubic stepped structure. The prepared Prussian blue material is applied to a positive electrode of a sodium ion battery, so that the capacity and thecycling stability of the sodium ion battery can be remarkably improved.
Owner:ELECTRIC POWER RES INST OF STATE GRID ZHEJIANG ELECTRIC POWER COMAPNY +5
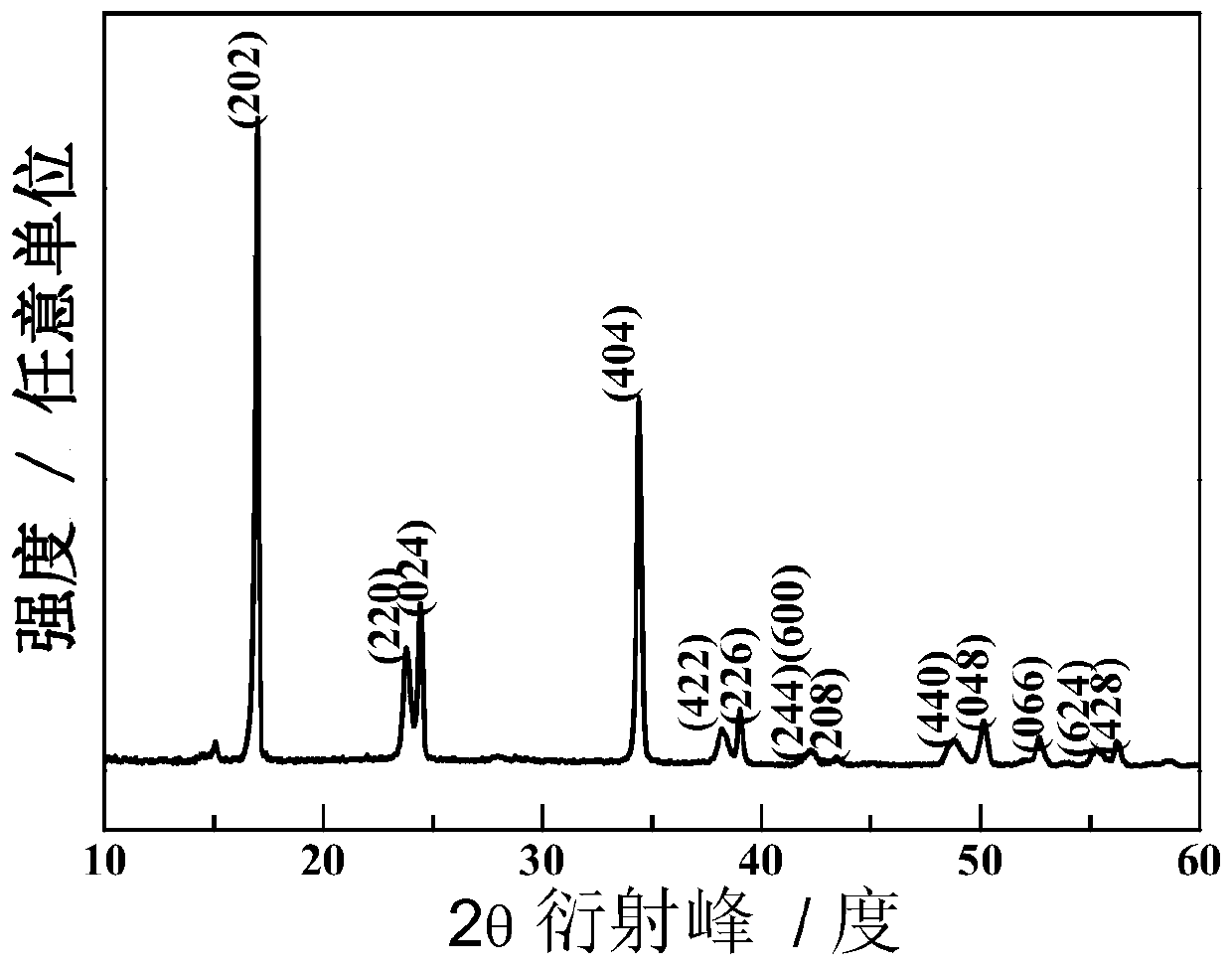
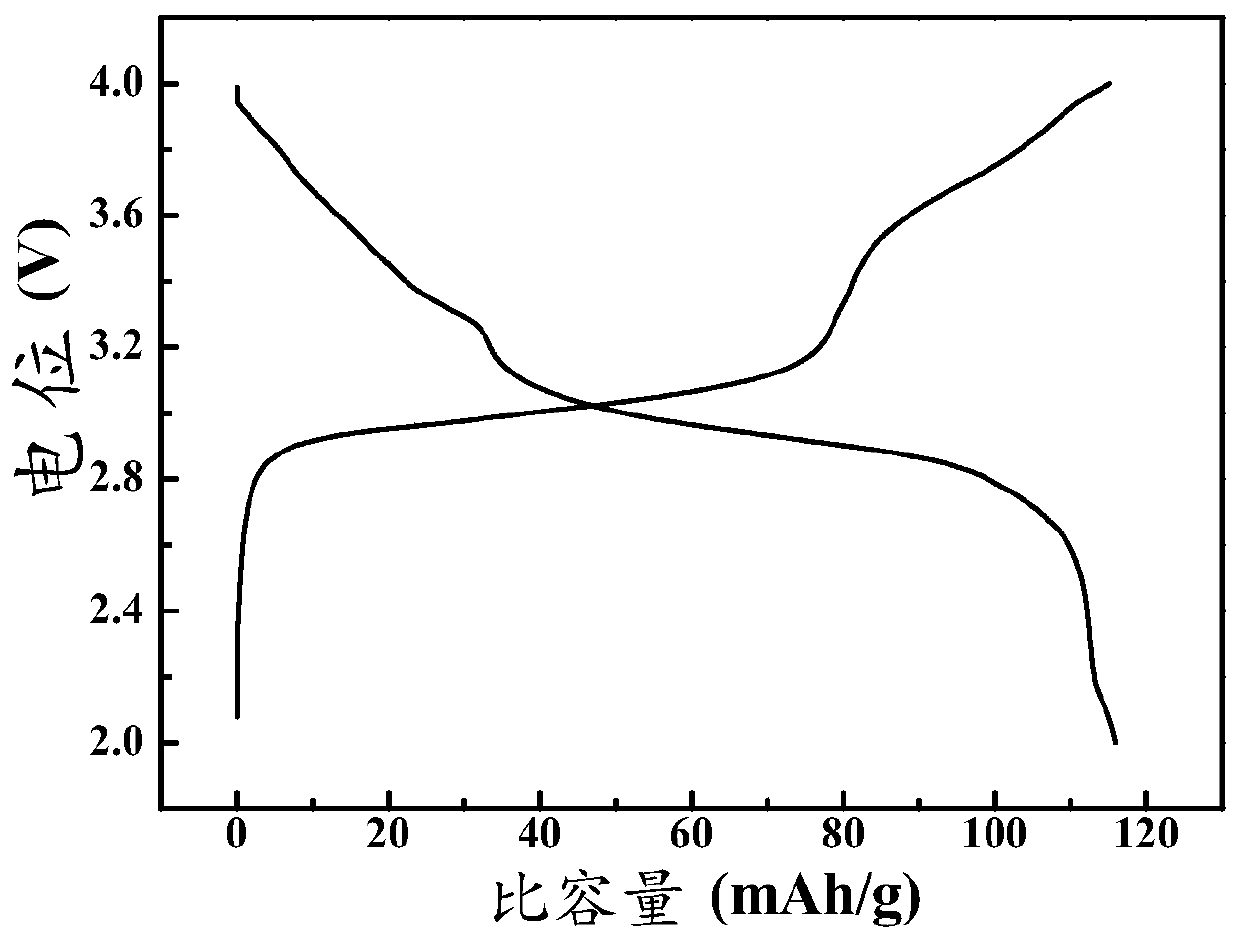
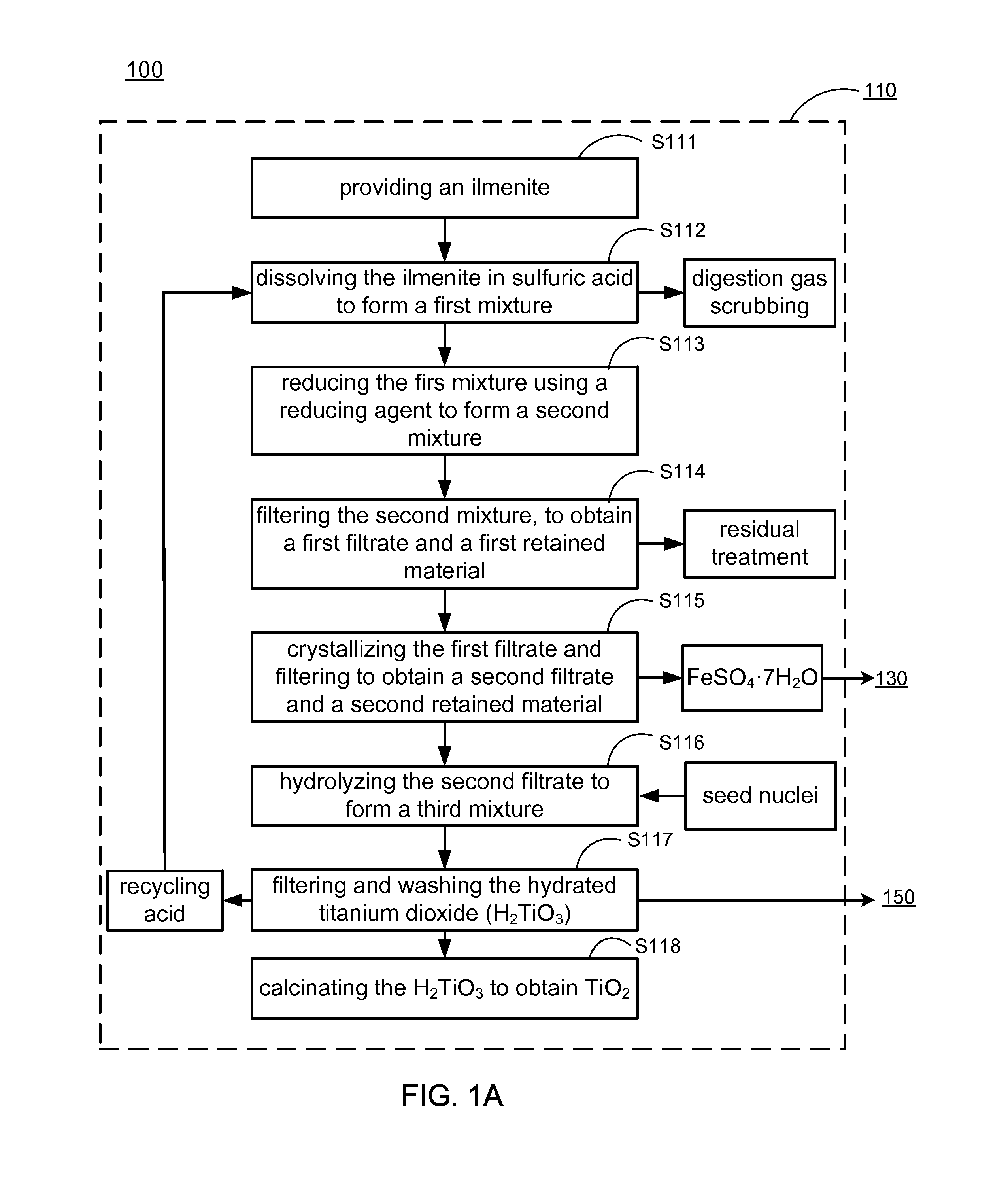
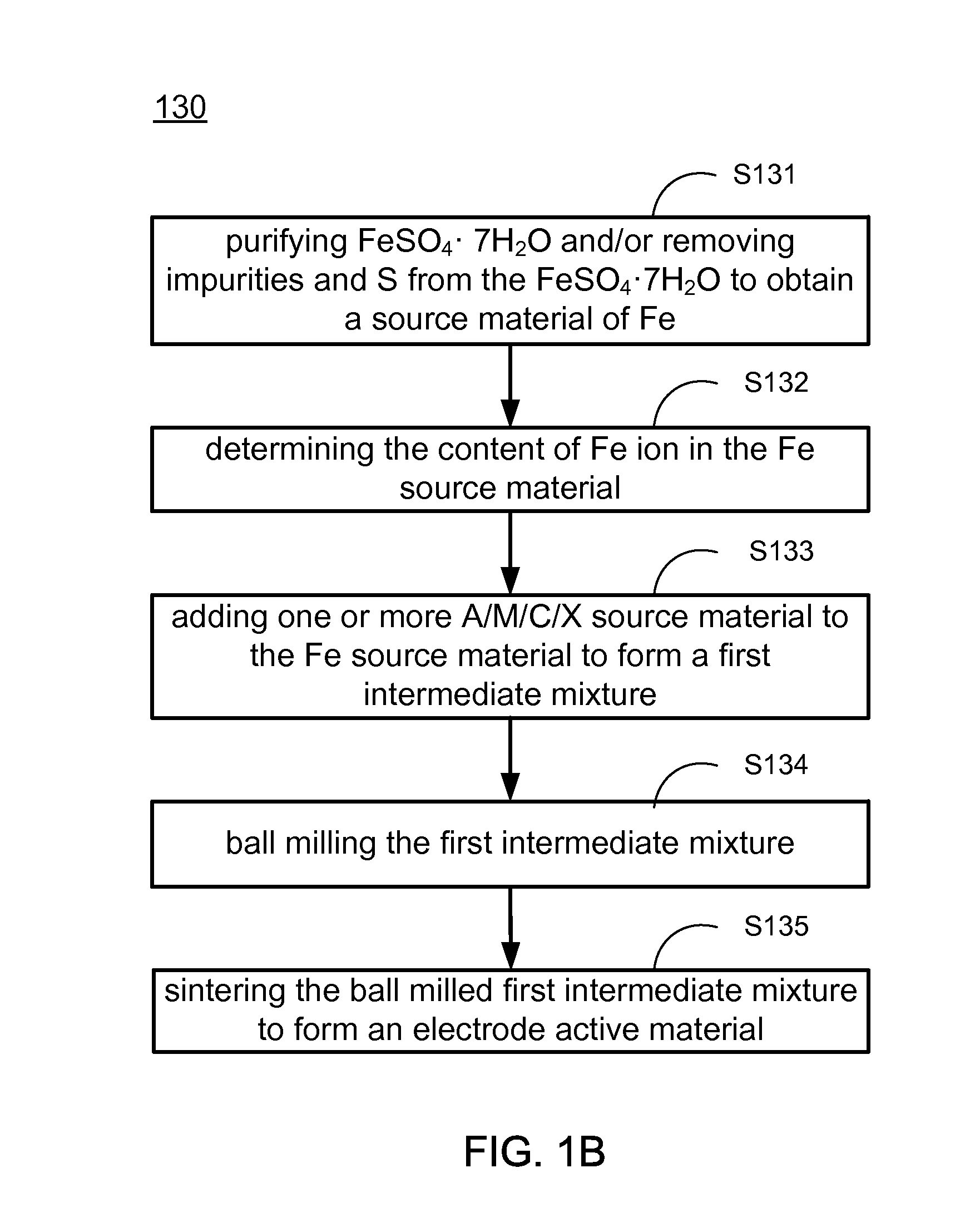
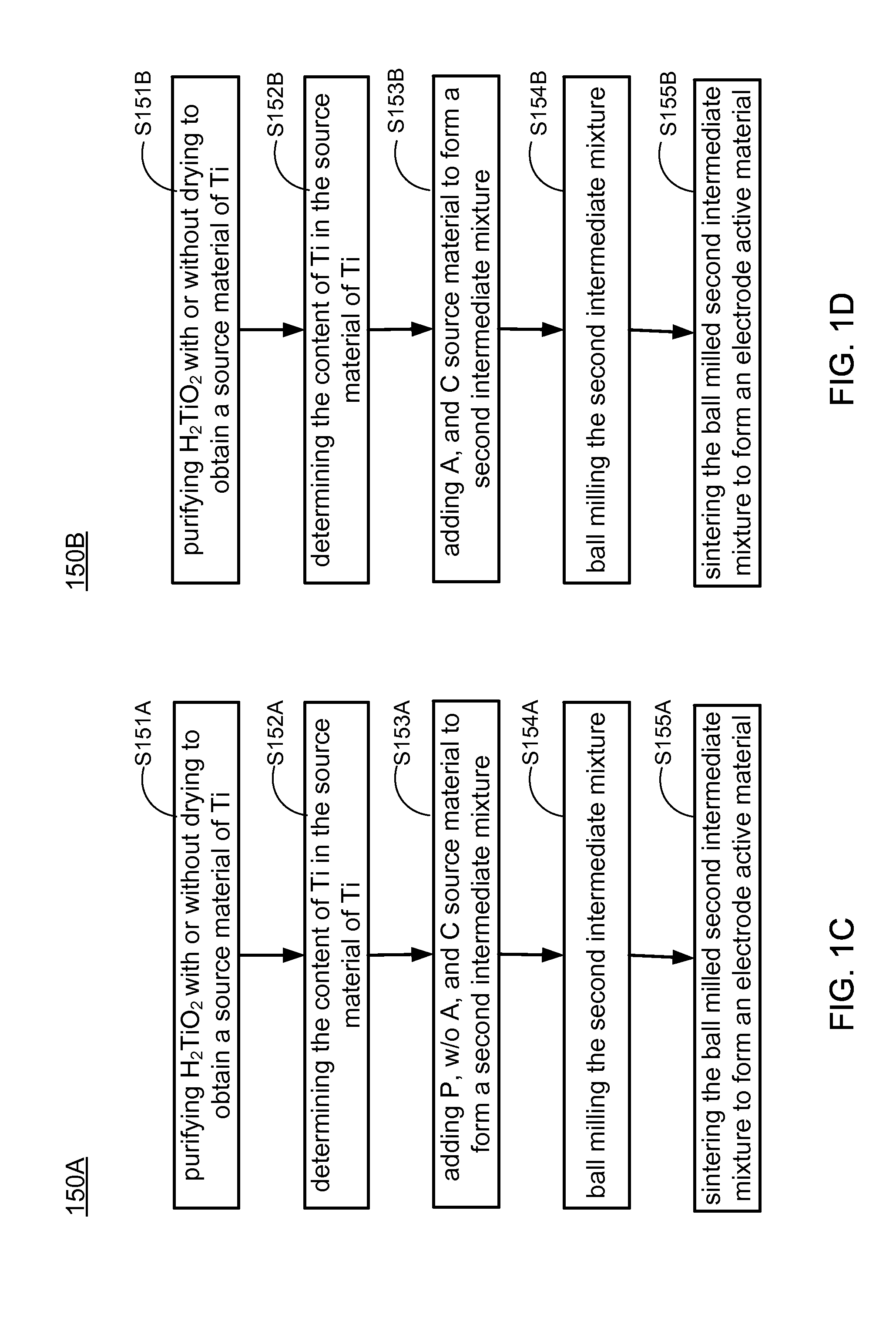
Methods of making low cost electrode active materials for secondary batteries from ilmenite
A method of producing electrode active materials includes generating a source material of titanium (Ti) and a source material of iron (Fe) from an ilmenite, and performing a operation to the source material of Fe and the source material of Ti. The operation includes determining a content of Fe or Ti in the source material of Fe or Ti, preparing an intermediate mixture having the source material of Fe or Ti and other required source materials, ball-milling and drying the intermediate mixture, and sintering the intermediate mixture to form the electrode active materials.
Owner:HUANG GUIQING +2
Multi-element Prussian blue sodium ion battery positive electrode material and preparation method thereof
PendingCN112607748AThe synthesis process is simpleReduce energy consumptionIron cyanidesCell electrodesPeristaltic pumpNickel acetate tetrahydrate
The invention relates to a multi-element Prussian blue sodium ion battery positive electrode material and a preparation method thereof. The preparation method comprises the following steps: mixing nickel acetate tetrahydrate Ni (AC) 2.4 H2O, cobalt acetate tetrahydrate Co (AC) 2.4 H2O, ferrous sulfate heptahydrate FeSO4. 7H2O and trisodium citrate dehydrate C6H5Na3O7. 2H2O to form a chelated solution A, mixing sodium ferrocyanide Na4Fe (CN) 6 and ascorbic acid to form a solution B, dissolving NaCl and PVP in deionized water, slowly dropwise adding the solution A and the solution B into the solution C by using a peristaltic pump, and stirring while dropwise adding; continuously stirring and reacting for 12 hours after dropwise adding to obtain turbid liquid, standing, ageing, centrifugally washing, and drying a filter cake in vacuum to obtain the multi-element Prussian blue sodium ion battery positive electrode material.
Owner:CHINA THREE GORGES UNIV
Popular searches
Antinoxious agents Pill delivery Animal repellants Aluminium/calcium/magnesium active ingredients Iodine compound active ingredients Plant growth regulators Secondary cells charging/discharging Non-aqueous electrolyte accumulator electrodes Group 3/13 element organic compounds Diagnostic recording/measuring
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
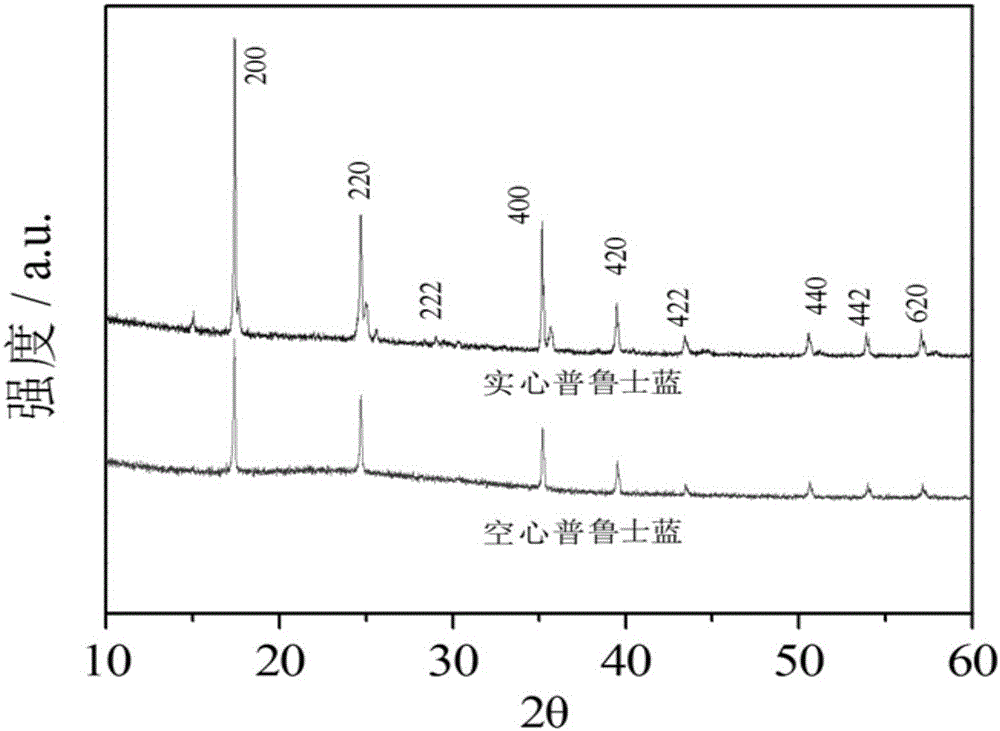
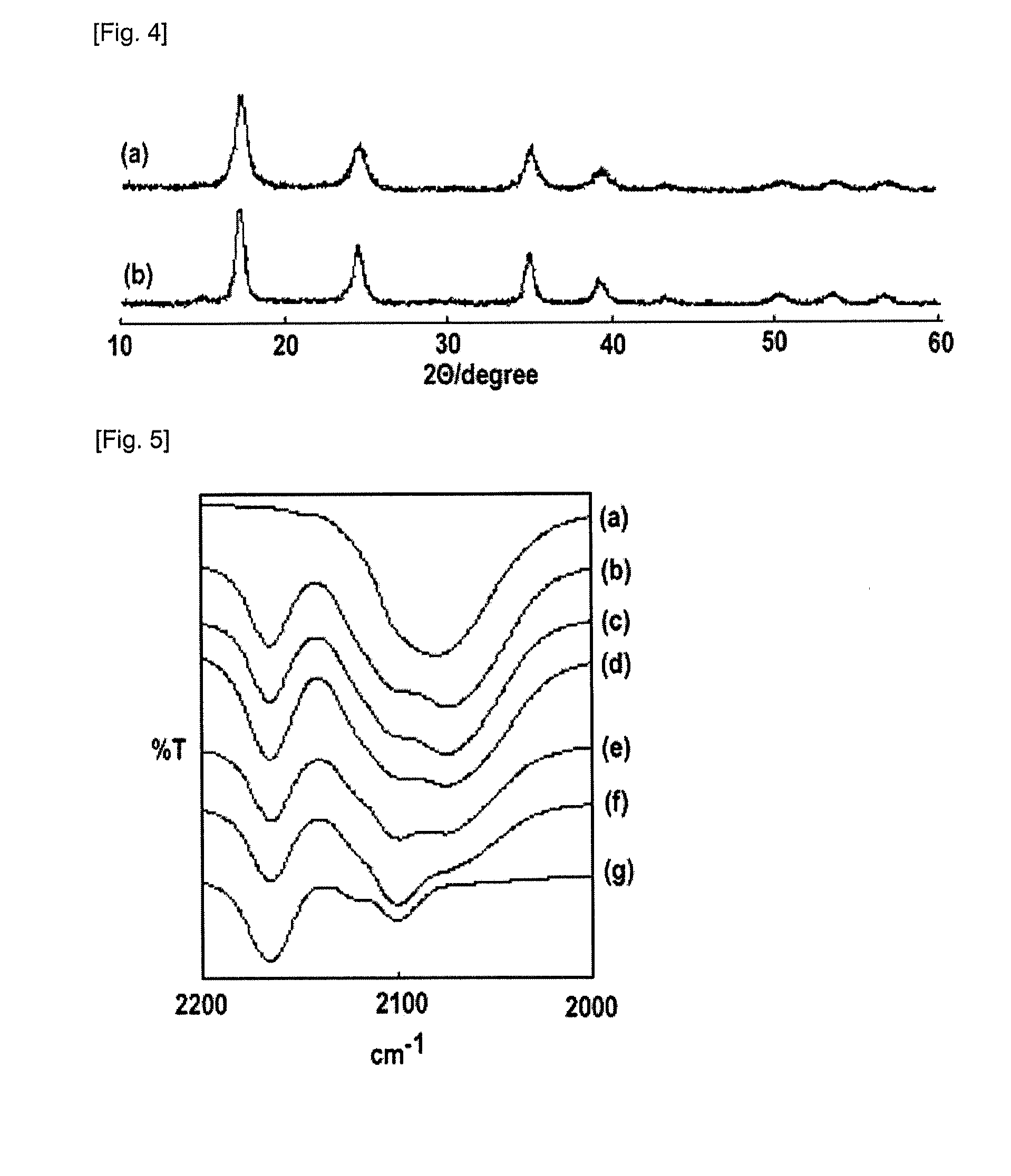

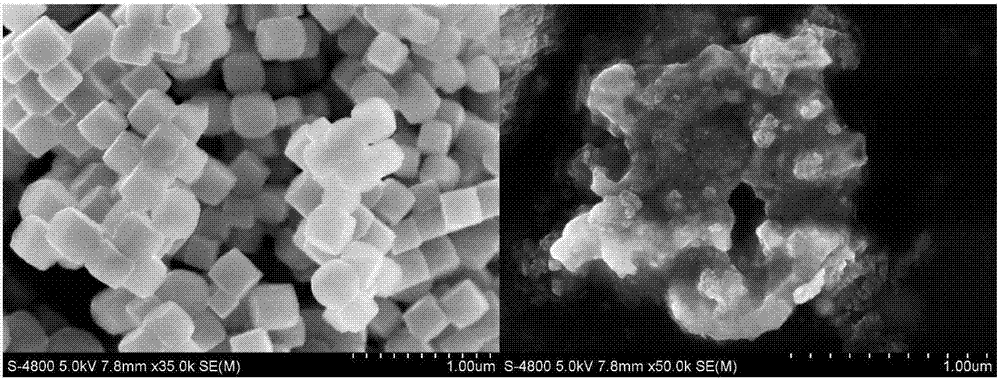
![Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof](https://images-eureka.patsnap.com/patent_img/00ecc6d4-9a9a-4fee-a376-13990313c933/1510121702471.PNG)
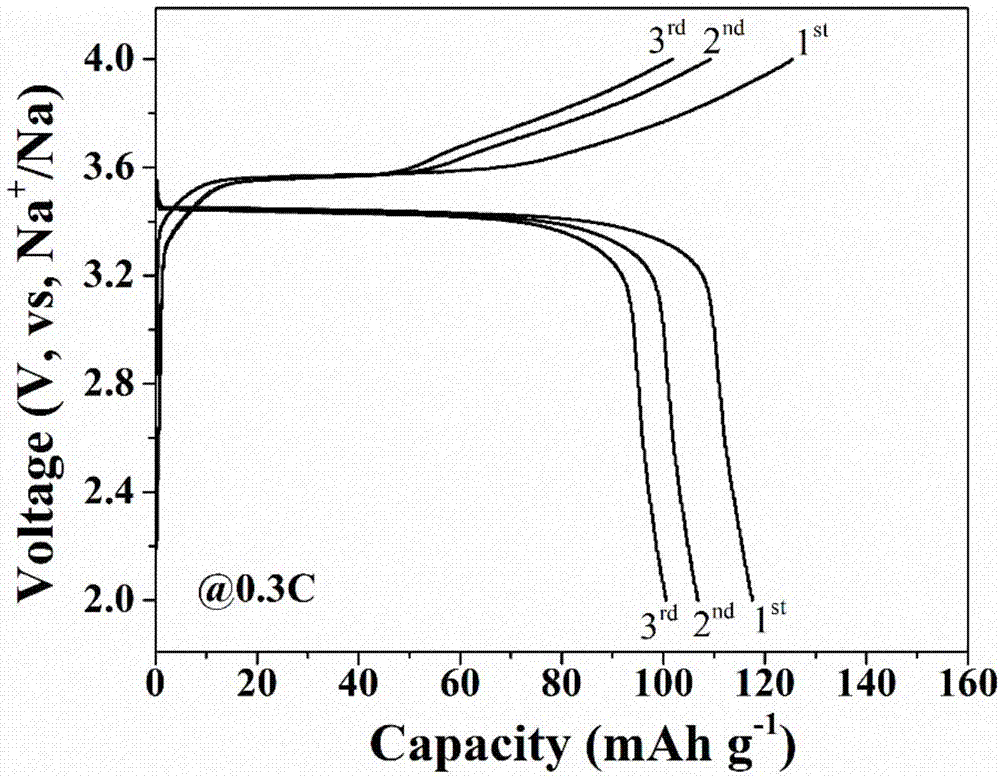
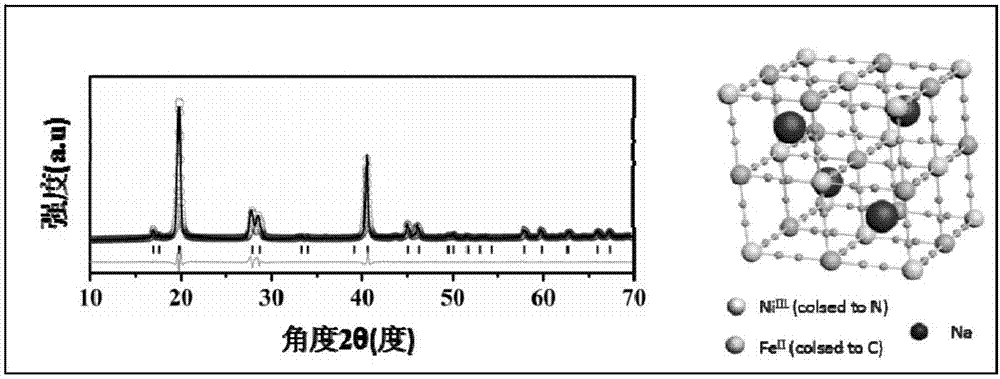
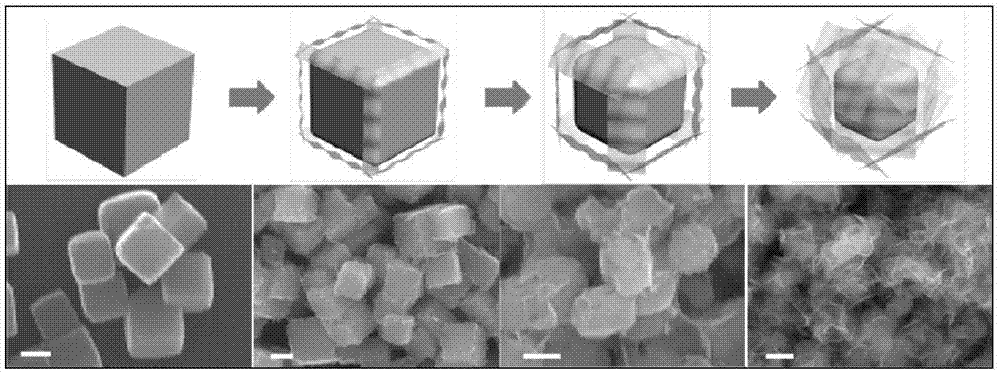
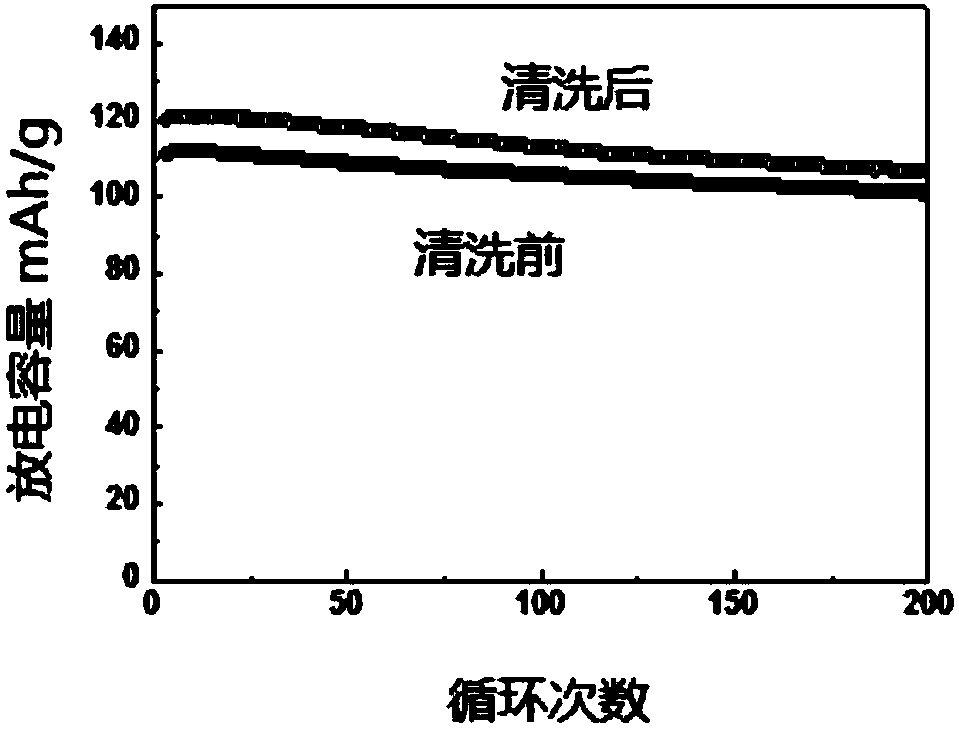
![Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof](https://images-eureka.patsnap.com/patent_img/00ecc6d4-9a9a-4fee-a376-13990313c933/1510121702472.PNG)
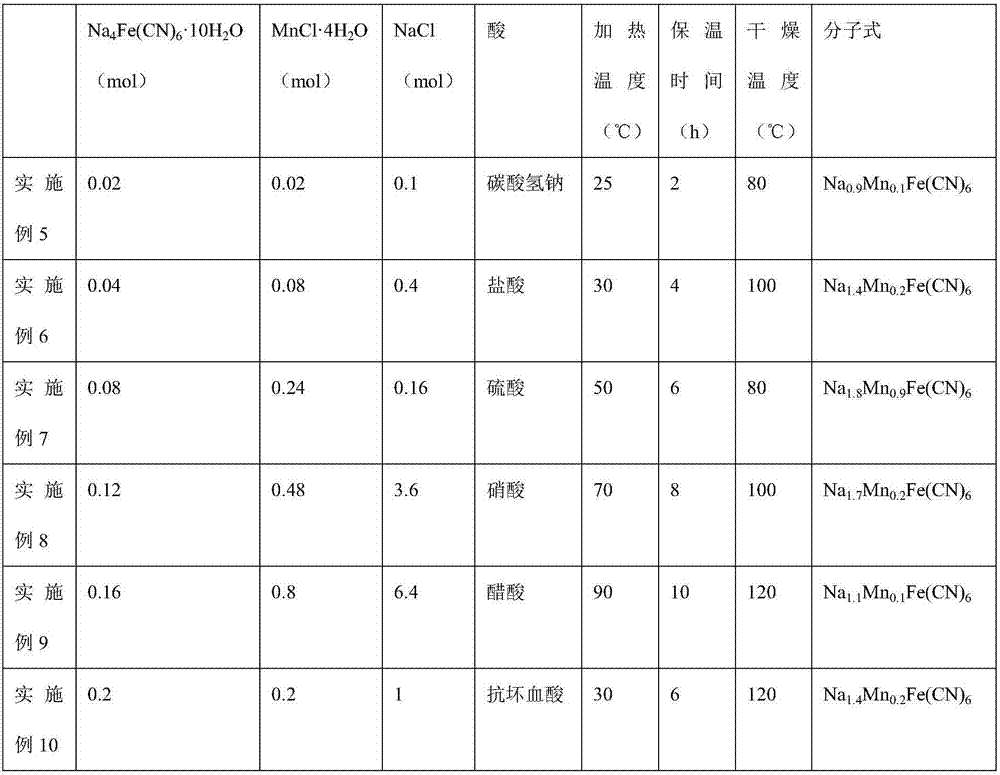
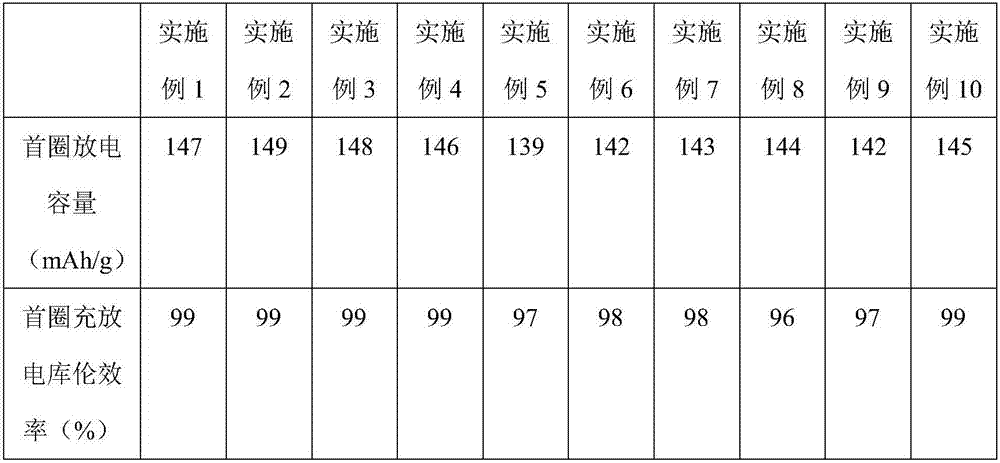
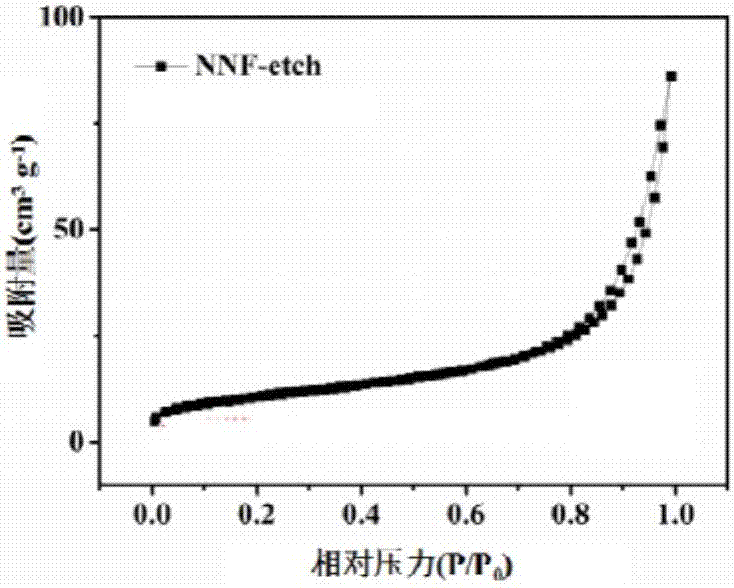
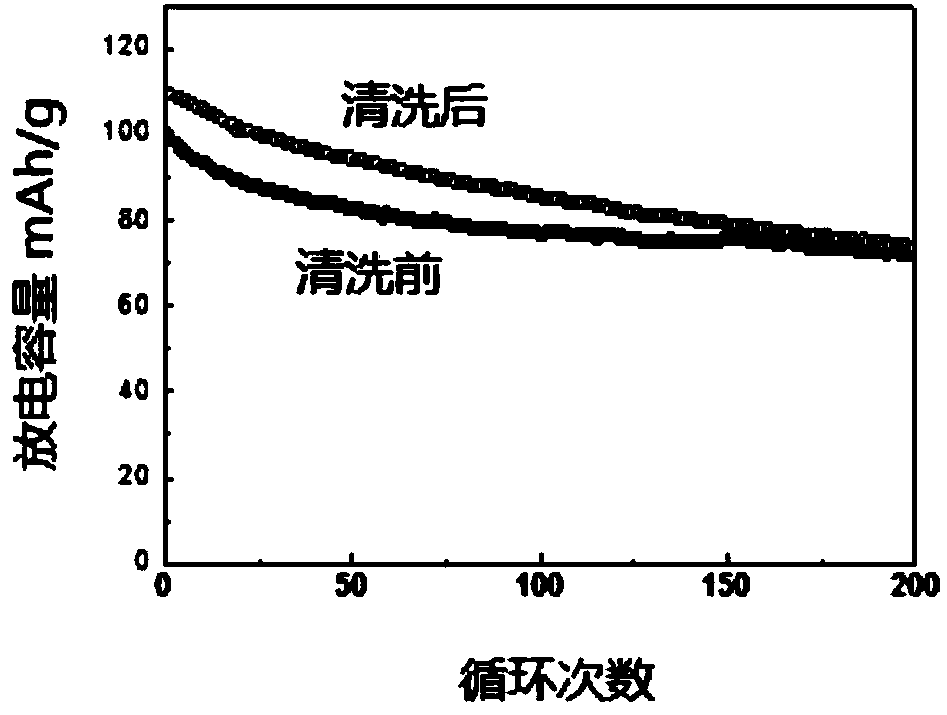
![Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof Prussian-blue derivative Cd2[Fe(CN)6] nanorod and preparation method thereof](https://images-eureka.patsnap.com/patent_img/00ecc6d4-9a9a-4fee-a376-13990313c933/1510121702473.PNG)