Metal-doped transition metal hexacyanoferrate (TMHCF) battery electrode
A metal doping, battery electrode technology, applied in the field of electrochemical batteries, can solve the problems of small capacity and reducing the concentration of mobile ions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Al 0.05 mn 0.95 -HCF
[0063] Solution 1 is Na 4 Fe(CN) 6 of aqueous solution. Solution 2 contains Mn 2+ and Al 3+ ion. Slowly drop solution 2 into solution 1 to form Al 0.05 mn 0.95 - Precipitation of HCF. After separation, washing and drying, the Al 0.05 mn 0.95 -HCF is used as an electrode in a sodium-ion battery with saturated NaClO 4 Ethylene carbonate / diethyl carbonate (EC / DEC) electrolyte. For comparison, Mn-HCF was synthesized under the same conditions.
[0064] Figure 5A and Figure 5B is Mn-HCF doped with aluminum (Al 0.05 mn 0.95 -HCF) electrode compared with Mn-HCF (undoped) electrode. The capacity was normalized by the maximum capacity of the Mn-HCF electrode during the first discharge. Figure 5A depicts the Mn-HCF and Al 0.05 mn 0.95 - Comparison of charge / discharge curves of HCF electrodes. Using Al 3+ Ion doping increased the capacity of the Mn-HCF electrode by about 15%. The electrodes were then cycled at currents of...
Embodiment 2
[0066] Example 2: NaKMn-HCF
[0067] Solution 1 contains Na 4 Fe(CN) 6 and K 4 Fe(CN) 6 . Solution 2 is Mn 2+The solution. Solution 1 and Solution 2 were mixed together to obtain NaKMn-HCF. For comparison, Mn-HCF was synthesized under the same conditions. NaKMn-HCF was evaluated as an electrode in a Na-ion battery with saturated NaClO 4 Ethylene carbonate / diethyl carbonate (EC / DEC) electrolyte.
[0068] Image 6 is a graph depicting the change in capacity of Mn-HCF and NaKMn-HCF electrodes cycled at different charge / discharge currents. Although the capacity of NaKMn-HCF is lower than that of Mn-HCF during the first cycle, it exhibits better capacity retention. After 100 cycles, the normalized capacity of KNaMn-HCF was 111%, but that of Mn-HCF at 0.1C was 91%. In other words, since K + The doping of ions increases the capacity retention by 20%. K + Ions greater than Na + ions, which support the structure of Mn-HCF and stabilize it during charge / discharge cycles....
Embodiment approach
[0081] US 13 / 897,492 is incorporated herein by reference.
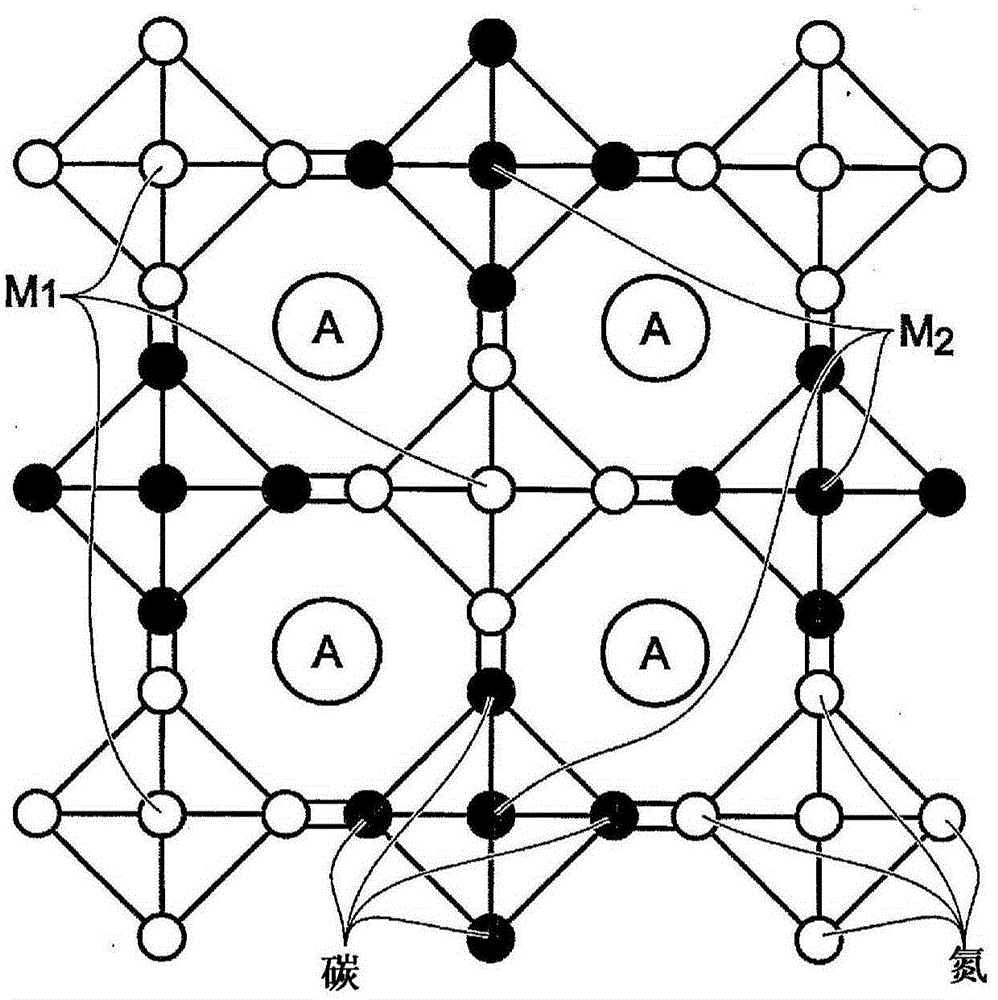
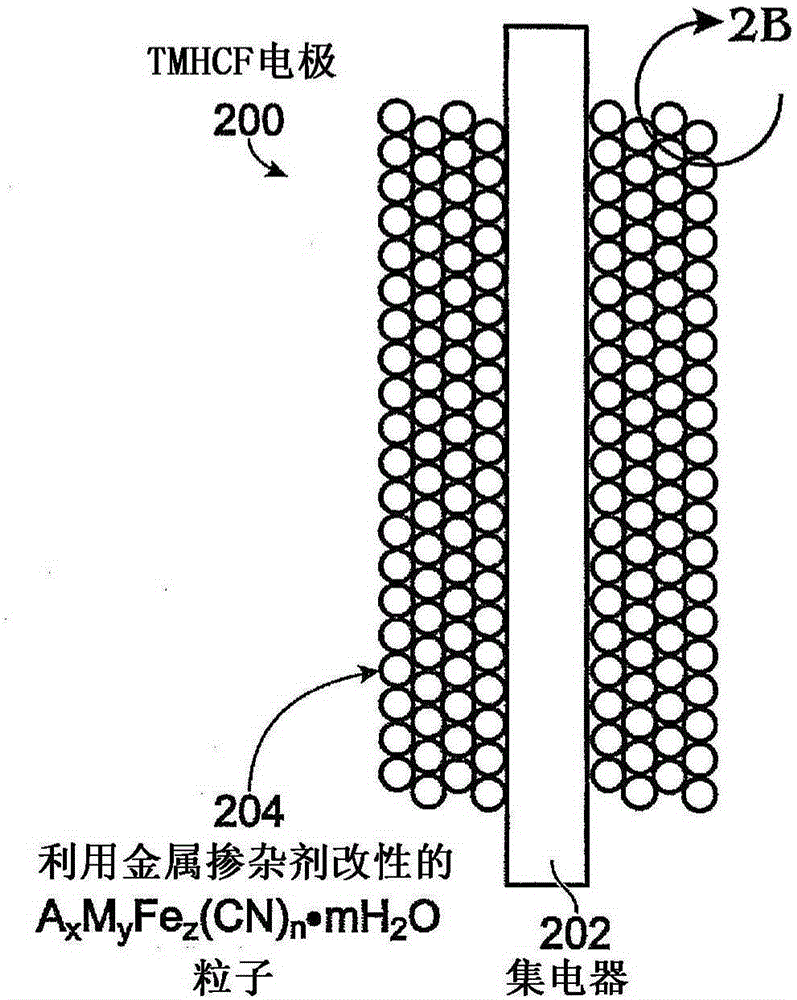
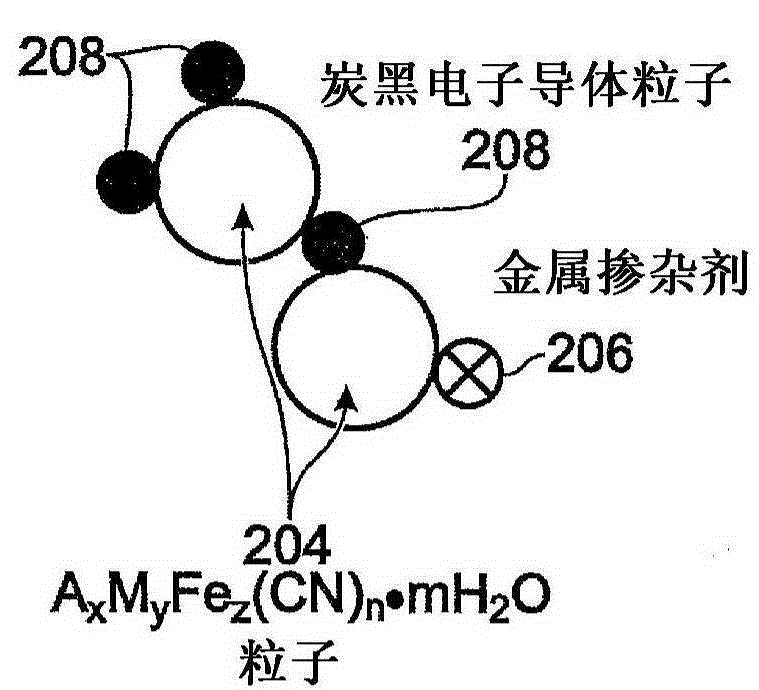
[0082] One with Fe(CN) 6 An additive transition metal hexacyanoferrate (TMHCF) battery electrode comprising: a metal current collector; A covering the current collector x m y Fe z (CN) n .mH 2 O particles; wherein the A cation is selected from alkali metal cations and alkaline earth metal cations; wherein M is a transition metal; wherein x is 0-2; wherein y is 0-2; wherein z is 0.1-2; wherein n is 1-6; Wherein m is 0~7; And Fe(CN) 6 Additive pair A x m y Fe z (CN) n .mH 2 O particles are modified.
[0083] The TMHCF battery electrode, wherein Fe(CN) 6 Additives are selected from ferrocyanide ([Fe(CN) 6 ] 4- ) and ferricyanide ([Fe(CN) 6 ] 3- ).
[0084] The TMHCF battery electrode, wherein the A cation is selected from sodium (Na), potassium (K), calcium (Ca) and magnesium (Mg).
[0085] A kind of synthesis has Fe(CN) 6 A method for additive transition metal hexacyanoferrate (TMHCF) battery electrod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com