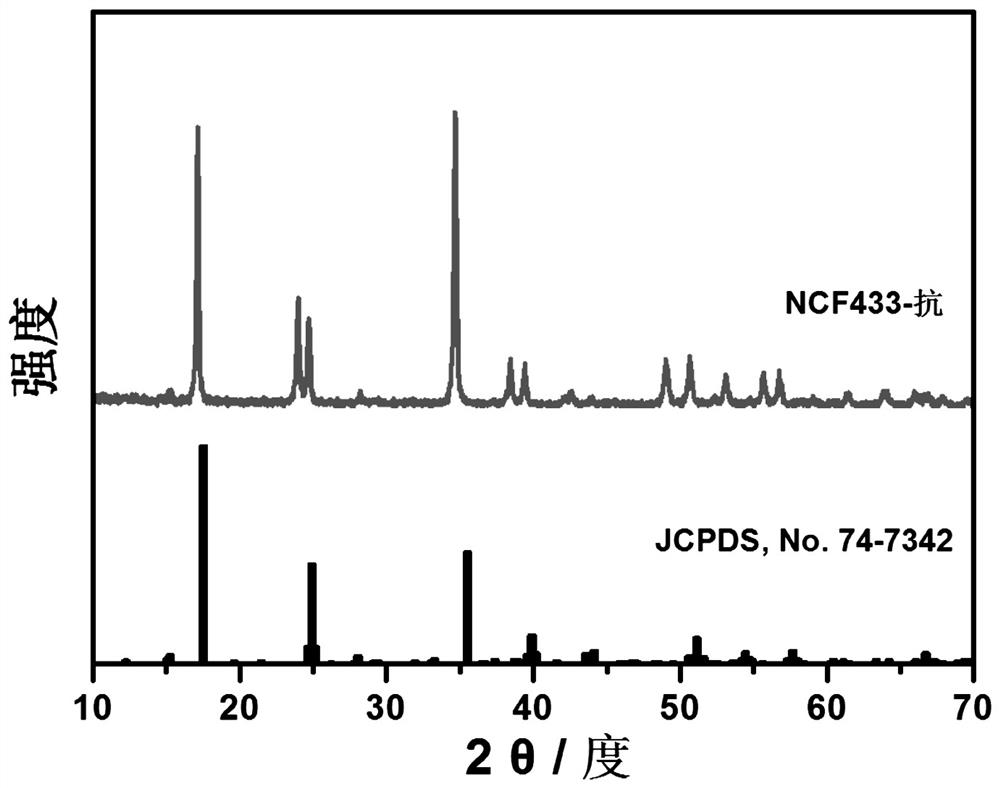
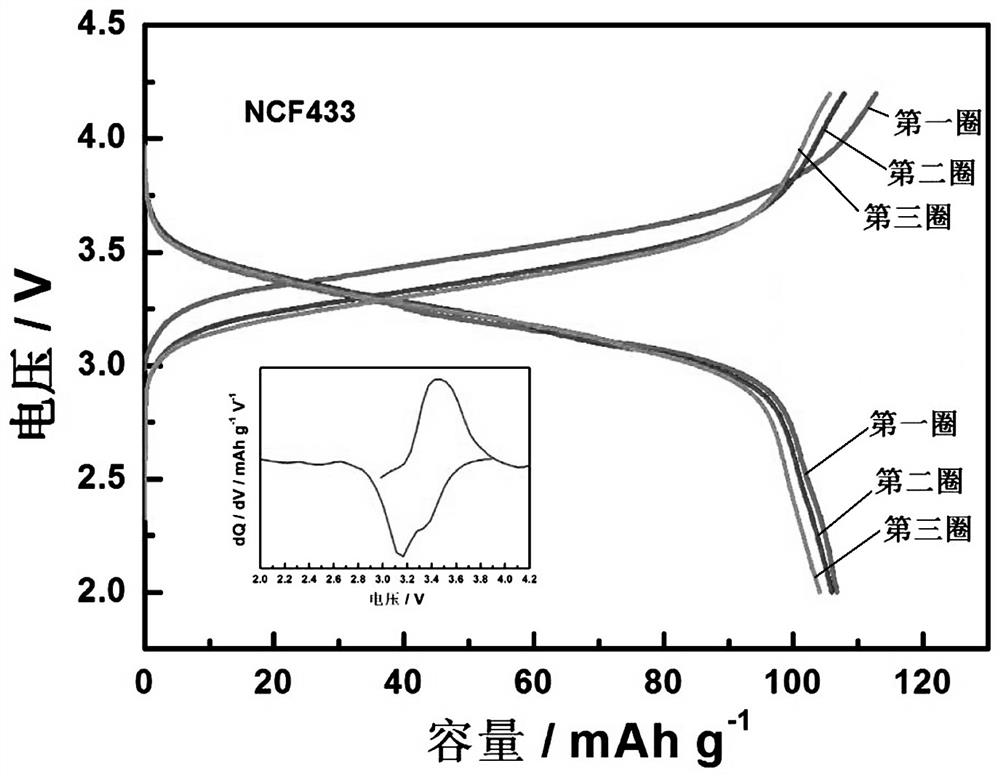
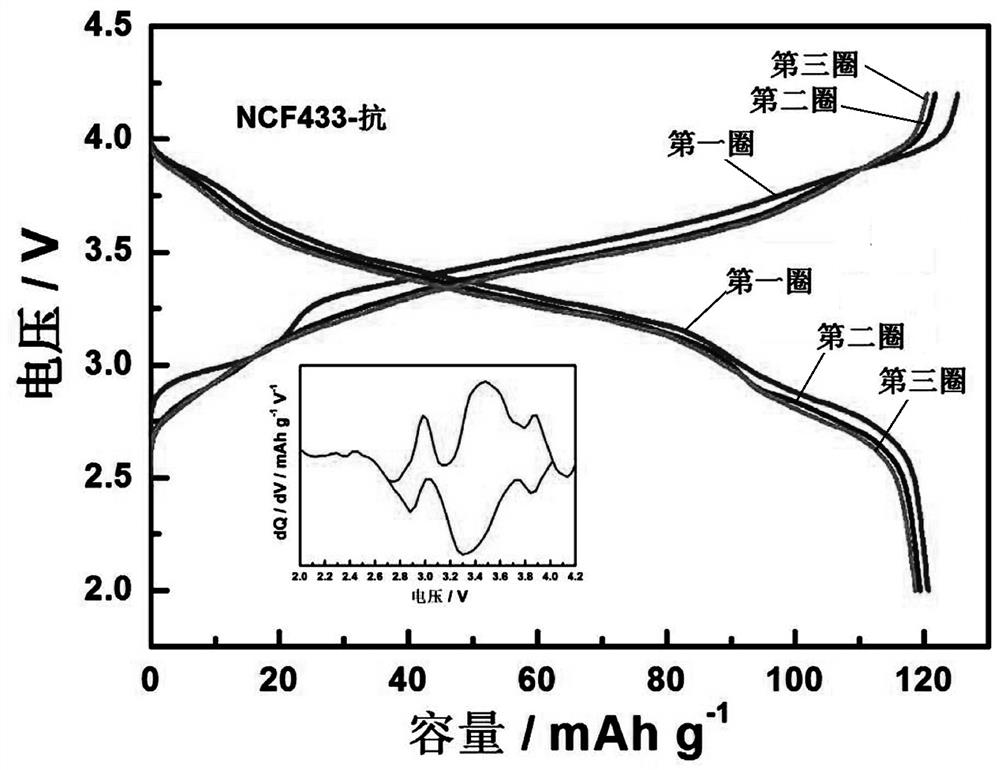
Multi-element Prussian blue sodium ion battery positive electrode material and preparation method thereof
A cathode material, Prussian blue technology, applied in the field of electrochemical power supply, can solve problems such as poor cycle stability, and achieve the effects of improving structural stability, simple synthesis process and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 3.5 mmol of nickel acetate tetrahydrate, 1.5 mmol of cobalt acetate tetrahydrate, and 5 mmol of trisodium citrate dihydrate in 50 ml of deionized water to form chelate solution A, and dissolve 5 mmol of sodium ferrocyanide and 1 g of ascorbic acid in A homogeneous solution B was formed in 50 ml of deionized water, and a homogeneous solution C was obtained by dissolving 3 g of NaCl and 1 g of PVP in 100 ml of deionized water. Mix solution A and solution B in 10 ml min -1 The dropping rate is added dropwise to solution C, and at the same time at 50 o N at a temperature of C 2 The reaction was stirred in the atmosphere, and the reaction solution was green. After the dropwise addition, continue to stir and react for 12 h, after standing still for 24 h, after washing with deionized water and alcohol several times, the filter cake was washed at 120 o After vacuum drying at C for 24 h, a blue-green Ni:Co:Fe 7:3:0 multi-component Prussian blue cathode material can b...
Embodiment 2
[0028] Dissolve 2.75 mmol of nickel acetate tetrahydrate, 2.25 mmol of ferrous sulfate heptahydrate and 5 mmol of trisodium citrate dihydrate in 50 ml of deionized water to form chelate solution A, and dissolve 5 mmol of sodium ferrocyanide with 1 g of ascorbic acid. A homogeneous solution B was formed in 50 ml deionized water, and 3 g NaCl and 1 g PVP were dissolved in 100 ml deionized water to obtain a homogeneous solution C. Mix solution A and solution B in 10 ml min-1 The dropping rate is added dropwise to solution C, and at the same time at 50 o N at a temperature of C 2 The reaction was stirred in the atmosphere, and the reaction solution was green. After the dropwise addition, continue to stir and react for 12 h, after standing still for 24 h, after washing with deionized water and alcohol several times, the filter cake was washed at 120 o After vacuum drying at C for 24 h, a blue-green Ni:Co:Fe 5.5:0:4.5 multi-component Prussian blue cathode material can be obtained,...
Embodiment 3
[0030] 2.5 mmol of nickel acetate tetrahydrate, 1 mmol of cobalt acetate tetrahydrate, 1.5 mmol of ferrous sulfate heptahydrate and 5 mmol of trisodium citrate dihydrate were dissolved in 50 ml of deionized water to form chelate solution A, and 5 mmol of sodium ferrocyanide was mixed with 1 g of ascorbic acid was dissolved in 50 ml of deionized water to form a homogeneous solution B, and 3 g of NaCl and 1 g of PVP were dissolved in 100 ml of deionized water to obtain a homogeneous solution C. Mix solution A and solution B in 10 ml min -1 The dropping rate is added dropwise to solution C, and at the same time at 50 o N at a temperature of C 2 The reaction was stirred in the air, and the reaction solution was green. After the dropwise addition was completed, the mixture was then stirred for 12 h, left to stand for 24 h, washed with deionized water and alcohol several times, and then heated at 120 o Dry in a vacuum oven at C for 24 h to obtain a blue-green Ni:Co:Fe multi-compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com