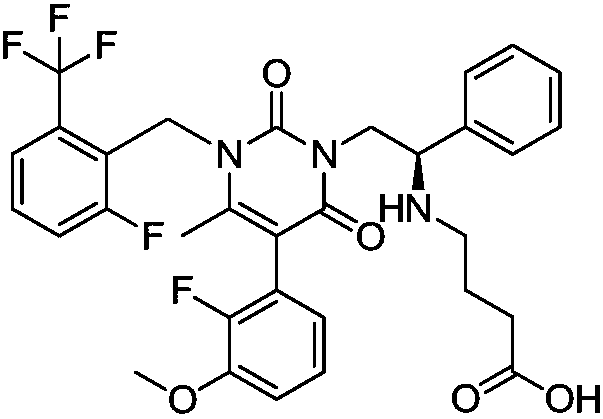
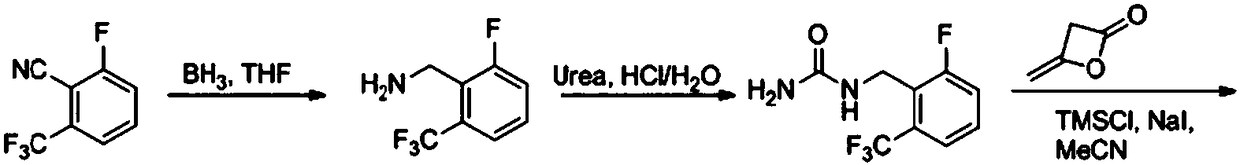
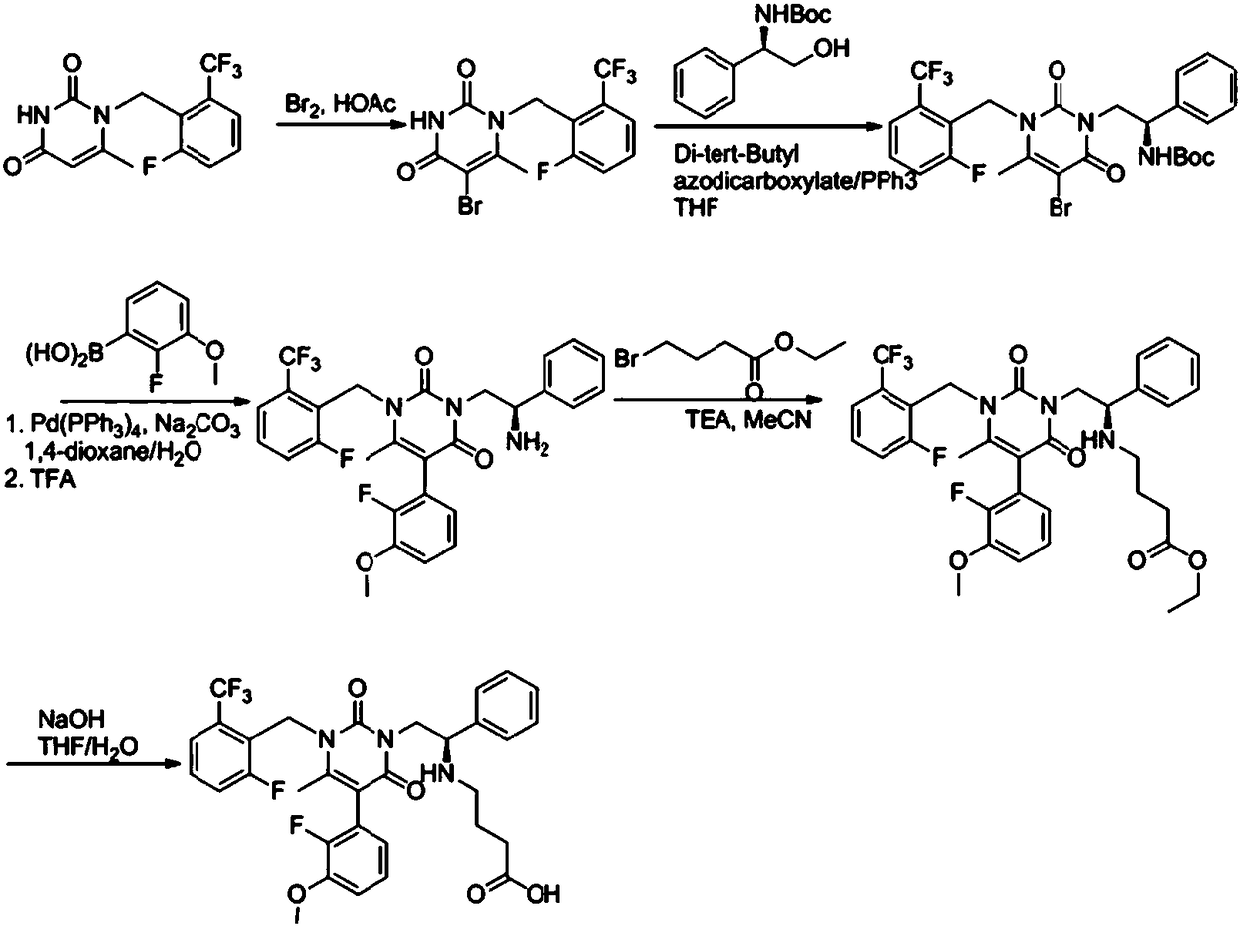
Elagolix synthesis method
A synthetic method, the technology of elagoli, is applied in the field of medicine and chemical industry, and can solve the problem of high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] Add 5-bromo-6-methylpyrimidine-2,4(1H,3H)-dione 1 (20.50g, 100mmol) and 2-(bromomethyl)-1-fluoro-3-trifluoromethane into a three-necked flask Benzene 2 (25.70g, 100mmol) and N,N-dimethylformamide (103mL), stirred and dissolved, then added potassium carbonate (27.64g, 200mmol), stirred evenly and heated to 75-85°C to react overnight. After the reaction was completed, water (205 mL) was added, and a large amount of solid was precipitated, which was filtered and dried to obtain compound 3 (30.88 g, 81%).
[0045] Potassium carbonate can be replaced by sodium carbonate, cesium carbonate, potassium tert-butoxide, sodium tert-butoxide, triethylamine or diisopropylethylamine, and the solvent N,N-dimethylformamide can be replaced by N,N-dimethylformamide Acetamide, N-methylpyrrolidone, acetonitrile, tetrahydrofuran, 2-methyltetrahydrofuran, 1,4-dioxane, toluene, xylene or chlorobenzene instead.
Embodiment 2
[0047]
[0048]Add compound formula 3 (38.11g, 100mmol) and tetrahydrofuran (190mL) into the three-necked flask, stir and dissolve, cool in an ice-salt bath to 0-5°C, add iron triacetylacetonate (1.77g, 5mmol), tetramethylethylenediamine (2.32g, 20mmol), switch the nitrogen gas 3 times under vacuum, add Grignard reagent 4 (200mmol, 190mL) tetrahydrofuran solution dropwise, and then raise the temperature to 40-45°C for 6-8 hours after the dropping. After the reaction was completed, dilute hydrochloric acid (2mol / L, 381mL) was added to quench the reaction, the precipitated solid was slurried, filtered and dried to obtain compound 5 (32.83g, 77%).
[0049] Here, iron triacetylacetonate can be replaced by ferric chloride or ferric bromide; tetramethylethylenediamine can be omitted, and can also be replaced by anhydrous lithium chloride or anhydrous lithium bromide.
Embodiment 3
[0051]
[0052] Add compound formula 6a (20.50g, 100mmol), 7a (15.62g, 120mmol) and dimethyl sulfoxide (102mL) in the three-necked flask, add cuprous iodide (1.90g, 10mmol) after stirring and dissolving, L-proline Acid (1.15g, 10mmol), potassium carbonate (27.64g, 200mmol), nitrogen was switched 3 times under vacuum, and the temperature was raised to 45-50°C for 6-8 hours. After the reaction was completed, ammonium chloride solution (204 mL) was added to quench the reaction, ethyl acetate (102 mL) was added to extract three times, the organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove part of the solvent, and added The product 8a (21.66g, 74%) was obtained after beating with petroleum ether, filtering, collecting and drying the solid.
[0053] Here cuprous iodide can be replaced by cuprous bromide or cuprous chloride; L-proline can be omitted, and can also be replaced by TMEDA or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com