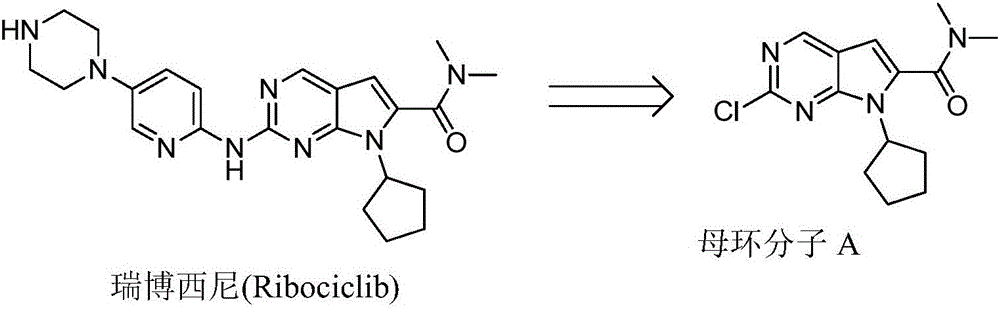
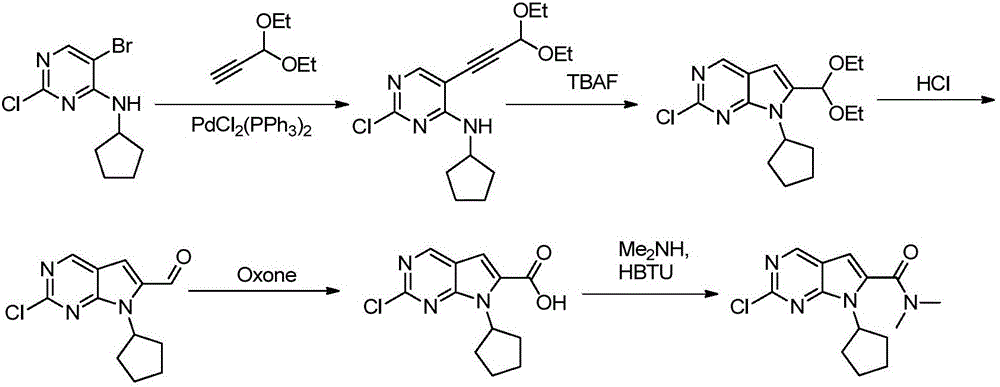
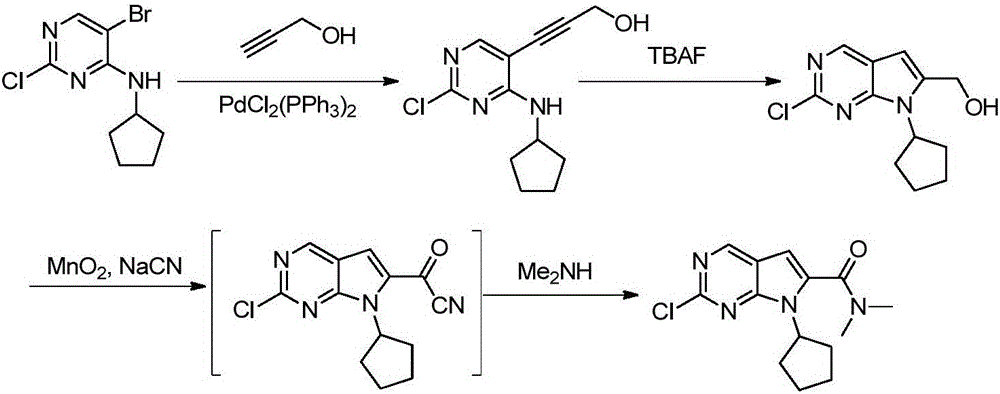
New synthesis method of ribociclib intermediate
A technology of ribociclib and intermediates, which is applied in the new synthesis field of intermediate compounds, can solve the problems of high risk of workers' operation, inconvenient process scale-up operation, unfavorable process scale-up, etc., so as to optimize the coupling conditions and shorten the reaction steps. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Add 5-bromo-2-chloro-4-(cyclopentylamino)pyrimidine 1a (27.66g, 100mmol) in the three-necked flask, ethyl propiolate 2a (19.62g, 200mmol), DABCO (2.24g, 20mmol) and Acetonitrile (277mL), stirred evenly, cooled to 0-5°C, switched nitrogen three times under vacuum, added newly prepared cuprous iodide (1.9g, 10mmol) and dichloroditriphenylphosphine palladium (702mg, 1mmol) under nitrogen protection After the addition, the temperature was raised to 55-60°C for 6-8 hours. After the reaction, most of the acetonitrile was rotated off, and 277 mL of water was added to quench the reaction. The aqueous phase was extracted 3 times with ethyl acetate (138 mL), and the combined organic phase was washed with saturated brine for 1 (138 mL), dried over sodium sulfate, concentrated and separated by column chromatography with a mixed solvent of dichloromethane and methanol to obtain compound 3a (21.44 g, 73%).
[0035] ESI m / z=294.10(M+1), 1 HNMR(CDCl3,400MHz)δ8.25(s,1H),4.2...
Embodiment 2
[0038]
[0039] Add 5-iodo-2-chloro-4-(cyclopentylamino) pyrimidine 1b (32.36g, 100mmol) in the three-necked flask, ethyl propiolate 2b (19.62g, 200mmol), DABCO (2.24g, 20mmol) and Acetonitrile (324mL), stirred evenly and cooled to 0-5°C, switched nitrogen three times under vacuum, added newly prepared cuprous iodide (1.9g, 10mmol) and dichloroditriphenylphosphine palladium (702mg, 1mmol) under nitrogen protection After the addition, the temperature was raised to 55-60°C for 6-8 hours. After the reaction, most of the acetonitrile was spun off, and 324 mL of water was added to quench the reaction. The aqueous phase was extracted 3 times with ethyl acetate (162 mL), and the combined organic phase was washed with saturated brine for 2 (162 mL), dried over sodium sulfate, concentrated and separated by column chromatography with a mixed solvent of dichloromethane and methanol to obtain compound 3b (23.71 g, 81%).
[0040] ESI m / z=293.11(M+1), 1HNMR(CDCl3,400MHz)δ8.27(s,1H),3.82...
Embodiment 3
[0043]
[0044] Add 3a (29.37g, 100mmol) and dimethylformamide (147mL) into the three-necked flask, stir evenly, switch nitrogen in vacuum for 3 times, and add new cuprous chloride (0.99g, 10mmol) and DBU (3.04g , 20mmol), after the addition was completed, the temperature was raised to 85-90°C to react overnight. At the end of the reaction, 294 mL of water was added to quench the reaction, the aqueous phase was extracted 3 times with ethyl acetate (147 mL), the combined organic phase was washed 2 times with saturated brine (147 mL), dried over sodium sulfate, concentrated and separated by column chromatography with dichloromethane methanol mixed solvent Compound 4a (25.26 g, 86%) was obtained.
[0045] Cuprous chloride in embodiment 3 can be replaced by cuprous bromide, and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) can be used diisopropylethylamine, triethylamine amine or triethylenediamine (DABCO) instead; the solvent dimethylformamide can be replaced by dimethylacetamide,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com