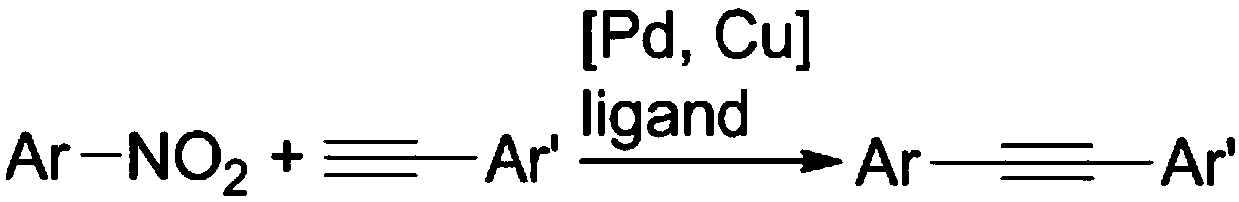Patents
Literature
193 results about "Sonogashira coupling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
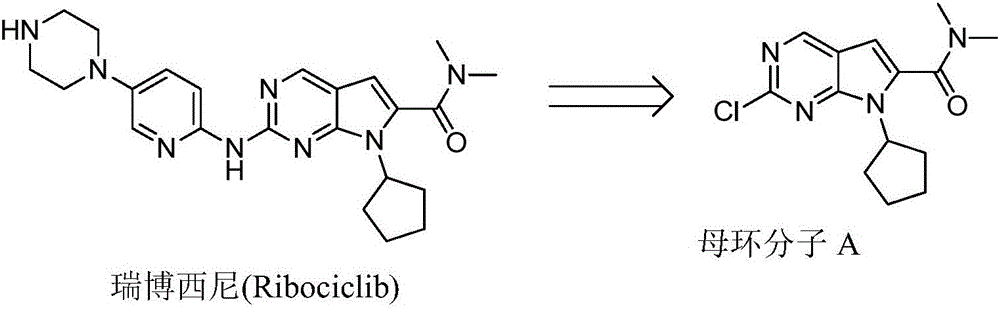
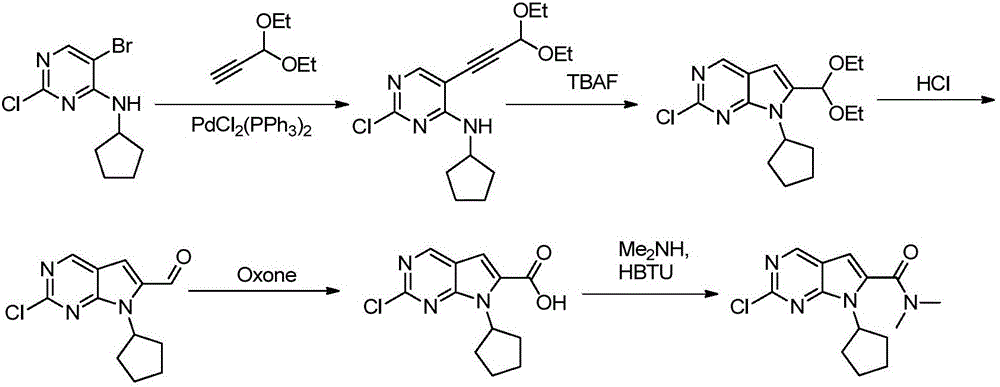
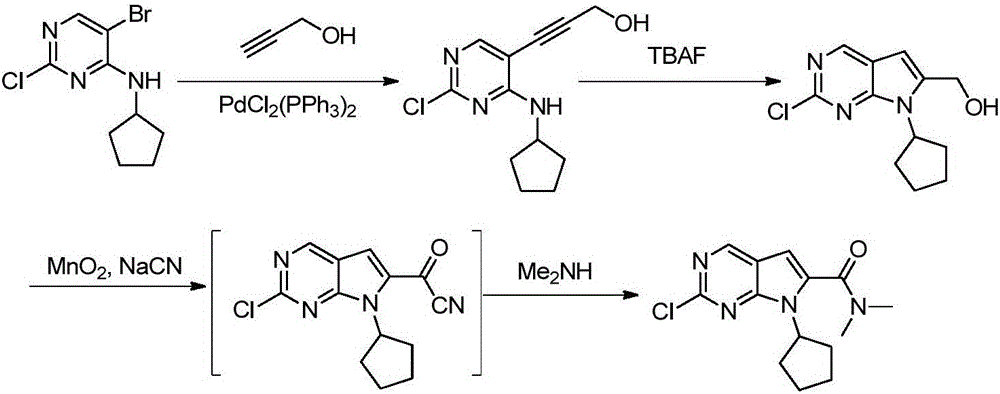
New synthesis method of ribociclib intermediate
ActiveCN106478641AEasy to operateImprove coupling conditionsOrganic chemistrySynthesis methodsSide chain
The invention discloses preparation of a ribociclib key intermediate A; propiolic acid ester or amide 2 is directly used as a Sonogashira coupling side chain, coupling conditions are optimized, an intermediate 3 is obtained with relatively high yield, the intermediate 3 is directly subjected to ring closing under simple conditions to complete construction of a mother ring molecule, to obtain a structural formula A or a precursor ester 4 of the structural formula A, and the precursor ester 4 is subjected to hydrolysis and condensation to obtain the structural formula A. The route has simple operation, reaction steps are shorted, the yield is relatively high, and the purity of the obtained product is relatively high, and the method is suitable for enlarged production, wherein the reaction route is described in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
In-situ nitrogen doped graphalkyne material and synthesizing and application methods thereof
ActiveCN108408715AImprove stabilityEasy to manufactureHybrid capacitor electrodesNano-carbonOrganic synthesisSynthesis methods
The invention belongs to the field of organic synthesis and relates to an in-situ nitrogen doped graphalkyne or graphdiyne material and synthesizing and application thereof to the field of energy storage. The synthesizing method of the in-situ nitrogen doped graphalkyne material comprises that, under the action of catalysts and solvent, a reaction monomer and tetrahaloethylene or cyanuric halogenperform Sonogashira coupling reaction at 60-150 DEG C under protection of inert gas to produce various types of nitrogen doped graphalkyne materials, wherein the reaction monomer is 2, 4, 6-triethinyl-1, 3, 5-triazine with a structural formula shown as the formula I. The synthesizing method of the in-situ nitrogen doped graphalkyne material has the advantages of being high in stability and simplein preparation, and meanwhile, is high in controllability and adjustability of reaction conditions; the applied catalysts are all commercialized catalysts and accordingly are wide in source and low incost; post-processing of polymerization is simple in process and beneficial to separation. The preparation process of the in-situ nitrogen doped graphalkyne material is applicable to medium and large-scale preparation and favorable to industrial production.
Owner:SICHUAN UNIV
Organic small molecular photoelectric functional material, and preparation method thereof
ActiveCN105017264AAbsorption red shiftPromote absorptionOrganic chemistrySolid-state devicesPorphyrinElectronic band structure
The invention discloses an organic small molecular photoelectric functional material. The organic small molecular photoelectric functional material containing porphyrin units and different dye units is prepared via selection of a plurality of dyes, and taking acetylenic bonds or aromatic ring or heterocyclic aromatic as bridges. According to a preparation method, the organic small molecular photoelectric functional material is prepared via Suzuki coupling reaction or Sonogashira coupling reaction. The organic small molecular photoelectric functional material possesses excellent processability, and appropriate energy band structures, and is high in energy conversion efficiency.
Owner:SOUTH CHINA UNIV OF TECH
Transition-metal-catalyzed carbon-nitrogen and carbon-carbon bond-forming reactions
ActiveUS20060173186A1More featureGroup 8/9/10/18 element organic compoundsCarboxylic acid amides preparationCarbon–carbon bondCoupling
One aspect of the present invention relates to ligands for transition metals. A second aspect of the present invention relates to the use of catalysts comprising these ligands in various transition-metal-catalyzed carbon-heteroatom and carbon-carbon bond-forming reactions. The subject methods provide improvements in many features of the transition-metal-catalyzed reactions, including the range of suitable substrates, number of catalyst turnovers, reaction conditions, and efficiency. For example, improvements have been realized in transition metal-catalyzed: aryl amination reactions; aryl amidation reactions; Suzuki couplings; and Sonogashira couplings. In certain embodiments, the invention relates to catalysts and methods of using them that operate in aqueous solvent systems.
Owner:MASSACHUSETTS INST OF TECH
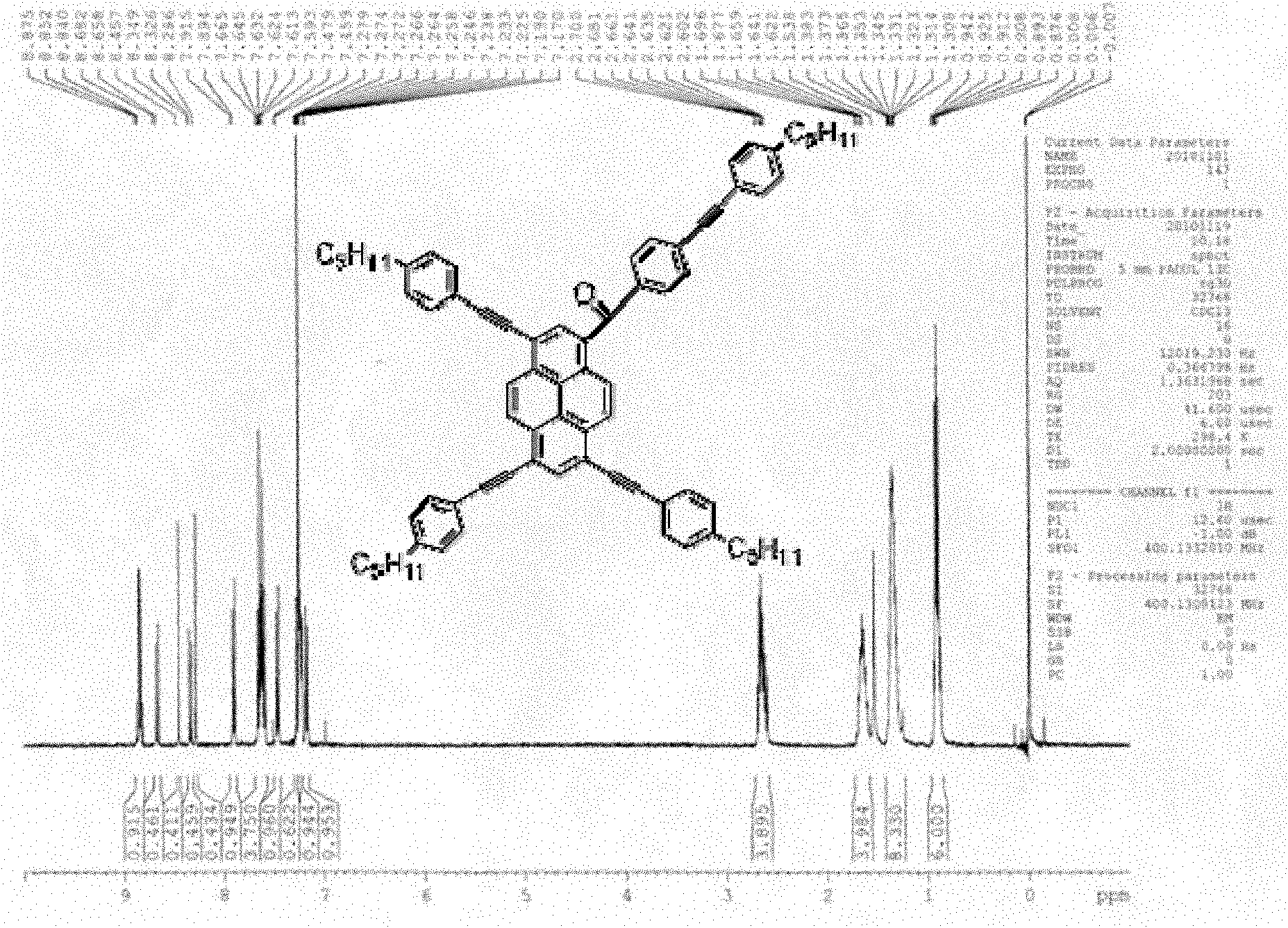
Pyrene asymmetrical double-shaft discotic liquid crystal compound and preparation method thereof
InactiveCN102061179AStrong fluorescenceFast refresh rateLiquid crystal compositionsOrganic compound preparationOrganic synthesisFluorescence
The invention belongs to the technical field of organic synthesis and relates to a pyrene asymmetrical double-shaft discotic liquid crystal compound and a preparation method thereof. The compound has a typical columnar hexagonal-phase discotic liquid crystal structure. The preparation method of the compound comprises the following steps of: carrying out a friedel-crafts acylation reaction on pyrene; carrying out a bromination reaction on an intermediate product; carrying out a Sonogashira coupling reaction; carrying out reaction for removing trimethyl silicon; and carrying out reaction for connecting bromination chains. The pyrene asymmetrical double-shaft discotic liquid crystal compound has biaxial orientation, i.e. a disk is used as a shaft, and an asymmetrical rod molecular chain is used as a shaft. Compared with the prior art, the invention has the advantages that the discotic liquid crystal compound with an asymmetrical structure is easier to form biaxial nematic discotic liquid crystal which has quicker refresh rate and low energy consumption, and has strong fluorescence and wide application prospect.
Owner:UNIV OF SCI & TECH BEIJING
Fluorescent sensor array based on conjugated polymers and application thereof
InactiveCN103525404AEasy to synthesizeEasy Ion DetectionFluorescence/phosphorescenceLuminescent compositionsSensor arrayAnalyte
The invention relates to a fluorescent sensor array based on conjugated polymers and application thereof. Three different synthetic routes are employed for synthesis of four aromatic hydrocarbons with halogens at two ends and two aromatic hydrocarbons with alkynyls at two ends; and different monomers are combined through Sonogashira coupling for synthesis of seven conjugated polymers which constitute the fluorescent sensor array applied to mode identification of nine metal ions. The array has similar response on same-group elements in the element periodic table and has relatively large response difference on different-group elements, so that the ions in different groups can be easily distinguished; and also, the array has slightly different response on the same kinds of ions which are in one same group but in different periods, so that the same-group different-period ions can be subjected to preliminary detection. During practical application, the response mode of a to-be determined standard substance can be stored, and the components of a to-be analyzed substance can be determined by determining the matching degree between the response mode of the to-be analyzed substance and the standard mode.
Owner:SUZHOU UNIV
Carbazole-based carborane derivative material, and preparation method and application thereof
InactiveCN107353302AEasy to prepareEasy to operateGroup 3/13 element organic compoundsTenebresent compositionsOrganic solar cellFluorescence
The invention discloses a carbazole-based carborane derivative material, and preparation and application methods thereof. The preparation method comprises the following steps: performing alkynylation on 9-(4-bromophenyl)-9-carbazole or 9-(3-bromophenyl)-9-carbazole by a Sonogashira coupling reaction to obtain 9-(4-acetenylphenyl)-9-carbazole or 9-(3-acetenylphenyl)-9-carbazole, and performing a reaction with carborane to synthesize a compound I or compound II. The invention gives out the specific conditions and illustrations on the reaction between the alkynyl derivatives of carbazole and the carborane, and the results show a high yield of the target products. The compounds are simple to prepare, the intermediates are low in cost, and the reaction processes are easy to control; and the products are easy to separate, have the advantages of high yield and high purity, and have potential application values in the aspects of sensors, electroluminescent devices, organic solar cells, organic field-effect transistors and the like. The carborane derivatives can be well applied to electrochemiluminescence (ECL), and can be used as a small-molecule biological fluorescent probe.
Owner:NANJING UNIV OF POSTS & TELECOMM
Tetracene derivative field effect transistor material and preparation method thereof
ActiveCN102659752AImprove performanceImprove stabilityOrganic compound preparationHydrocarbon by hydrocarbon and non-hydrocarbon condensationField effectStructural formula
The invention discloses a tetracene derivative field effect transistor material and a preparation method thereof. The tetracene derivative field effect transistor material has a general structural formula I, wherein Ar represents aryl, substituted aryl, heterocyclic aryl or substituted heterocyclic aryl and R represents one of alkyl, alkoxy, alkyl sulphanyl and the like. The tetracene derivative field effect transistor material can be synthesized by a Sonogashira coupling reaction and a Bergman cyclization reaction. The tetracene derivative field effect transistor material has high stability and high dissolvability and can improve a mobility ratio of OFETs devices.
Owner:NANJING UNIV OF POSTS & TELECOMM
Transition-metal-catalyzed carbon-nitrogen and carbon-carbon bond-forming reactions
ActiveUS7560596B2More featureGroup 8/9/10/18 element organic compoundsCarboxylic acid amides preparationCarbon–carbon bondCoupling
Owner:MASSACHUSETTS INST OF TECH
Beta-graphdiyne and synthesis method and application thereof in field of energy storage
ActiveCN106865526AImprove stabilityEasy to manufactureHybrid capacitor electrodesCell electrodesOrganic synthesisSynthesis methods
The invention discloses a beta-graphdiyne and a synthesis method and application thereof in the field of energy storage, and belongs to the field of organic synthesis. The synthesis method of the beta-graphdiyne provided by the invention comprises the following steps: using 3-(dibromomethenyl)-1,4-pentadiine as a reaction monomer, and undergoing an Sonogashira coupling reaction under the action of a catalyst and a solvent and the protection of an inert gas at a temperature of 60 to 150 DEG C to obtain beta-graphdiyne, wherein the catalyst is a mixture of a palladium catalyst and a Cu (I) salt with a molar ratio of (1 to 10) to (1 to 1), and the solvent is a mixed solvent of a conventional solvent and an organic amine solvent; and the molar ratio of the reaction monomer to the palladium catalyst is (1 to 0.05) to (1 to 0.2). The synthesis method of the beta-graphdiyne provided by the invention has the advantages that raw materials are cheap and easy to obtain, the process is simple, the cost is relatively low, and the yield is relatively high, and can be used for realizing industrial production.
Owner:SICHUAN UNIV
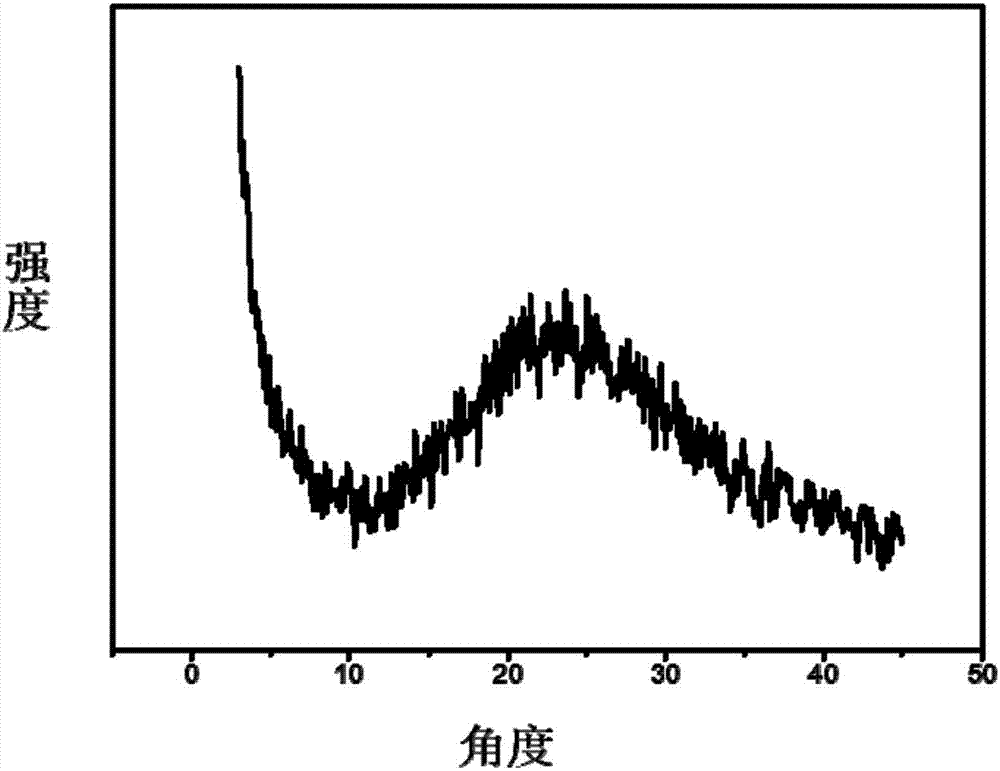
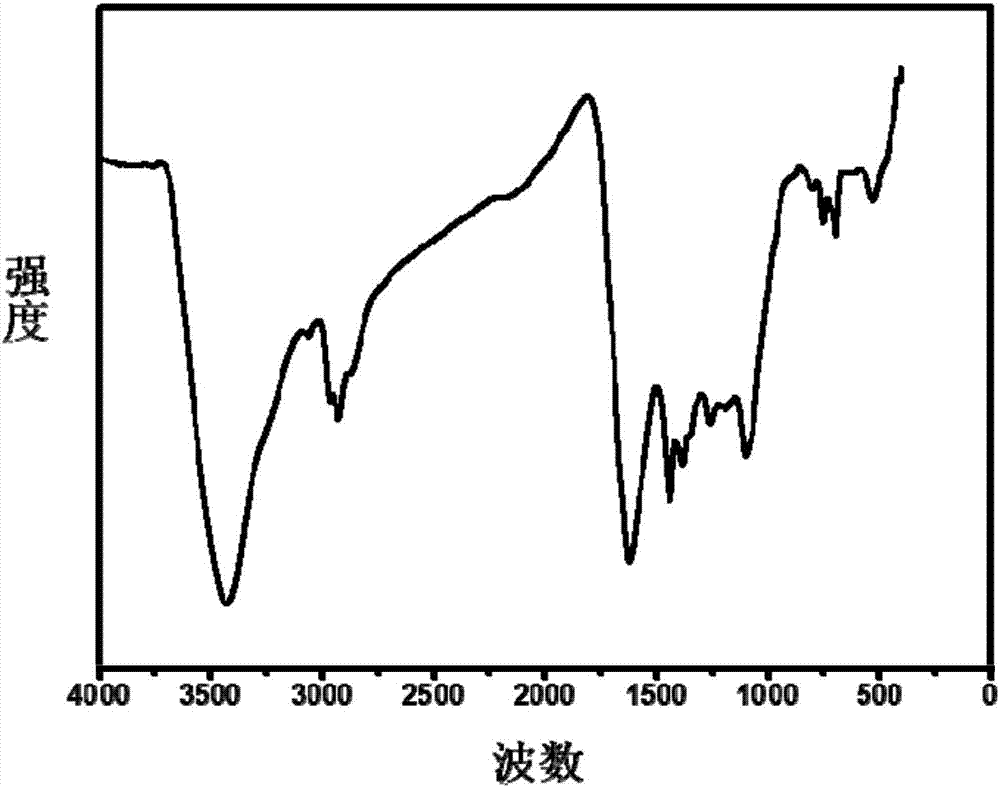
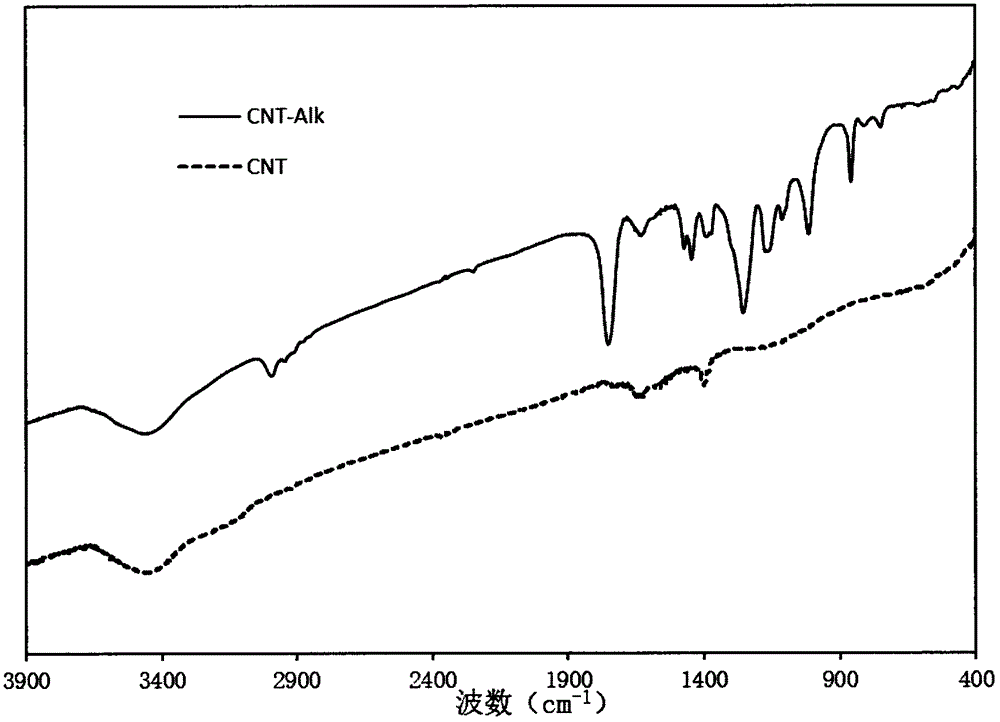
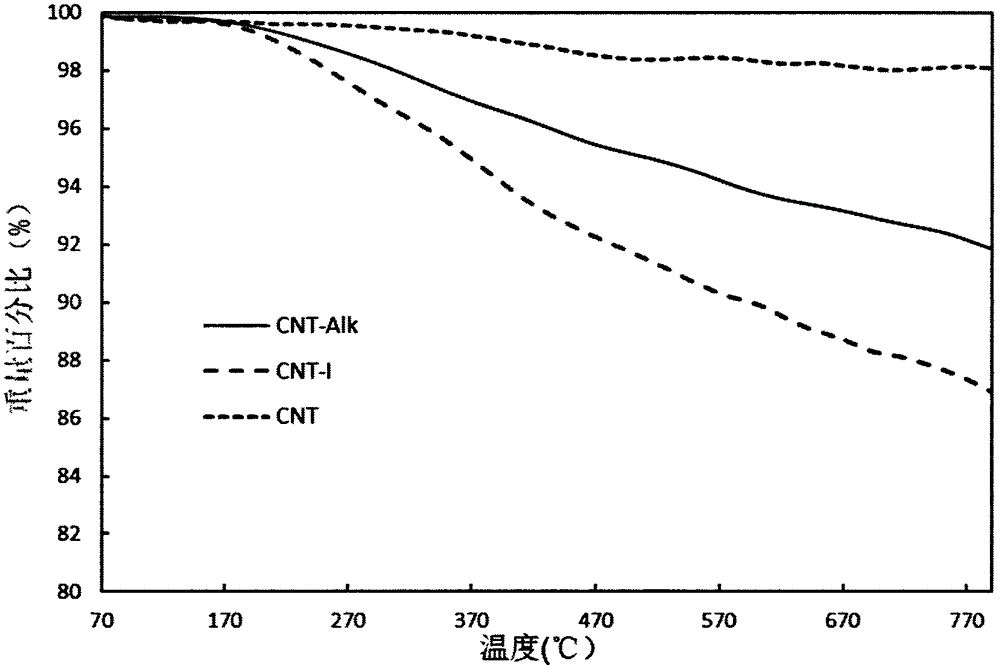
Preparation method of carbon nanotube modified by alkynyl on surface
The invention discloses a preparation method of a carbon nanotube modified by alkynyl on the surface. The preparation method comprises the following steps: carrying out surface modification on the carbon nanotube by virtue of a Pschorr-type arylation method to obtain the carbon nanotube which is halogen modified on the surface; then, converting the surface halogen of the carbon nanotube into alkynyl by virtue of Sonogashira coupling reaction; and carrying out alkynyl modification on the surface of the carbon nanotube by virtue of a joint application of Pschorr-type arylation and Sonogashira coupling reaction. The preparation method provides a simple path for introducing the carbon nanotube into preparation of a biosensor electrode or a biological nano material further by virtue of click chemistry. The method disclosed by the invention is simple, few in byproduct and can be prepared on a large scale, and the raw materials are easily available.
Owner:CHENGDU BIOSENSING TECH CO LTD
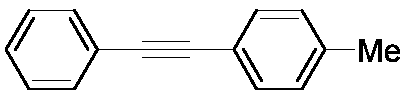
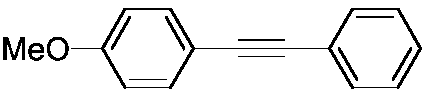
Method for preparing aromatic alkynes through cross-coupling of nitroaromatic hydrocarbons and aryl-terminated alkynes under catalysis of transition metal
ActiveCN108610225AReduce usageWide range of toleranceCarboxylic acid nitrile preparationOrganic compound preparationPalladium catalystAlkyne
The invention discloses a method for preparing aromatic alkynes through cross-coupling of nitroaromatic hydrocarbons and aryl-terminated alkynes under the catalysis of transition metal. The method comprises a step of subjecting nitroaromatic hydrocarbons and aryl-terminated alkynes to a cross-coupling reaction in a solution system of a palladium-containing catalyst, a copper-containing catalyst, aphosphine ligand and an amine ligand in a protective atmosphere so as to obtain aromatic alkynes. According to the method, the cheap, easily available and polar nitroaromatic hydrocarbons are used asan electrophilic reagent for synthesis of the aromatic alkynes, so a series of advantages in traditional Sonogashira coupling can be compensated. The method of the invention has the following advantages: (1) usage of expensive halogenated hydrocarbons hard to prepare can be avoided; (2) in the process of multiple coupling reactions, the polarity of nitro groups allows coupling products to have polarity different from the polarity of raw materials and by-products, so the coupling products can be easily separated through column chromatography; and (3) the transition metal is employed for homogeneous catalysis, so cross-coupling is expected to be smoothly carried out under mild conditions, and the tolerance range of function groups is wide.
Owner:HUNAN UNIV OF SCI & TECH
Liquid crystal compound containing acetal ring and preparation method of liquid crystal compound
ActiveCN104745200APositive dielectric anisotropyWith nematic phase liquid crystal intervalLiquid crystal compositionsOrganic chemistrySolubilityCrystallography
The invention discloses a liquid crystal compound containing acetal. A structural formula of the compound is as shown in the specification, wherein R is C2-C5 linear alkyl, m is 0 or 1, and cyclohexyl is trans-cyclohexyl. The liquid crystal compound is obtained by enabling substituted aryl alkynol and substituted benzenepropanal acetal to generate sonogashira coupling reaction, and the synthesis method is simple, relatively low in cost, and suitable for industrial production. The liquid crystal compound disclosed by the invention has positive dielectric anisotropy and a certain nematic phase liquid crystal region, a relatively high clearing point and proper optical anisotropy, is excellent in solubility with other liquid crystal compounds, and can be applied to an IPS display mode and a TN display mode.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
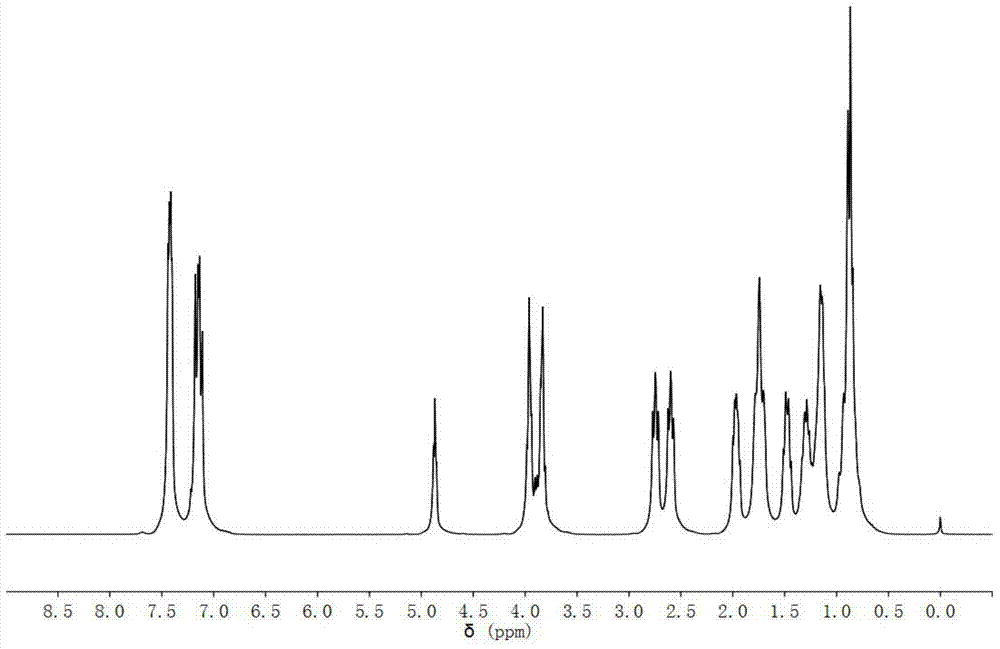
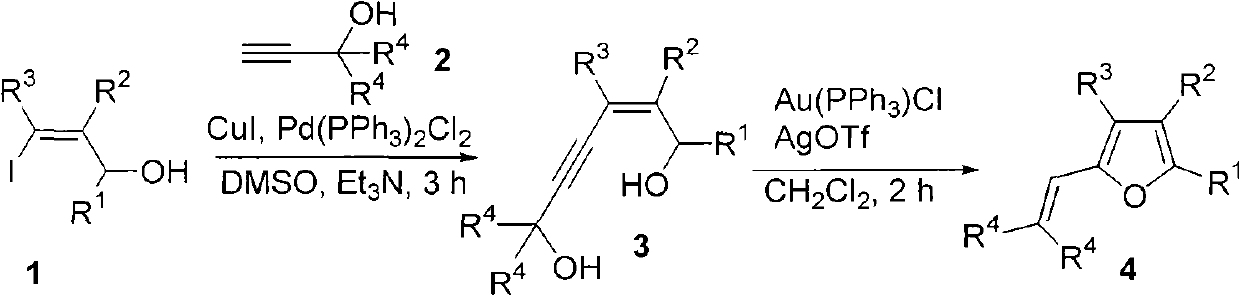
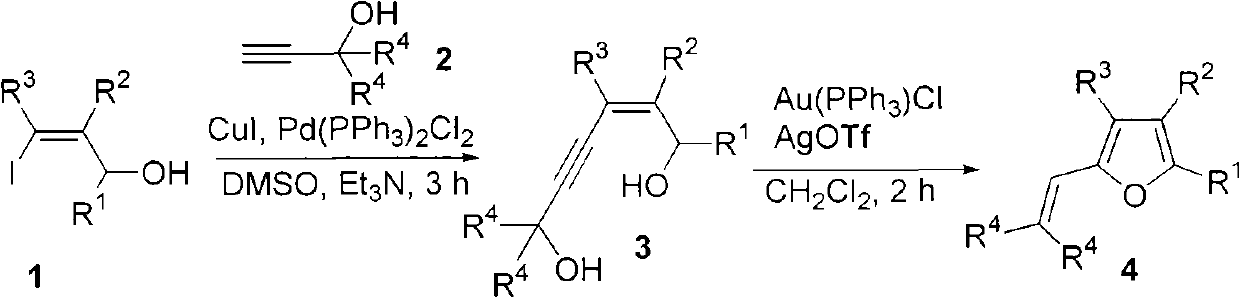
Method for combining polysubstituted furan
InactiveCN101792427AEasy to separate and purifyMild reaction conditionsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSonogashira couplingTriphenylphosphine gold chloride
The invention relates to a method for combining polysubstituted furan; polysubstituted 3-iodine-2-propylene-1-alcohol and propargyl alcohol at tail end carry out Sonogashira coupling reaction to generate 4-alkyne-2-hexene-1, and 6-glycol carries out isomerism cyclization reaction to generate a series of polysubstituted furan compounds under the common catalysis of triphenylphosphine-gold chloride and silver trifloromethanesulfonic acid; the method has simple operation and is easy to obtain raw material and reagents, the reaction has high yield, multiple substituent groups can be introduced, the product is easy to separate and purify, therefore, the method is suitable for combining various polysubstituted furan compounds.
Owner:ZHEJIANG UNIV
MXene two-dimensional material and Cu/MXene catalyst, and preparation methods and applications thereof
ActiveCN110841721ATypical two-dimensional morphologyIncrease productionCatalyst carriersCatalystsPtru catalystPhysical chemistry
The invention discloses an MXene two-dimensional material and a Cu / MXene catalyst, and preparation methods and application thereof. The preparation method of the MXene two-dimensional material comprises the following steps: subjecting a uniformly-mixed reaction system containing lithium fluoride, acid and an Mxene precursor to a reaction, oscillating a mixture of a reaction product and water by adopting a mechanical oscillation technology, and carrying out ultrasonic treatment to obtain the MXene two-dimensional material. The invention also discloses the preparation method of the Cu / MXene catalyst. The preparation method of the Cu / MXene catalyst comprises the following steps: carrying out a mixed reaction on a copper source and an MXene two-dimensional material solution, carrying out centrifugal treatment, and drying a solid obtained after centrifugation so as to obtain the Cu / MXene catalyst. The two-dimensional catalyst material of the Cu / MXene catalyst has the characteristics of highdispersity and accurate control of the site position of an active component, and has good application prospects in the fields of a Sonogashira coupling reaction, reduction of 4-NP and the like.
Owner:SHANGHAI NORMAL UNIVERSITY
Process for the production of palladium supported catalysts for catalyzing heck, suzuki-miyaura sonogashira coupling and buchwald-hartwig reactions
InactiveCN101678330AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventElectrical polarity
A method of producing a heterogeneous catalyst suitable for catalyzing Heck, Suzuki-Miyaura and Buchwald-Hartwig reactions, comprising the steps of: a) providing a porous carrier, said porous carrierconsisting of a core and a plurality of ion exchange groups covalently bonded to the surface of said core, where at least 90 % of the ions bound to said carrier are formate ions; b) providing a palladium (II) salt; c) suspending said carrier in an organic solvent thereby obtaining a suspension; d) adding said palladium salt to said suspension and allowing the resulting mixture to react at a temperature within the range of 30 - 70 DEG C until said carrier has turned black; e) washing said carrier in water; and f) drying said carrier under vacuum; characterised in that the resulting carrier subsequently is resuspended in dimethyl formamide and heated under inert atmosphere to at least 90 DEG C for 2 hours followed by washing with a polar solvent and drying. The invention also relates to acatalyst produced by the method and to processes where the catalyst is used for catalysing Heck, Suzuki-Miyaura, and Buchwald-Hartwig reactions.
Owner:NORDIC CHEMQUEST AB
Three-dimensional nanometer graphene based on triptycene and preparation method thereof
ActiveCN103373892AStrong fluorescenceHigh yieldHydrocarbon by isomerisationFluorescence/phosphorescenceOrganic solventCoronene
The invention discloses three-dimensional nanometer graphene based on triptycene and a synthetic method thereof. The three-dimensional nanometer graphene based on the triptycene is a novel coronene-modified triptycene derivative. The preparation method comprises the following steps of: firstly carrying out a Sonogashira coupling reaction on triiodo triptycene to obtain a tri-acetenyl triptycene derivative; then carrying out a Diels-Alder reaction on the tri-acetenyl triptycene derivative to obtain a coronene triptycene derivative; finally carrying out an FeCl3 oxidation and cyclization reaction in an organic solvent (dichloromethane) under the conditions of gas protection (Ar) and normal temperature for 15-240 minutes to obtain the three-dimensional nanometer graphene based on the triptycene. The preparation method disclosed by the invention is novel and higher in yield. The prepared three-dimensional nanometer graphene based on the triptycene disclosed by the invention has an outstanding effect on the aspect of cell imaging.
Owner:HUAZHONG UNIV OF SCI & TECH
Novel two-dimensional covalent organic framework material and preparation and application thereof
ActiveCN111269432ASave raw materialsThe synthesis process is simpleFuel cellsTert-Butyloxycarbonyl protecting groupElectrical battery
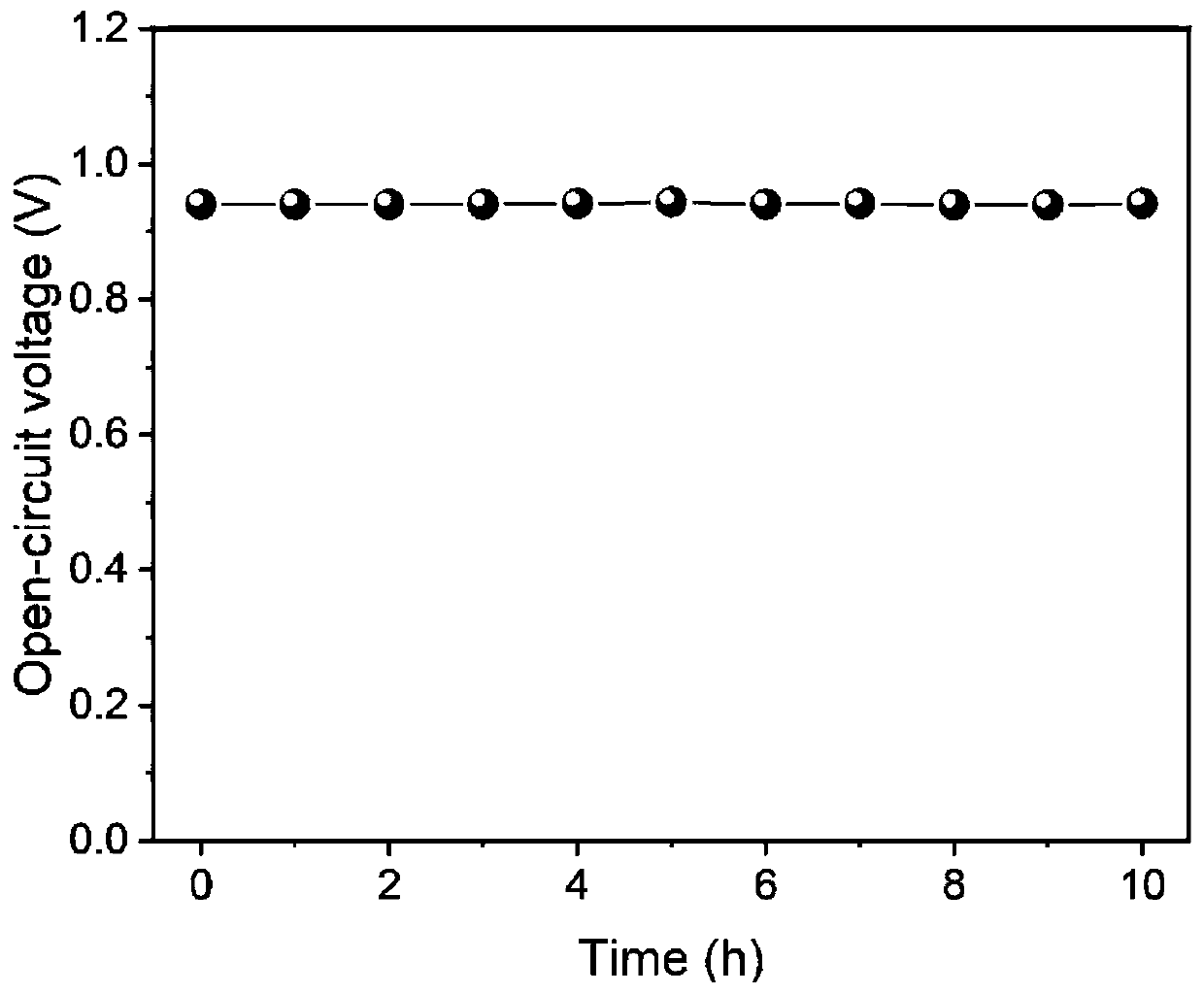
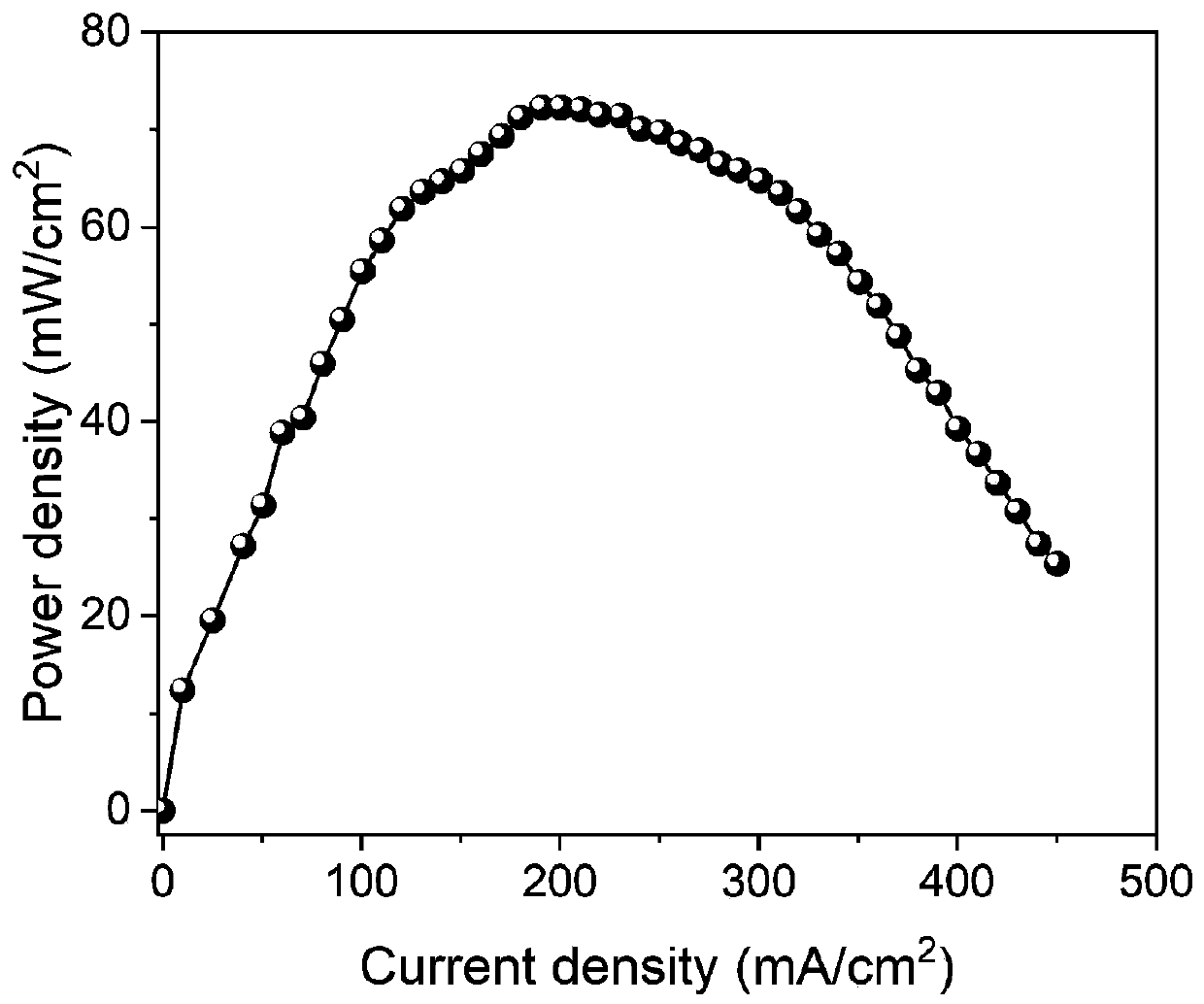
The invention discloses a novel two-dimensional covalent organic framework material and preparation and application thereof, belongs to the field of new energy, and particularly relates to an organicframework material and preparation and application thereof. The two-dimensional covalent organic framework material is prepared by the following steps: carrying out Sonogashira coupling reaction and deprotection on a benzene compound 1, 3, 5-tri (4-bromophenyl) benzene containing bromine atoms, and finally, carrying out Schiff base reaction on the product and a tert-butyloxycarbonyl protecting group. The covalent organic framework material is low in raw material cost, simple in synthesis process and convenient to purify. When the covalent organic framework material is used as a proton exchangemembrane, the battery can keep excellent voltage working stability in a normal operation state; when the current density is 188mA / cm <2 >, the power density of the fuel cell is maximum and reaches 72mw / cm <2 >, and the fuel cell has higher power density at room temperature.
Owner:吉林中科研伸科技有限公司
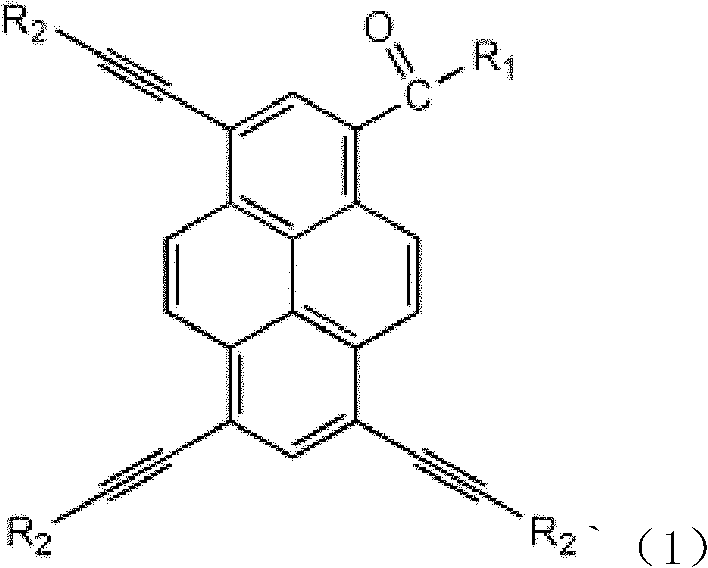
Symmetric discotic pyrene compounds and preparation method thereof
InactiveCN102503854AStrong modifiabilityHighly innovativeCarboxylic acid nitrile preparationOrganic compound preparationChemical reactionOrganic synthesis
The invention belongs to the technical field of organic synthesis and relates to symmetric discotic pyrene compounds and a preparation method of the symmetric discotic pyrene compounds. The compounds have the following general formula, wherein R1 and R2 are the same or R1 is H and R2 is C1-C20 p-acetylene-dialkyl phenylamine, triazoles or conjugated butadiene phenylamines. The preparation method of the compounds comprises the following steps of: bromination of pyrene, Sonogashira coupling reaction, de-trimethylsilyl reaction, bromide chain grafting reaction and click chemistry reaction. The symmetric discotic pyrene compounds prepared by the invention have a strong discotic conjugation structure and strong electron-withdrawing groups, so that the compounds have good nonlinear optical effects. Besides, owing to the strong side chain modifiability, the compounds can be grafted with different conjugate groups and chromophoric groups, to provide great support for preparing novel nonlinear optical materials. Therefore, the compounds have great potential application values.
Owner:UNIV OF SCI & TECH BEIJING
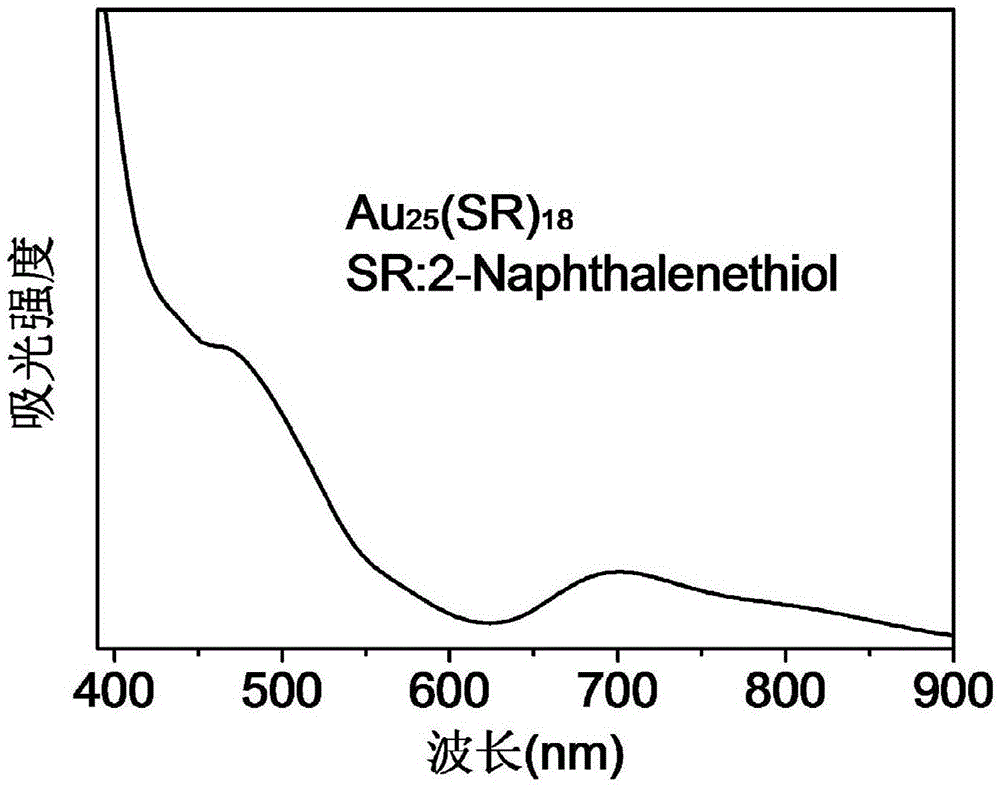
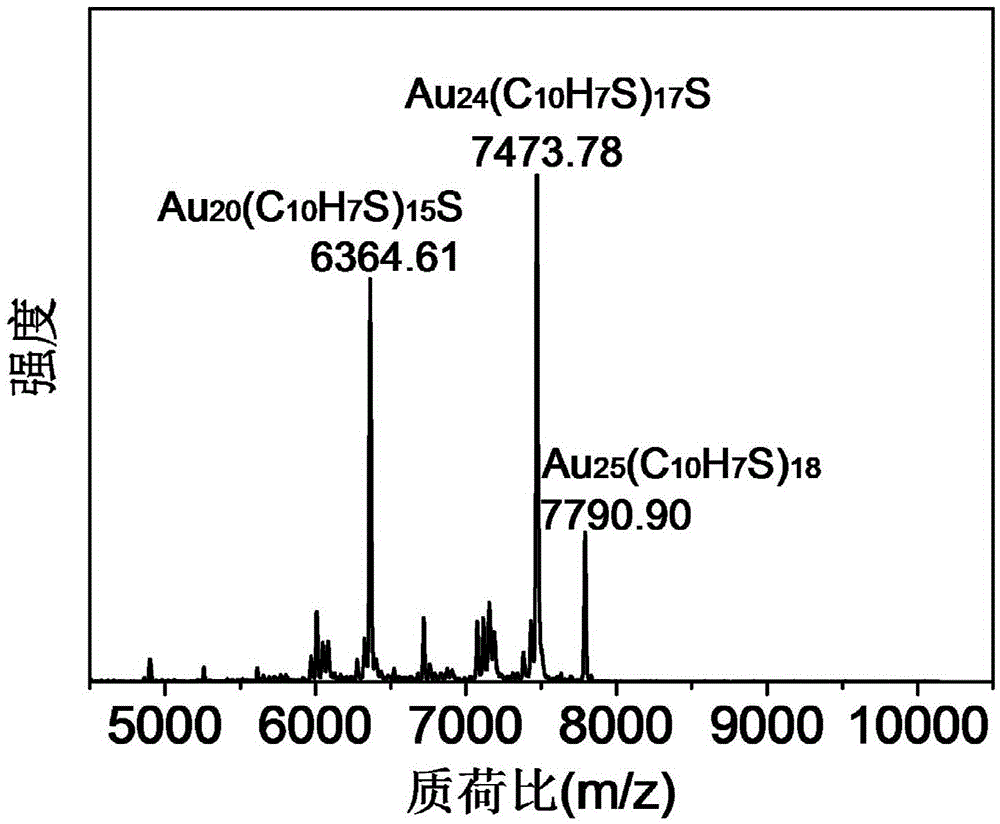
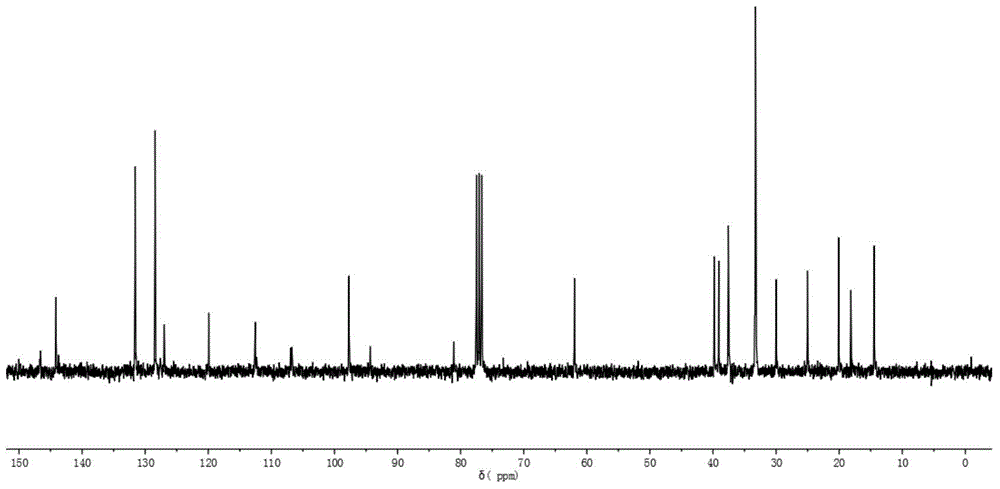
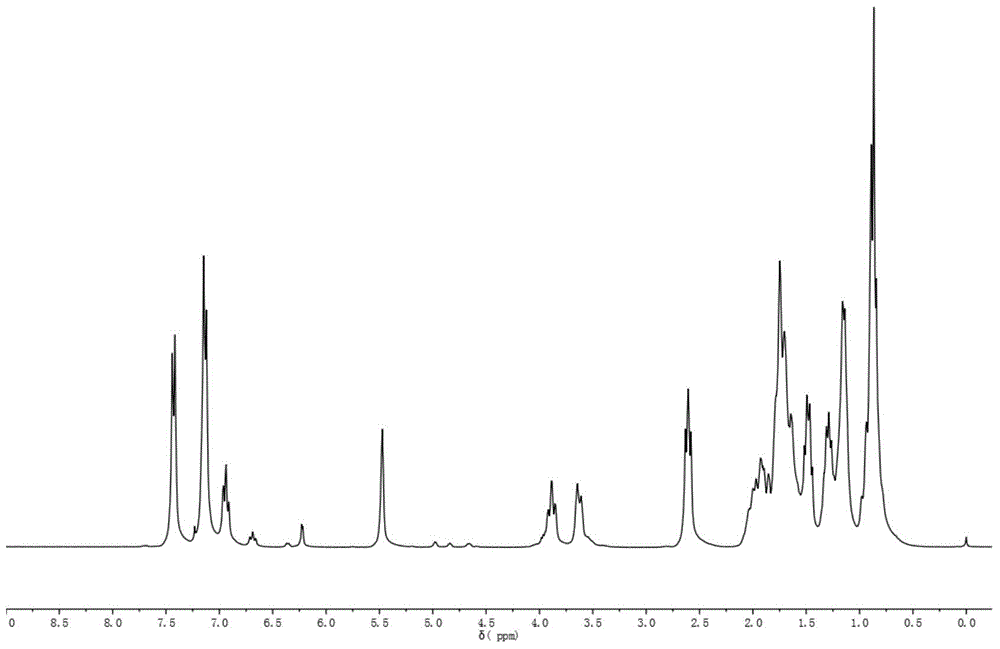
Au25 nanosphere synthetic method and Au25 nanospheres
InactiveCN106807952AHigh activityImprove catalytic performanceNanotechnologyGold clusterOrganic reaction
Preparation and synthesis of Au25 nanospheres with precise atom number and structure; a preparation method may synthesize the Au25 nanospheres simply and efficiently and may also serve for preparing a supported catalyst and for catalyzing organic reactions, such as Sonogashira coupling reaction. These gold cluster nanomaterials have precise microscopic structure. A synthetic method includes: dissolving chloroauric acid trihydrate (HAuCl4.3H2O) and tetraoctyl ammonium bromide (TOABr) in acetone, adding suitable 2-naphthalenethiol (2-NapSH) ligand, adding a strong reducing agent sodium borohydride for reduction, adding massive 2-naphthalenethiol at suitable temperature to allow for etching reaction to obtain the product Au25(2-NapS)18. The synthetic route related herein has the advantages that the materials are simple and easily accessible, reaction conditions are simple and feasible, and controlling is simple.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Pyran ring-containing negative liquid crystal compound and preparation method thereof
InactiveCN104789232AHas negative dielectric anisotropyWith nematic phase liquid crystal intervalLiquid crystal compositionsOrganic chemistrySonogashira couplingSolubility
The invention discloses a pyran ring-containing negative liquid crystal compound. The structural formula of the compound is shown in the description, wherein R represents C2-C5 linear alkyl, values of x and y are 0 or 1, cyclohexyl is trans cyclohexyl. A preparation method for the liquid crystal compound comprises the following steps: reacting substituted phenol with 3,4-2H-dihydropyran at normal temperature to obtain pyran ring-protected halogenated hydrocarbon; performing sonogashira coupling reaction on pyran ring-protected halogenated hydrocarbon and substituted aryl alkynol to obtain the liquid crystal compound; the liquid crystal compound is simple in synthesis method, lower in cost and suitable for industrial production. The liquid crystal compound has negative dielectric anisotropy and a certain nematic liquid crystal range, has excellent low-temperature stability and appropriate optical anisotropy, has excellent solubility with other liquid crystal compounds, and can be applied to an IPS display mode, a VA-TFT display mode and a dual-frequency liquid crystal display mode.
Owner:SHAANXI NORMAL UNIV
Alkynyl-containing ruthenium complex as well as synthesis method and application thereof
InactiveCN108570076AGood antitumor activityFacilitate transmembrane absorptionRuthenium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSide effectSynthesis methods
Theinvention provides an alkynyl containing ruthenium complex, and also relates to a synthesis method and application of the alkynyl-containing ruthenium complex. The alkynyl-containing ruthenium complex is a new type of ruthenium complex, and alkynyl is introduced into the DPPZ type ruthenium complex through Sonogashira coupling reaction, and is favorable for promoting the transmembrane absorption effect of drug molecules, improving the probability of drugs entering cells and enhancing the medical efficacy while lowering the toxic and side effects of the drugs. The alkynyl-containing ruthenium complex provided by the invention has significant anti-tumor activity, especially anti-breast cancer activity, provides a new idea for molecule design of future anti-tumor drugs, can also be used asa fluorescent probe, and thus has broad application prospects in the field of medicinal chemistry.
Owner:GUANGDONG PHARMA UNIV
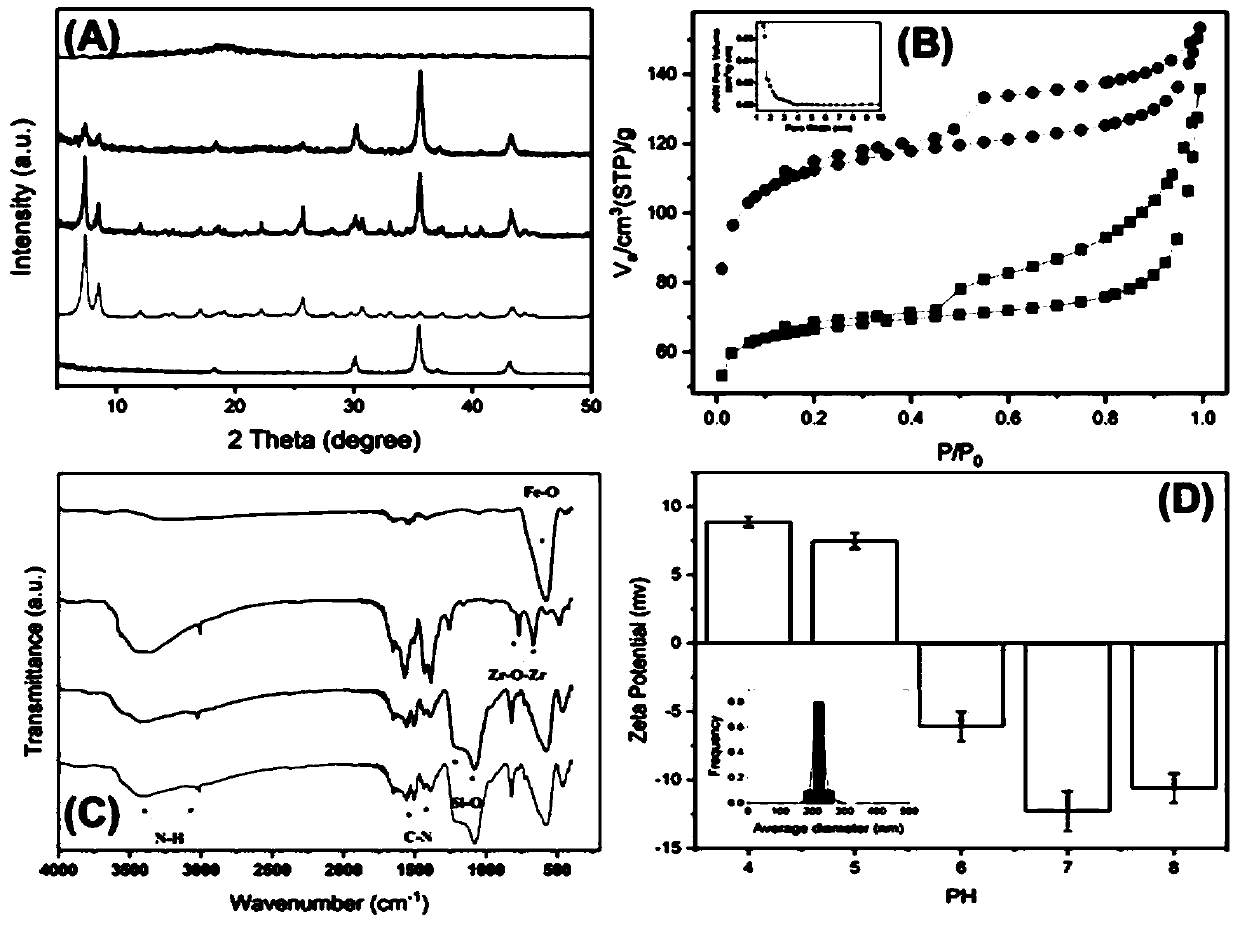
Preparation and applications of magnetic composite porous network adsorbing material
ActiveCN110586052AImprove uniformityImprove stabilityOther chemical processesAlkali metal oxides/hydroxidesOrganic solventSolid phase extraction
The invention relates to preparation and applications of a magnetic composite porous network adsorbing material, wherein the preparation method comprises: (a) preparing a magnetic porous organic framework material with strong adsorbability and large specific surface area by adopting an in-situ growth method, and (b) synthesizing a novel composite porous network adsorbing material with a core-shellstructure through a sonogashira coupling reaction so as to achieve the selective adsorption and efficient enrichment of food and environment pollutants. According to the present invention, the prepared magnetic composite porous network adsorbing material has good particle size uniformity and significantly efficient adsorption capacity on target pollutants, the separation step during solid-phase extraction is greatly simplified due to the magnetic characteristic of the material, and the microporous organic coating changes the hydrophobicity of the surface of the material, such that the material has high stability in a humid environment, can well play a role in an organic solvent, and is suitable for adsorption and enrichment of various food pollutants.
Owner:NANKAI UNIV
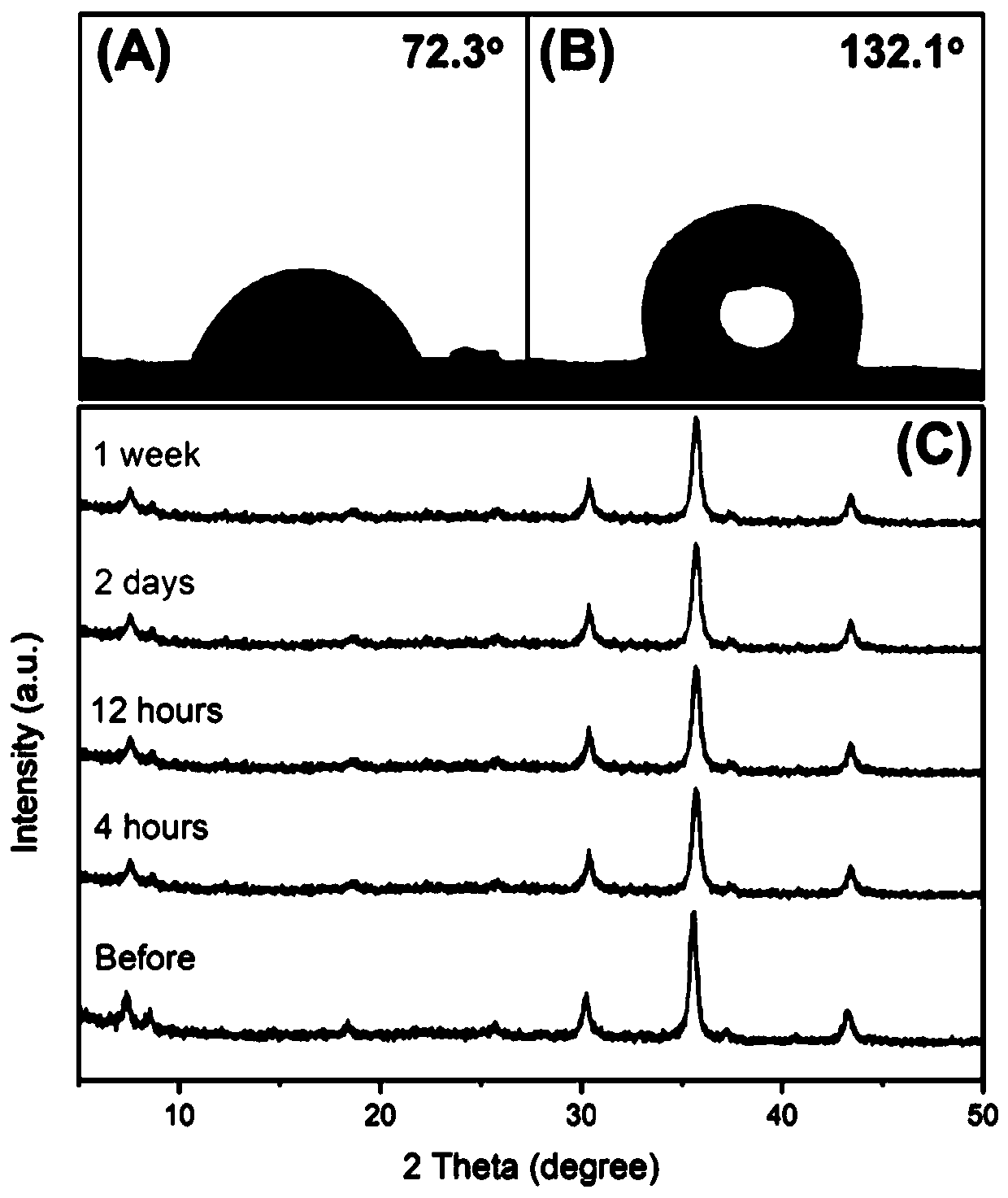
Oleophylic super-hydrophobic porous aromatic framework material as well as preparation method and application thereof
ActiveCN111286010AEfficient separationThe method is simpleFatty/oily/floating substances removal devicesOther chemical processesPolyesterBenzene
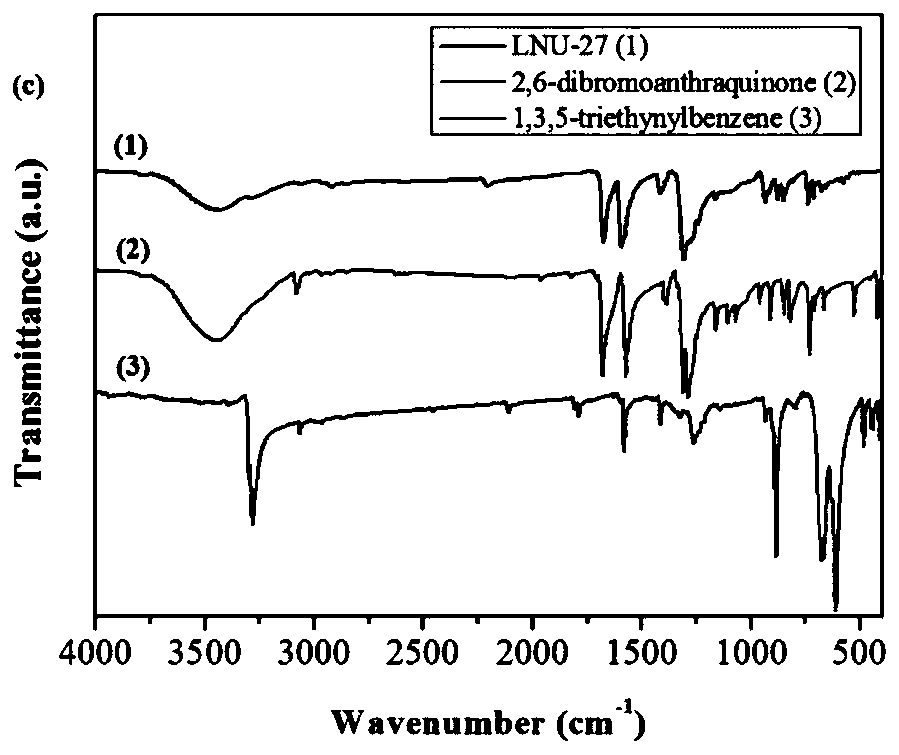
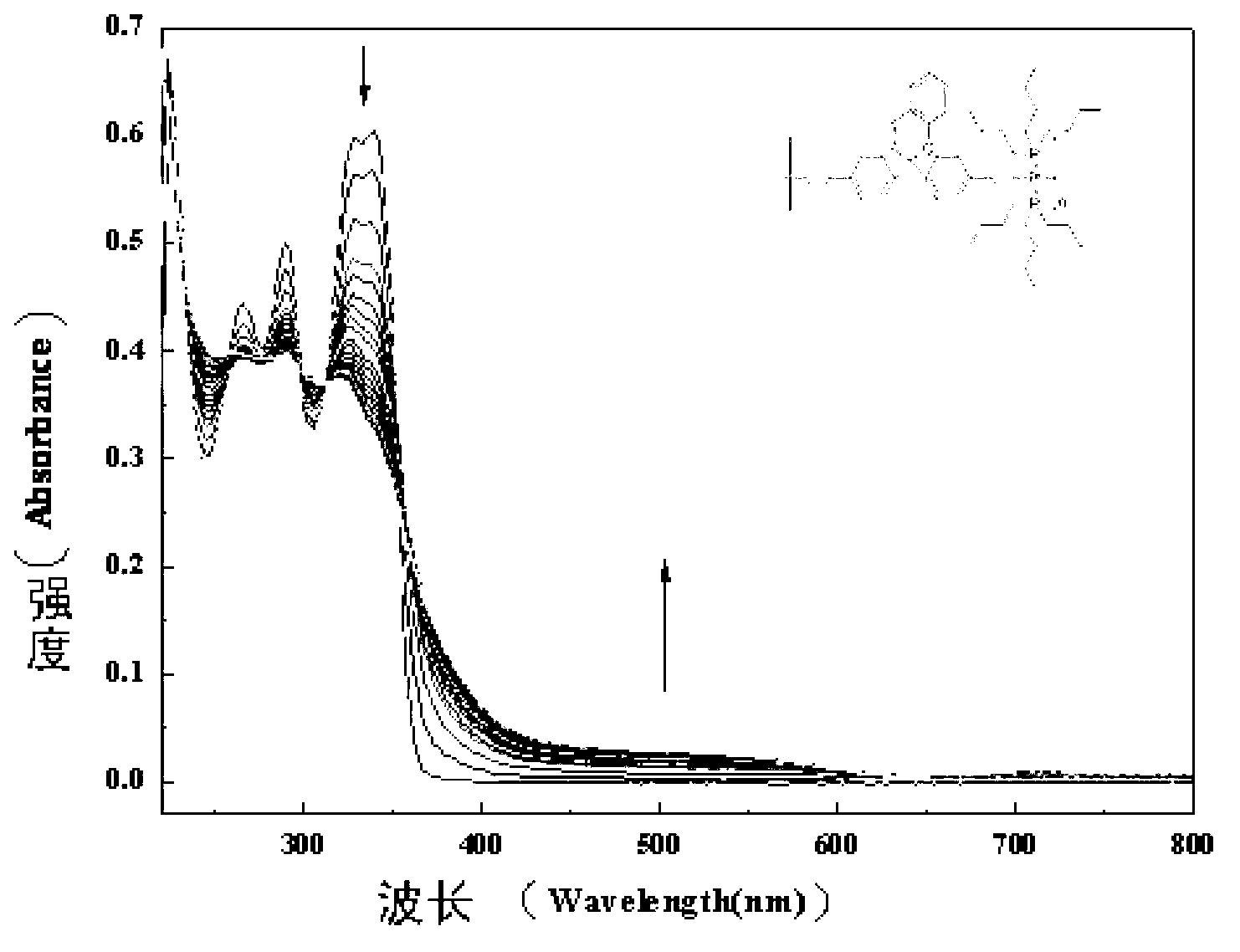
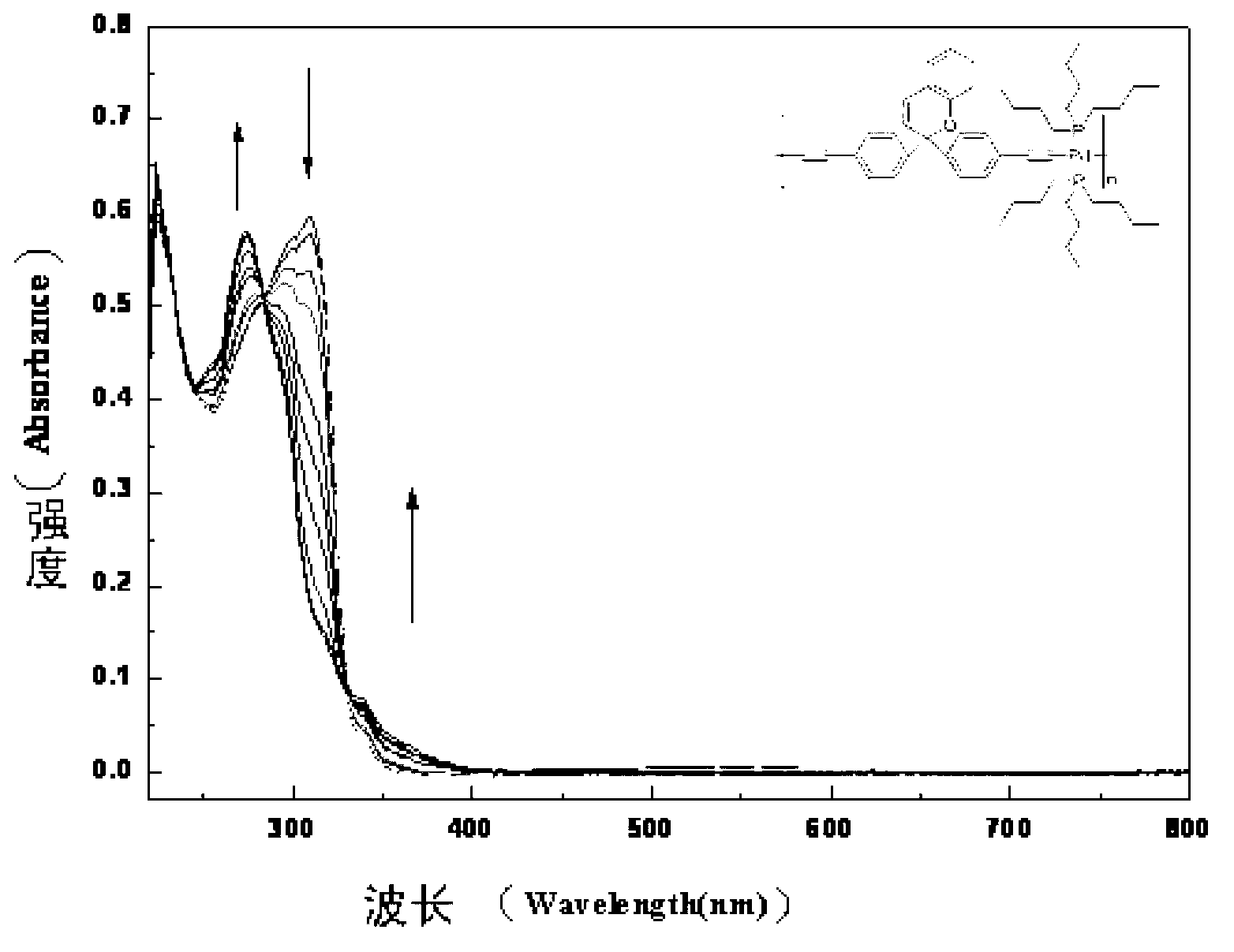
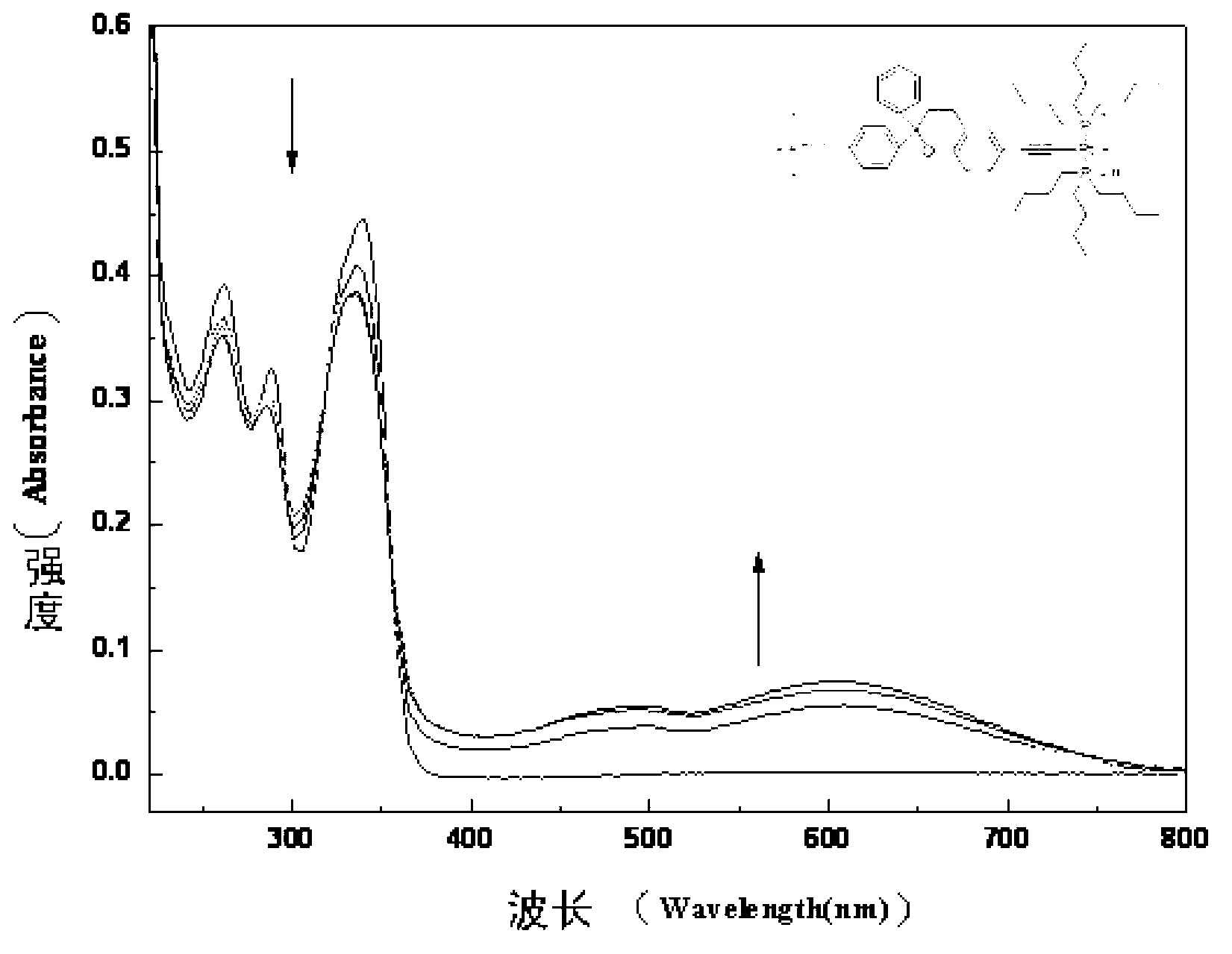
The invention belongs to the technical field of chemistry and new materials, and particularly relates to an oleophylic super-hydrophobic porous aromatic framework material as well as a preparation method and application thereof. The oleophylic super-hydrophobic porous aromatic framework material LNUs is synthesized by carrying out Sonogashira coupling reaction on 1, 3, 5-triacetylene benzene as aconstruction element and different brominated benzene ring-containing compounds, and the contact angles of the materials of the kind are all greater than 150 degrees. The materials can be successfullyloaded on polyester fabric in a simple soaking mode, and the prepared oleophylic super-hydrophobic polyester fabric can be used for effective separation of an oil / water mixture or an organic solvent / water mixture. The synthesis method of the oleophylic super-hydrophobic porous aromatic framework material provided by the invention is simple in process and remarkable in hydrophobic effect. The prepared oleophylic super-hydrophobic polyester fabric can keep excellent hydrophobic performance under severe conditions such as strong acid, strong alkali or high temperature, and has a good applicationprospect in the field of oil / water separation.
Owner:LIAONING UNIVERSITY
Photochromic polymers containing spiro group and its synthetic method
InactiveCN103265706AThe synthesis process is simpleReduce manufacturing costTenebresent compositionsCarbonyl groupSonogashira coupling
Photochromic polymers containing spiro-class radical group and synthetic method thereof are provided. The polymers consist of eight photochromic polymers formed by four spiro photochromic units polymerize with trans-dichloro bis (triphenylphosphine) platinum / palladium respectively. The synthetic method is as follows: (1) benzophenone carbonyl addition reaction; (2) photochromic units gained by cyclization reaction; (3) a Carbon-carbon triple bond that is gained by sonogashira coupling reaction; (4) terminal alkyne hydrogen that is gained by hydrolysis reaction; and (5) carrying out carbonyl addition reaction of benzophenone, carrying out to obtain photochromic units, carrying out a sonogashira coupling reaction to obtain a carbon-carbon triple bond, carrying out hydrolysis reaction to obtain terminal alkyne hydrogen and carrying out polymerization reaction. The preparation method of the photochromic polymer is simple with low preparation cost and high productivity, and has a wider range of application than the prior polymers.
Owner:XI AN JIAOTONG UNIV
Fluoroboron pyrrole liquid crystal compound containing 8-(diphenylethinyl)-ester group flexible multi-element rings, preparation method and application thereof
Owner:DALIAN UNIV OF TECH
Method of preparing poly(ADP-ribose) polymerases inhibitors
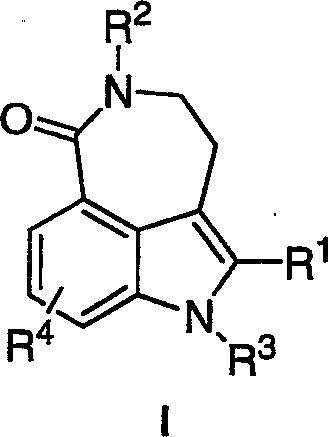
The invention relates to a new and convergent route to small molecule inhibitors of poly(ADP-ribose) polymerase, such as 8-fluoro-2{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino [5,4,3-cd]indol-6-one, via a key Sonogashira coupling reaction and a Cul-promoted indole formation.
Owner:PFIZER INC +1
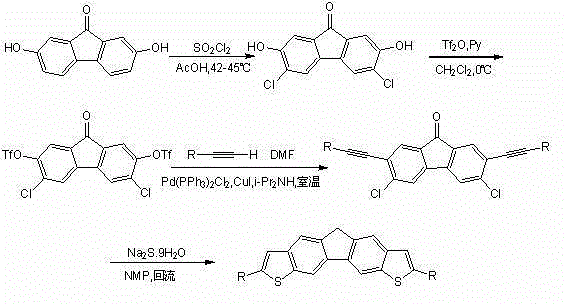
Bithienofluorene, its derivative and preparation method
The invention discloses bithienofluorene, its derivative and a preparation method. The compounds are characterized in that: a fluorene ring is in connection with thieno rings, and the 2- position of thiophene has a substituent group, which can be hydrogen, alkyl or aryl. The preparation method consists of: taking 2, 7-dyhydroxyl-9-fluorenone as a raw material, employing sulfonyl chloride to conduct chlorination so as to synthesize 3, 6-dichloro-2, 7-dyhydroxyl-9-fluorenone; and then adopting trifluoromethanesulfonic anhydride to carry out esterification, and performing a Sonogashira coupling reaction to obtain series of 2, 7-disubstituted ethynyl-3, 6-dichloro-9-fluorenone, and finally conducting ring closing by sodium sulfide and reduction to obtain bithienofluorene and its derivative. The series of compounds involved in the invention provides a new option for organic field effect transistor materials, and the preparation method provides a new method for aromatic ketone carbonyl reduction.
Owner:EAST CHINA NORMAL UNIV
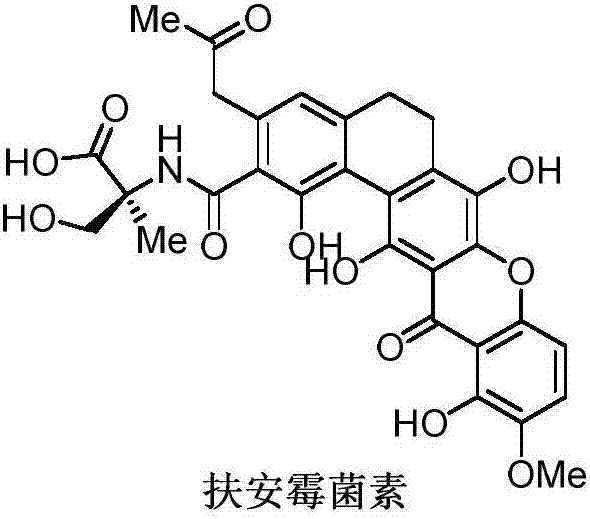
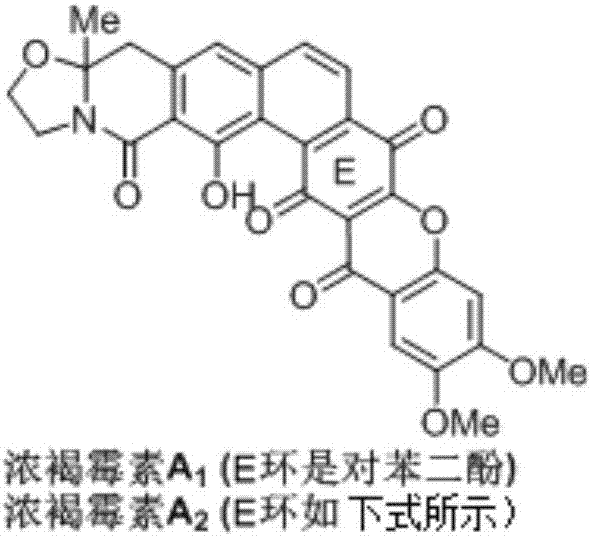
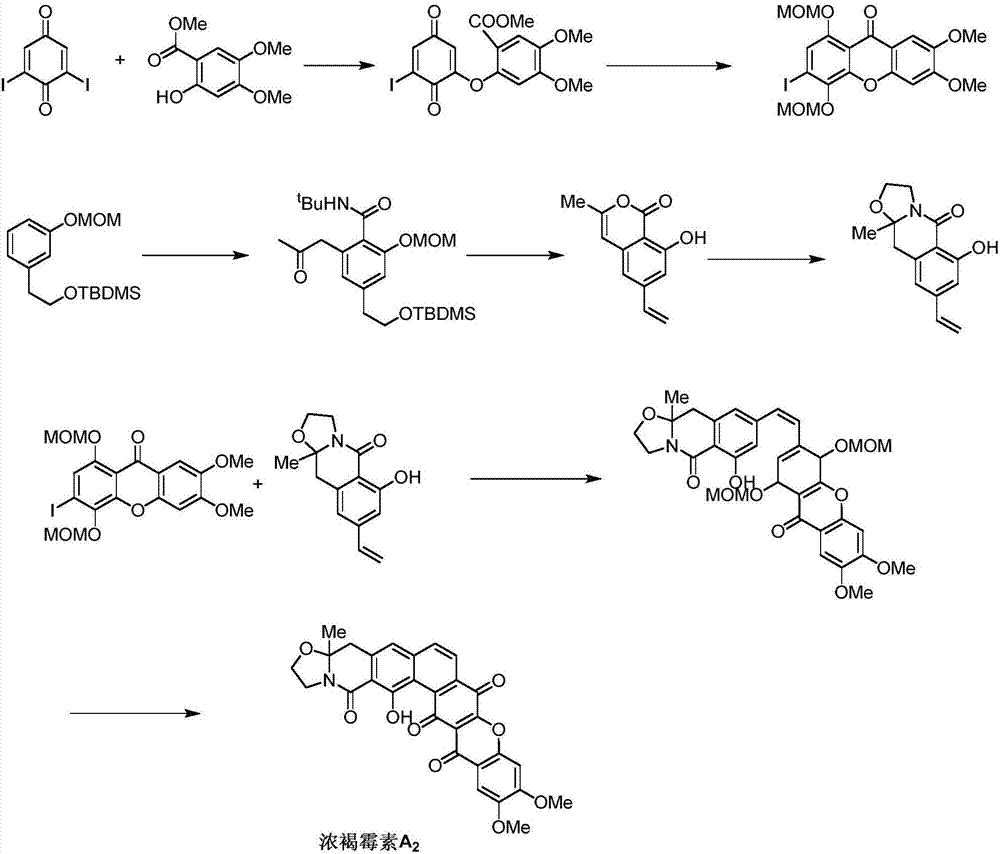
Synthetic method of Fuan mycin skeleton
InactiveCN107235952AEasy to operateResponse requirements are lowOrganic chemistryBulk chemical productionNatural productKetone
The invention discloses a synthetic method of a Fuan mycin skeleton. The method adopts a compound of a formula 1 as a starting material, and a compound of a formula 3 is obtained by removing a silicon protection group in a two-step conversion manner by virtue of Sonogashira coupling reaction. The compound of formula 4 is also used as a starting raw material to obtain the compound of formula 6 by virtue of pinic oxidization and methyl esterification. The compound of formula 3 and the compound of formula 6 have the Sonogashira coupling reaction, a phenolic hydroxyl group is protected by a silicon group, methoxyl is selectively removed, an acetylenic bond is reduced, 6pai electrocyclization is carried out, the phenolic hydroxyl group is activated to have the secondary Sonogashira coupling reaction, the silicon-group protective group is removed, a Ullmann reaction is carried out, methyl protection, pinic oxidization and Fourier-krafz acylation are carried out, esters are hydrolyzed and condensated with amino acid, the acetylenic bond is hydrolyzed into ketone under the gold catalysis, thus obtaining Fuan mycin skeleton protected by pentamethoxyl, and synthesizing the Fuan mycin skeleton. The synthetic route is reasonable in design, raw materials are cheap and easy to get, the operation is simple and easy, a key reaction midbody is easy to modify, and the synthetic research of various polycyclic xanthenone natural products can be realized by utilizing the method.
Owner:EAST CHINA NORMAL UNIV
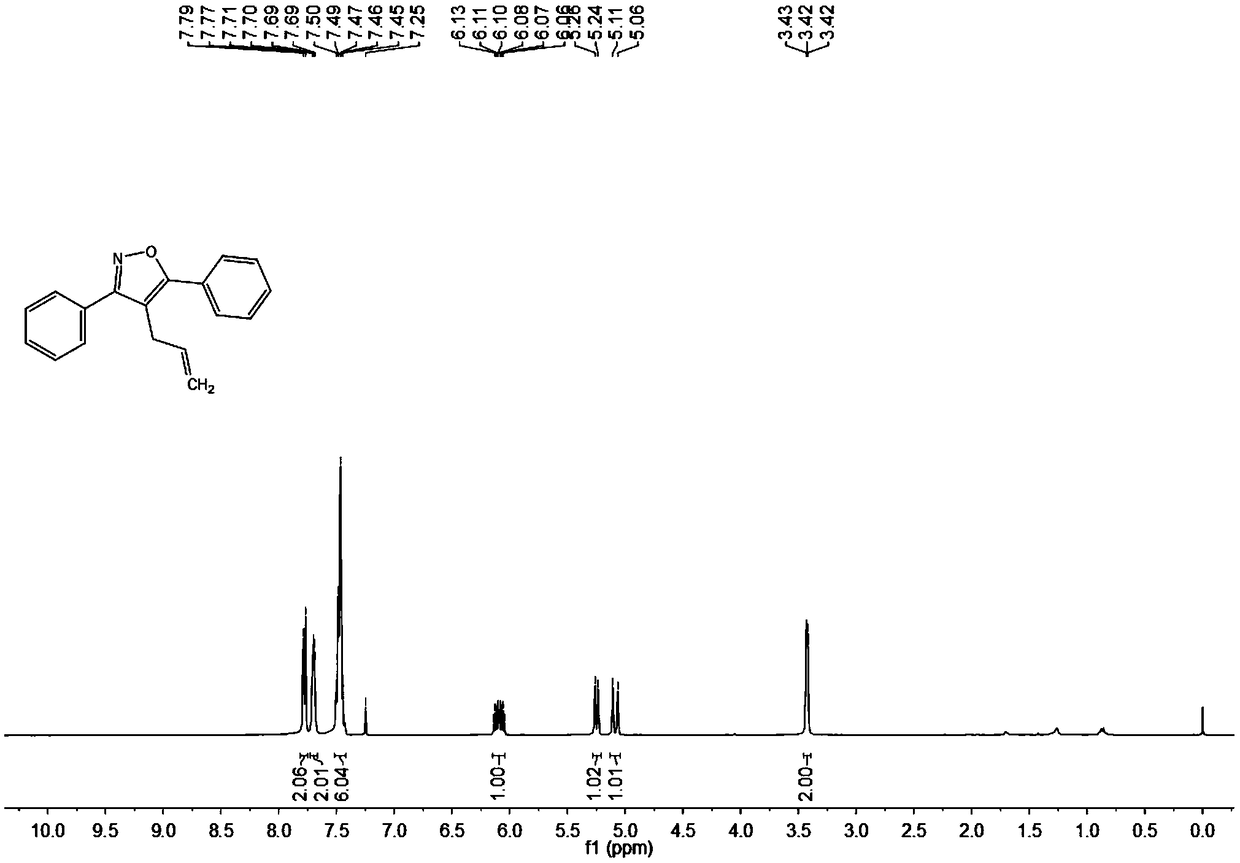
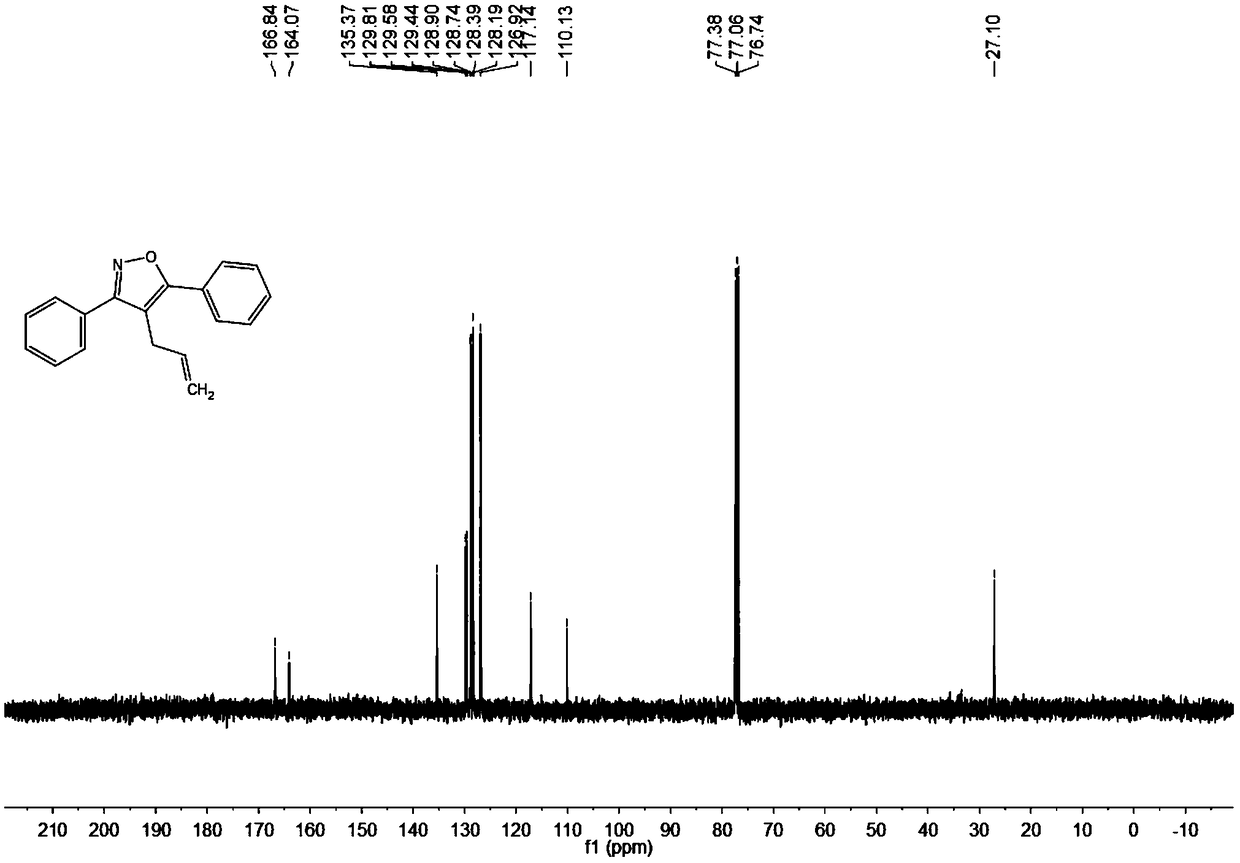
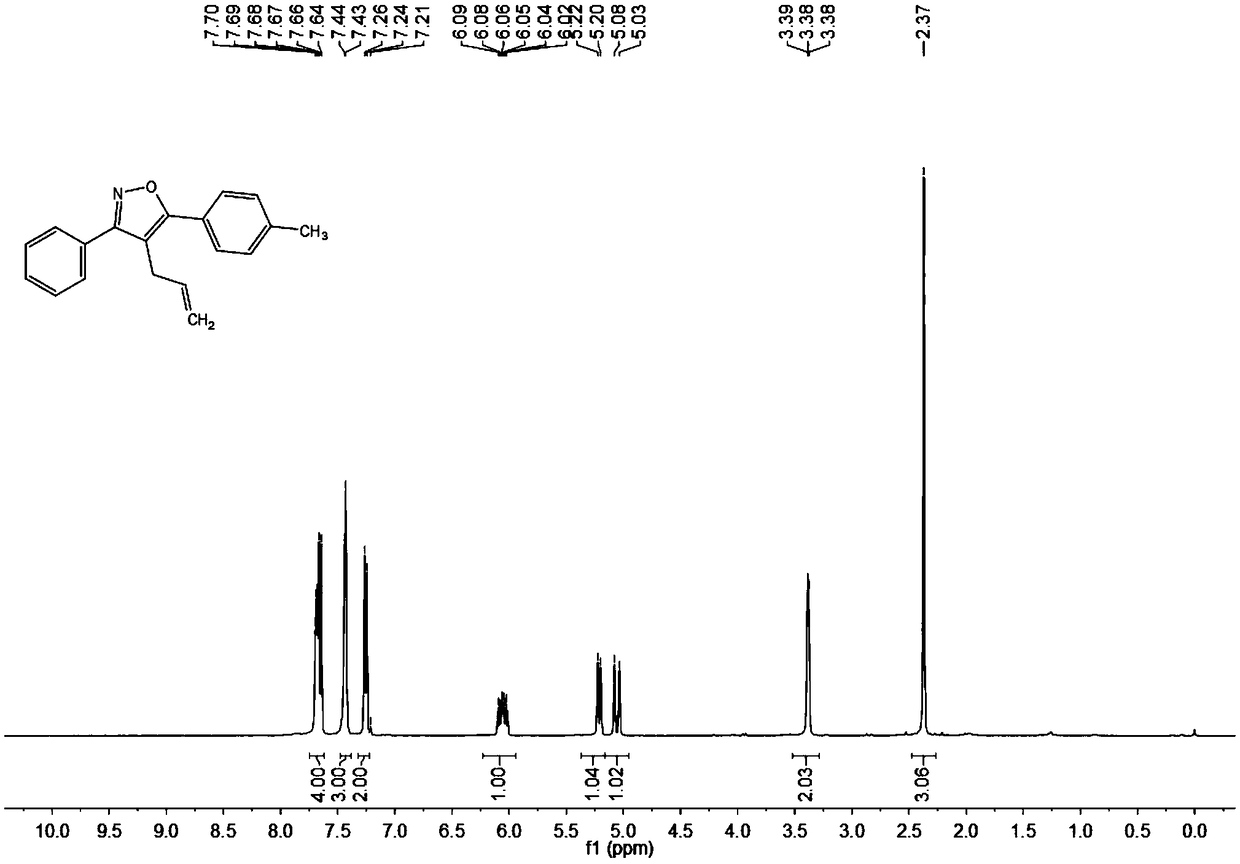
Synthesis method of 4-allyl-3,5-disubstituted isooxazole
ActiveCN108863969ARaw materials are easy to getInnovativeOrganic chemistryOrganic synthesisSynthesis methods
The invention discloses a synthesis method of 4-allyl-3,5-disubstituted isooxazole, and belongs to the technical field of organic synthesis. The synthesis method is characterized in that in a reactor,acetyenic ketone oxime ether substrates, 3-bromopropylene, palladium catalysts, additives and solvents are added; stirring reaction is performed at 70 to 80 DEG C; a reaction product is separated andpurified to obtain the 4-allyl-3,5-disubstituted isooxazole. The method has the advantages that a product obtained by performing Sonogashira coupling on simple and easy-to-obtain acyl chloride and alkyne, and methoxylamine hydrochloride react to obtain a series of norethisteroneoxime ether; the reaction conditions are mild; no environment pollution exists; a potential functional 4-allyl-3,5-disubstituted isooxazole compound is built. The method has innovativeness and atom economy; the conditions are mild; the operation is safe; the scale can be magnified to 5g level scale without influencingthe yield, so that potential practical values are realized.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com