Beta-graphdiyne and synthesis method and application thereof in field of energy storage
A synthesis method and graphdiyne technology, which can be used in hybrid capacitor electrodes, electrical components, battery electrodes, etc., can solve problems such as the fact that the synthesis of graphdiyne has not been substantively solved, and achieve good chemical and thermal stability and good purity. , easy-to-prepare effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention provides a method for synthesizing β-graphyne, using 3-(dibromomethylenyl)-1,4-pentadiyne as the reaction monomer, under the action of a catalyst and a solvent, and under the protection of an inert gas Perform coupling reaction at 60-150°C to obtain β-graphyne; wherein, the catalyst is a mixture of palladium catalyst and Cu(I) salt, and the molar ratio of palladium catalyst and Cu(I) salt is 1:10-1: 1; The solvent is a mixed solvent of solvent 1 and organic amine solvent, the volume ratio of solvent 1 and organic amine solvent is 1:1 to 6:1, and solvent 1 is N,N-dimethylformamide , At least one of N-methylpyrrolidone, tetrahydrofuran, dioxane, toluene or chloroform; the molar ratio of the reaction monomer to the palladium catalyst is: reaction monomer: palladium catalyst=1:0.05~1:0.2 . In the present invention, the solvent is added as long as the reaction monomer is fully dissolved, and its amount is not particularly limited; Cu(I) salt refers to a...
Embodiment 1
[0049] 1. Preparation of 1,5-bis(trimethylsilyl)-1,4-pentadiyn-3-ol:
[0050] Under argon protection and ice water bath, add 9mL (63.37mmol) trimethylethynyl silicon and 150mL freshly distilled tetrahydrofuran (THF) into a 500ml reaction flask; continue to add 39.6mL (63.37mmol) of n-butyl lithium (n- BuLi), stirring for 15min; under ice-water bath, add 1.96mL (31.69mmol) of methyl formate (HCO 2 Me), remove the ice-water bath, cool to room temperature naturally after heating; pour the mixed solution into a separatory funnel filled with ethyl acetate and saturated ammonium chloride solution for extraction, wash, dry, filter with suction, spin-evaporate, and perform column Chromatographic separation gave a yellow oil with a yield of 80%.
[0051] 1 H NMR(CDCl 3 ): 5.09ppm(d,1H), 2.24ppm(d,1H), 0.19ppm(s,18H).
[0052] 13 C NMR(CDCl 3 ): 101.59ppm, 89.71ppm, 53.01ppm, -0.35ppm.
[0053] 2. Preparation of 1,5-bis(trimethylsilyl)-1,4-pentadiyn-3-one:
[0054] Under the protection of argon...
Embodiment 2
[0072] Example 2 Preparation of β-graphyne:
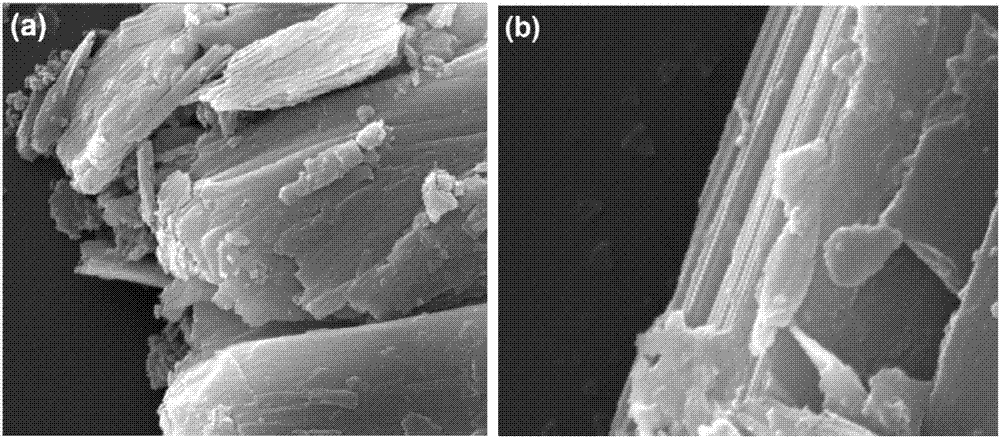
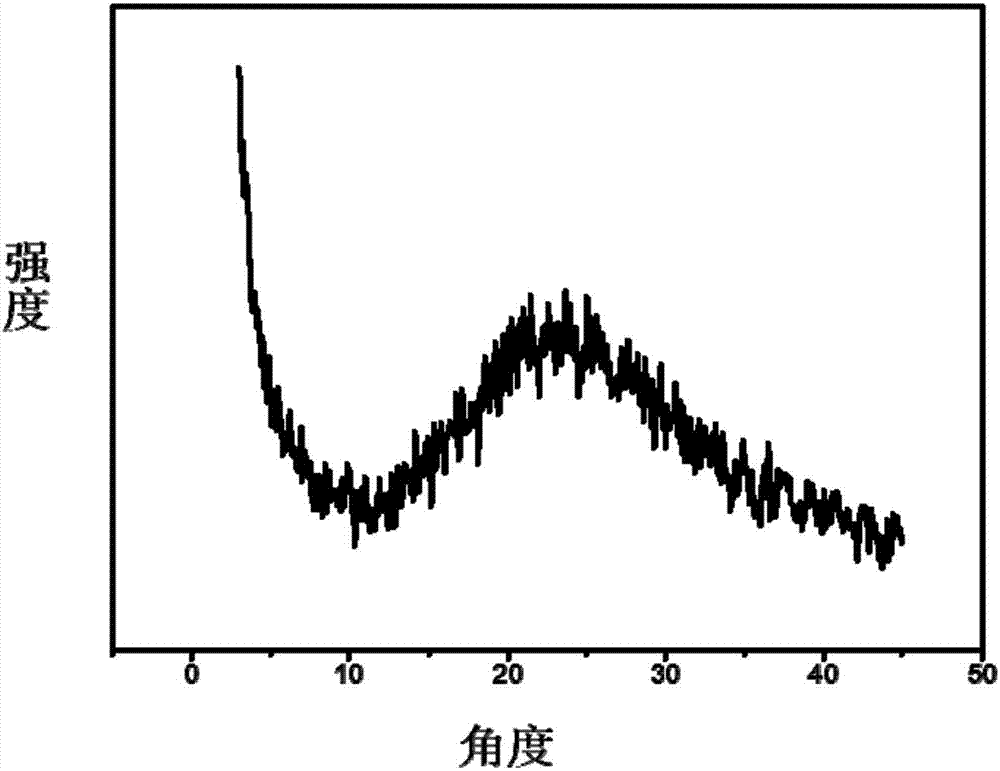
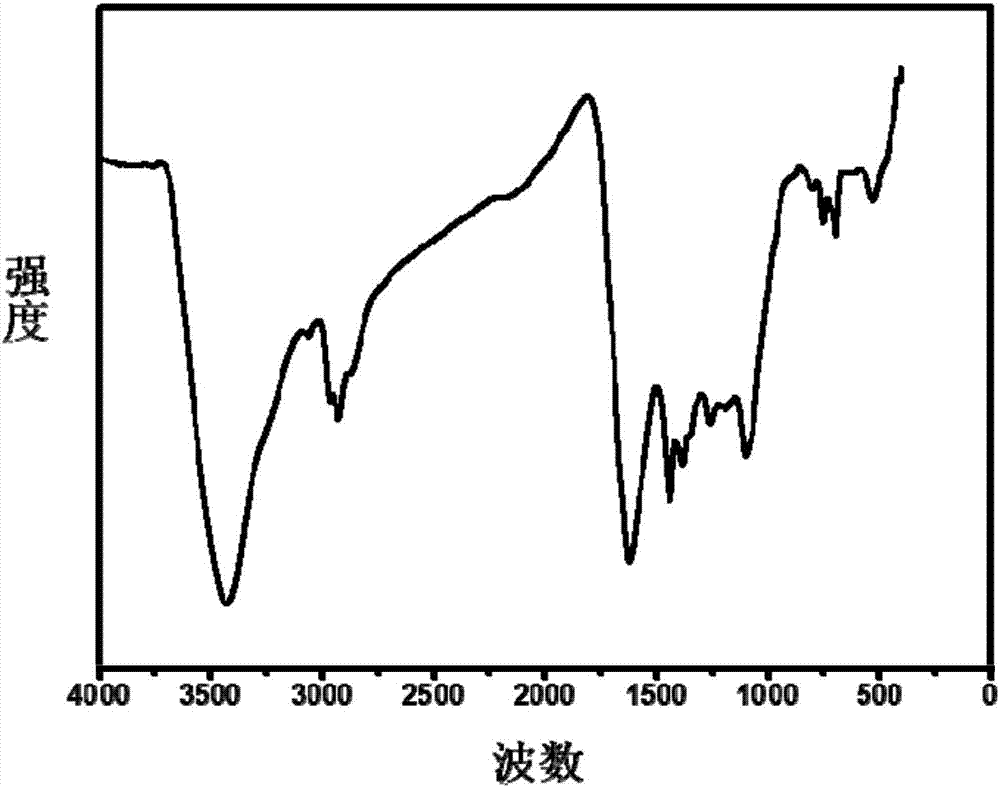
[0073] Under the protection of argon, add 10mL of 3-(dibromomethylenyl)-1,4-pentadiyne solution (60.6mg / mL, DMF) into a 250ml reaction flask, and then add 40mL of N,N-dimethyl Dimethylformamide and 10mL n-butylamine were dissolved and diluted, 149.4mg (0.1295mmol) of tetrakis(triphenylphosphine) palladium and 24.65mg (0.1295mmol) of cuprous iodide were added, and the reaction was stirred at 120°C for three days. Suction filtration, washing, extraction, and drying were performed to obtain black β-graphyne powder with a yield of 86%. The specific surface area of the obtained β-graphyne is 437m 2 / g, the pore volume is 0.45cm 3 / g, it has a micropore diameter of 1.5 nm, and a mesopore diameter of 2 to 6 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com