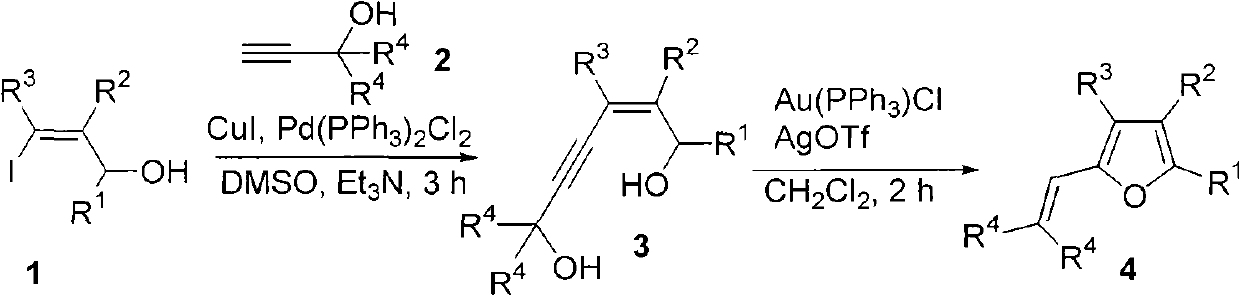
Method for combining polysubstituted furan
A multi-substituted furan and compound technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of poor substrate compatibility and harsh conditions, and achieve the reaction Mild conditions and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add cuprous iodide (3.0mg, 0.015mmol, 6mol%), dichloroditriphenylphosphopalladium (7.0mg, 0.010mmol, 4mol%), 4-phenyl-3-n-pentyl to a dry reaction tube yl-4-iodo-3-butyn-2-ol (85.5mg, 0.25mmol), triethylamine (1mL), 2-methyl-3-butyn-2-ol (124.1mg, 1.5mmol, 6.0 equiv), dimethyl sulfoxide (1 mL), reacted at 40°C for 3 hours, quenched by adding 5 mL of saturated ammonium chloride solution, and extracted with diethyl ether (3 x 15 mL). The organic phase was washed once each with 5% hydrochloric acid, saturated sodium bicarbonate solution, and brine, dried over anhydrous sodium sulfate, filtered, spin-dried, and subjected to flash column chromatography (eluent petroleum ether / ethyl acetate=2 / 1), Collect the obtained crude product 7-methyl-4-phenyl-3-n-pentyl-5-yne-3-octene-2,7-diol in a bottle, and add 1 ml at a time to another bottle Dichloromethane, triphenylphosphine gold chloride (2.5mg, 0.005mmol, 2mol%), silver trifluoromethanesulfonate (1.6mg, 0.006mmol, 2mol%), stir...
Embodiment 2
[0023] According to the method described in Example 1, the difference is that the substrates and reagents used are: cuprous iodide (11.0mg, 0.058mmol, 2mol%), dichloroditriphenylphosphopalladium (3.7mg, 0.005mmol, 2mol%) %), 2-butyl-3-hexyl-4-iodo-2-propen-1-ol (80.0mg, 0.25mmol) / triethylamine (1mL), 2-methyl-3-butyne-2- Alcohol (43.3 mg, 0.52 mmol, 2 equiv) / dimethyl sulfoxide (1 mL), react at 40°C for 3 hours. The second step: two milliliters of dichloromethane, triphenylphosphine monochloride gold (7.2mg, 0.015mmol, 6mol%) / silver trifluoromethanesulfonate (4.0mg, 0.015mmol, 6mol%), stirred at room temperature for 3 hours , to obtain the product 46.5 mg, 72% yield, colorless liquid.
[0024] 1 H NMR (300MHz, CDCl 3 )δ7.08(s, 1H), 5.91(s, 1H), 2.36-2.28(m, 4H), 2.04(s, 3H), 1.88(s, 3H), 1.59-1.24(m, 12H), 0.98 -0.84(m, 6H); 13 C NMR (CDCl 3 , 75MHz) δ149.3, 136.6, 133.2, 126.0, 121.5, 112.2, 31.7, 31.6, 30.6, 29.2, 27.3, 23.5, 23.4, 22.7, 22.6, 20.0, 14.1, 13.9; IR (neat...
Embodiment 3
[0026]According to the method described in Example 1, the difference is that the substrates and reagents used are: cuprous iodide (1.0mg, 0.005mmol, 2mol%), dichloroditriphenylphosphopalladium (3.6mg, 0.005mmol, 2mol%) %), 2-n-pentyl-1-p-methylphenyl-3-iodo-2-propen-1-ol (85.2 mg, 0.25 mmol) / triethylamine (1 mL), 2-methyl-3- Butyn-2-ol (45.1 mg, 0.54 mmol, 2 equiv) / dimethyl sulfoxide (1 mL), react at 40°C for 10 hours. The second step: two milliliters of dichloromethane, triphenylphosphine gold chloride (2.4mg, 0.005mmol, 2mol%) / silver trifluoromethanesulfonate (1.4mg, 0.005mmol, 2mol%), stirred at room temperature for 1 hour , to obtain the product 61.1 mg, 87% yield, colorless liquid.
[0027] 1 H NMR (300MHz, CDCl 3 )δ7.50(d, J=7.8Hz, 2H), 7.19(d, J=8.1Hz, 2H), 6.13(s, 1H), 6.05(s, 1H), 2.62(t, J=7.8Hz, 2H), 2.35(s, 3H), 2.06(s, 3H), 1.91(s, 3H), 1.70-1.55(m, 2H), 1.44-1.27(m, 4H), 0.94-0.85(m, 3H) ; 13 C NMR (CDCl 3 , 75MHz) δ151.5, 146.5, 136.1, 134.6, 129.2, 125.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com