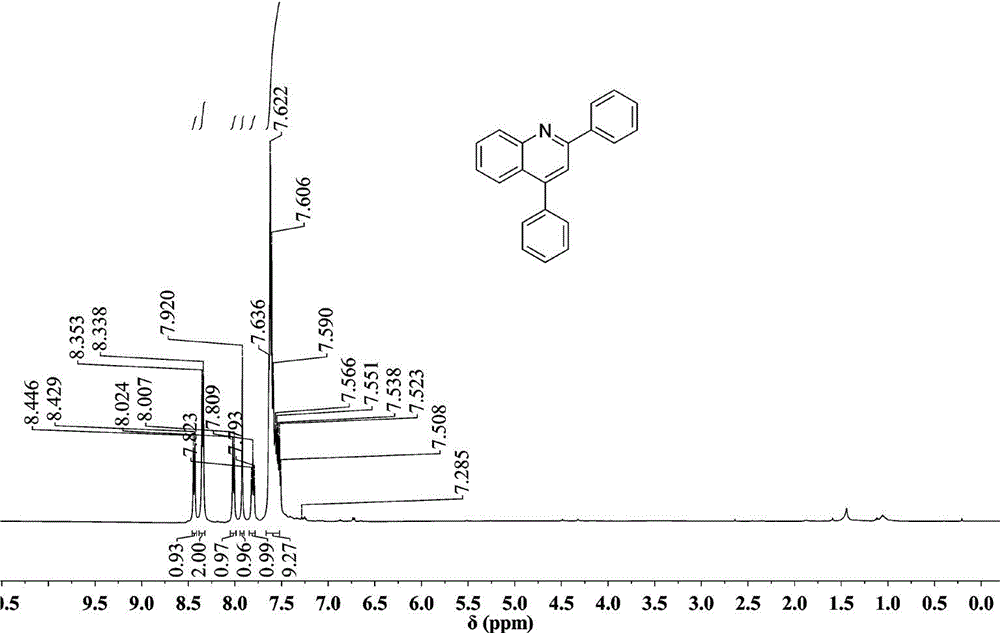
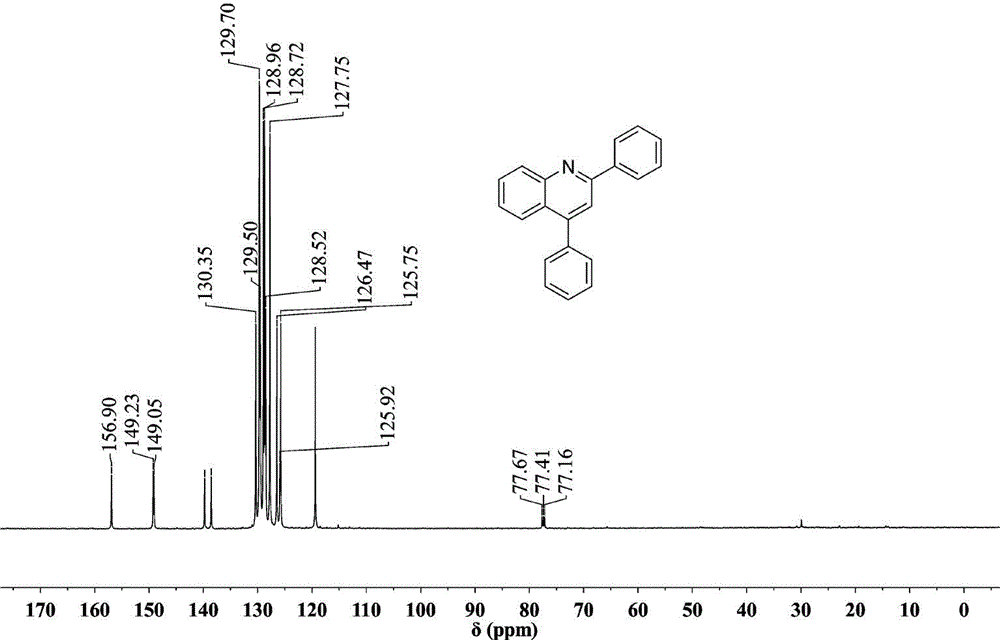
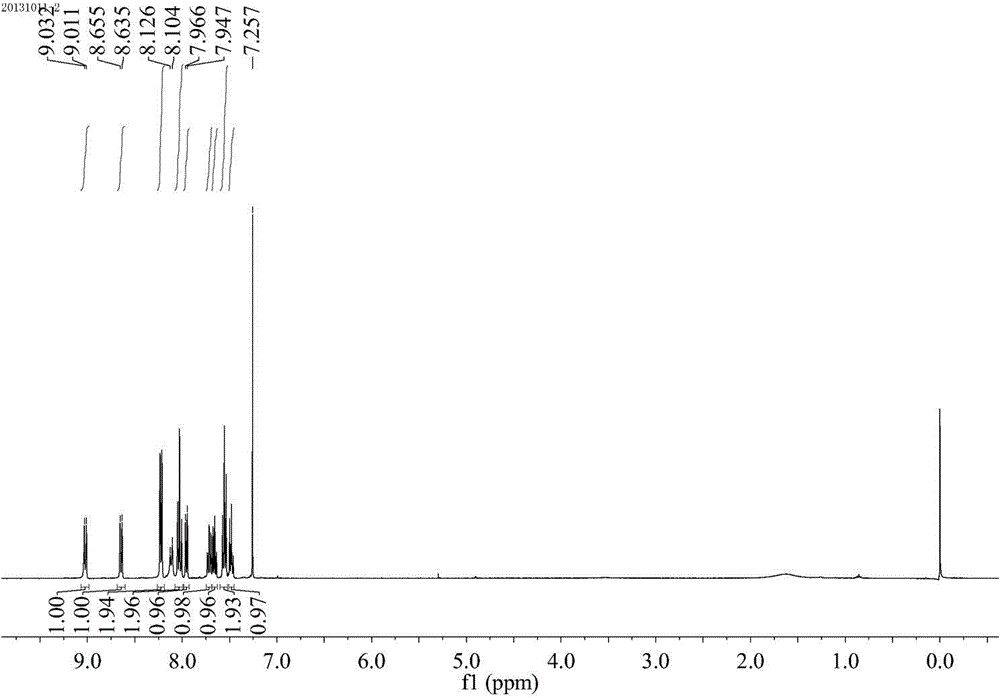
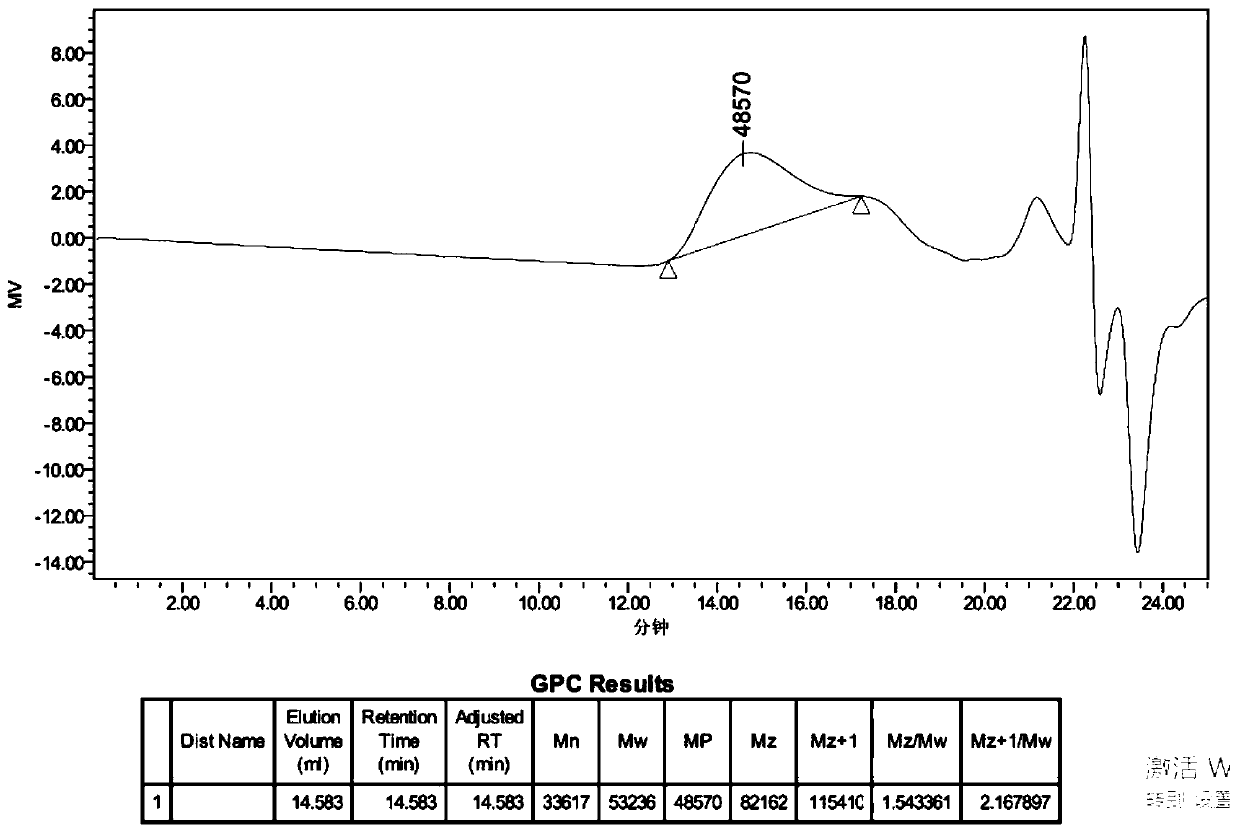
Patents
Literature
55 results about "Silver trifluoromethanesulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
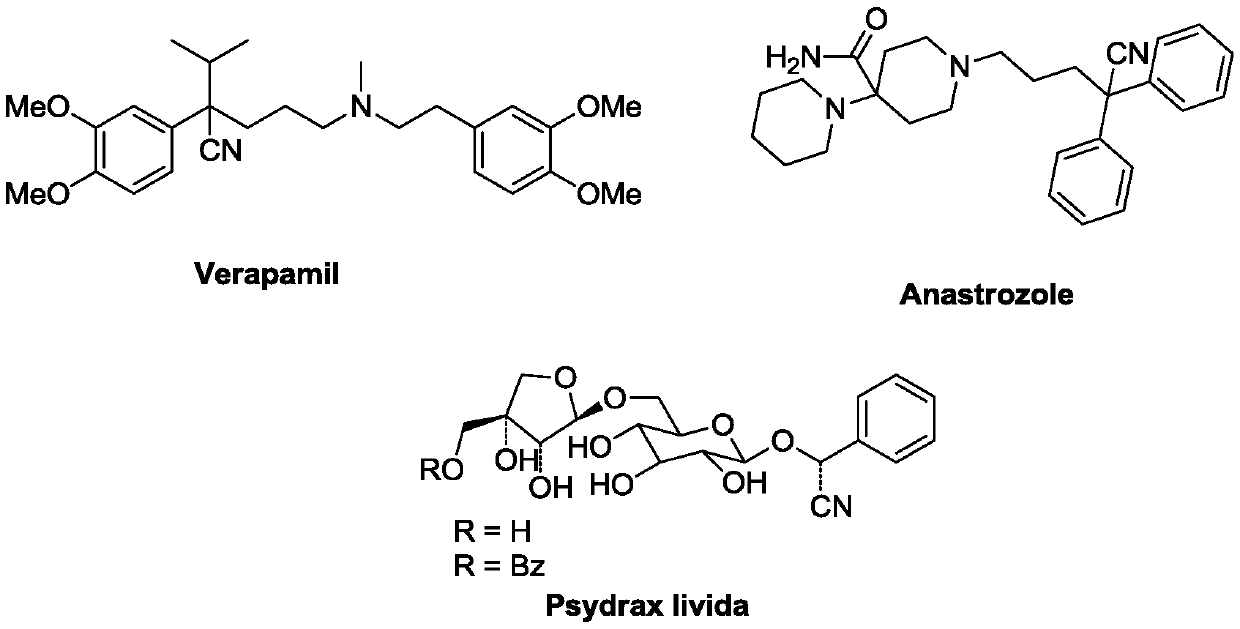
Silver trifluoromethanesulfonate, or silver triflate is the triflate (CF₃SO₃⁻) salt of Ag⁺. It is a white or colorless solid that is soluble in water and some organic solvents (most interestingly, benzene). It is a reagent in the synthesis of organic and inorganic triflates.
Electrospray ionization mass spectrometry methodology
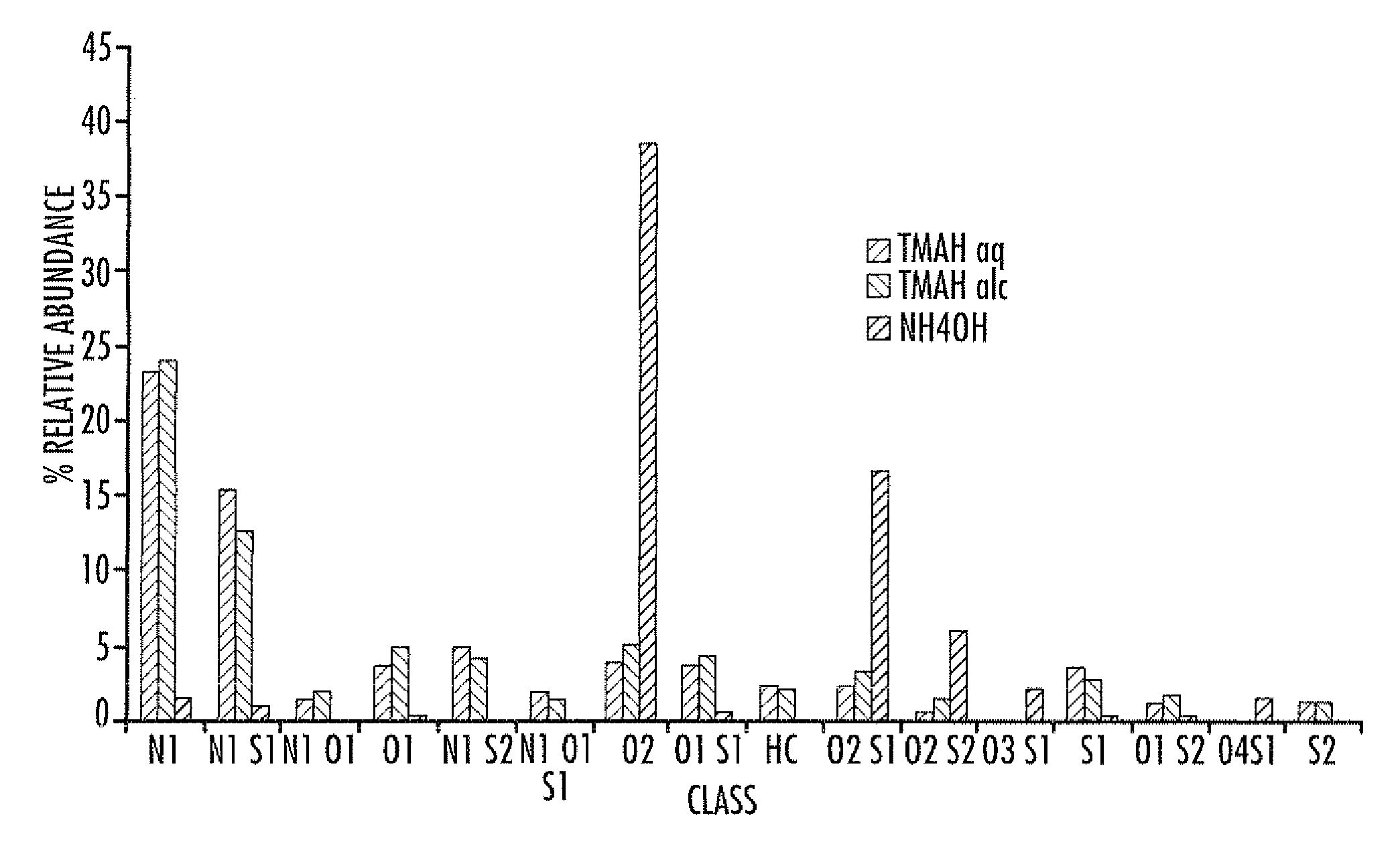
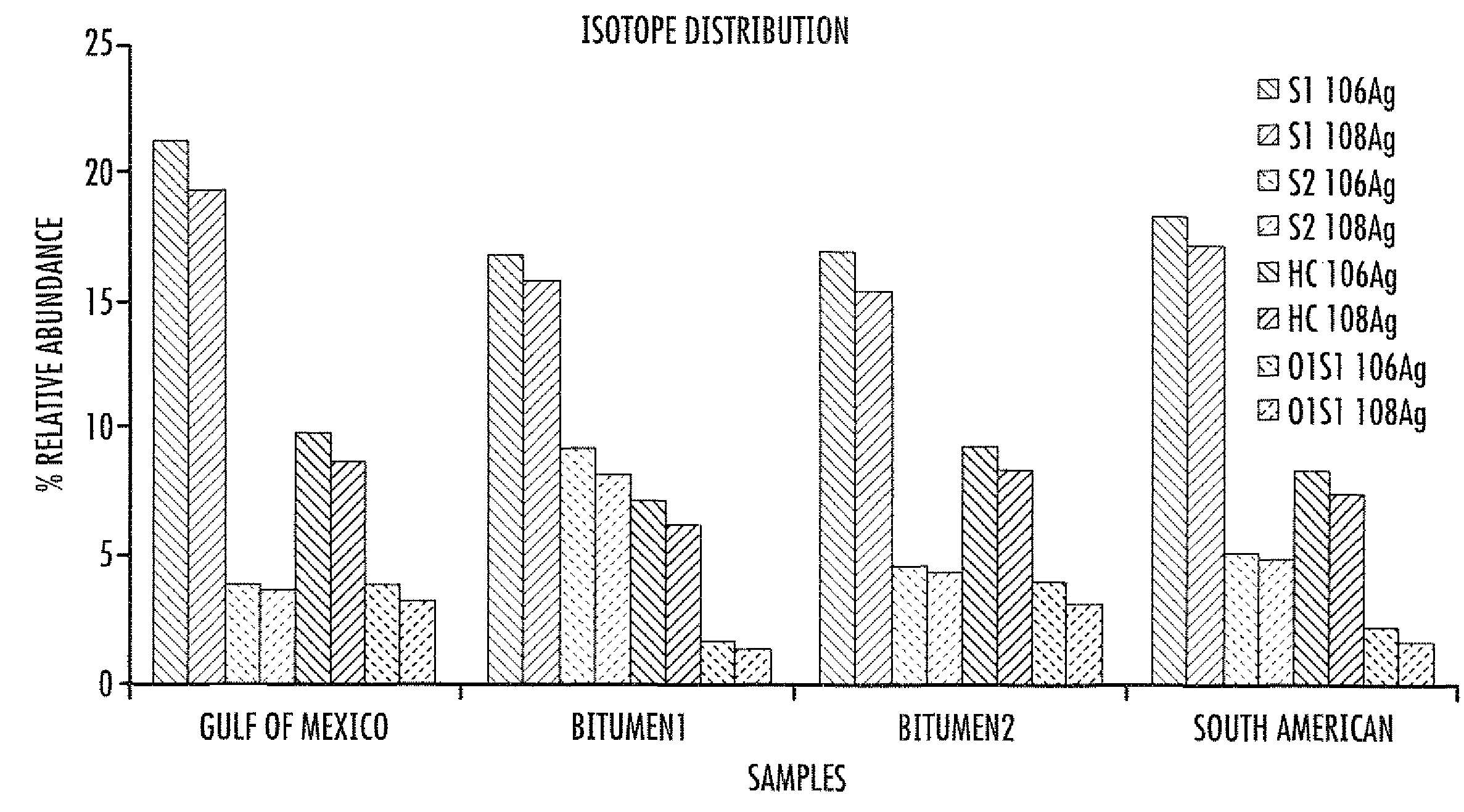
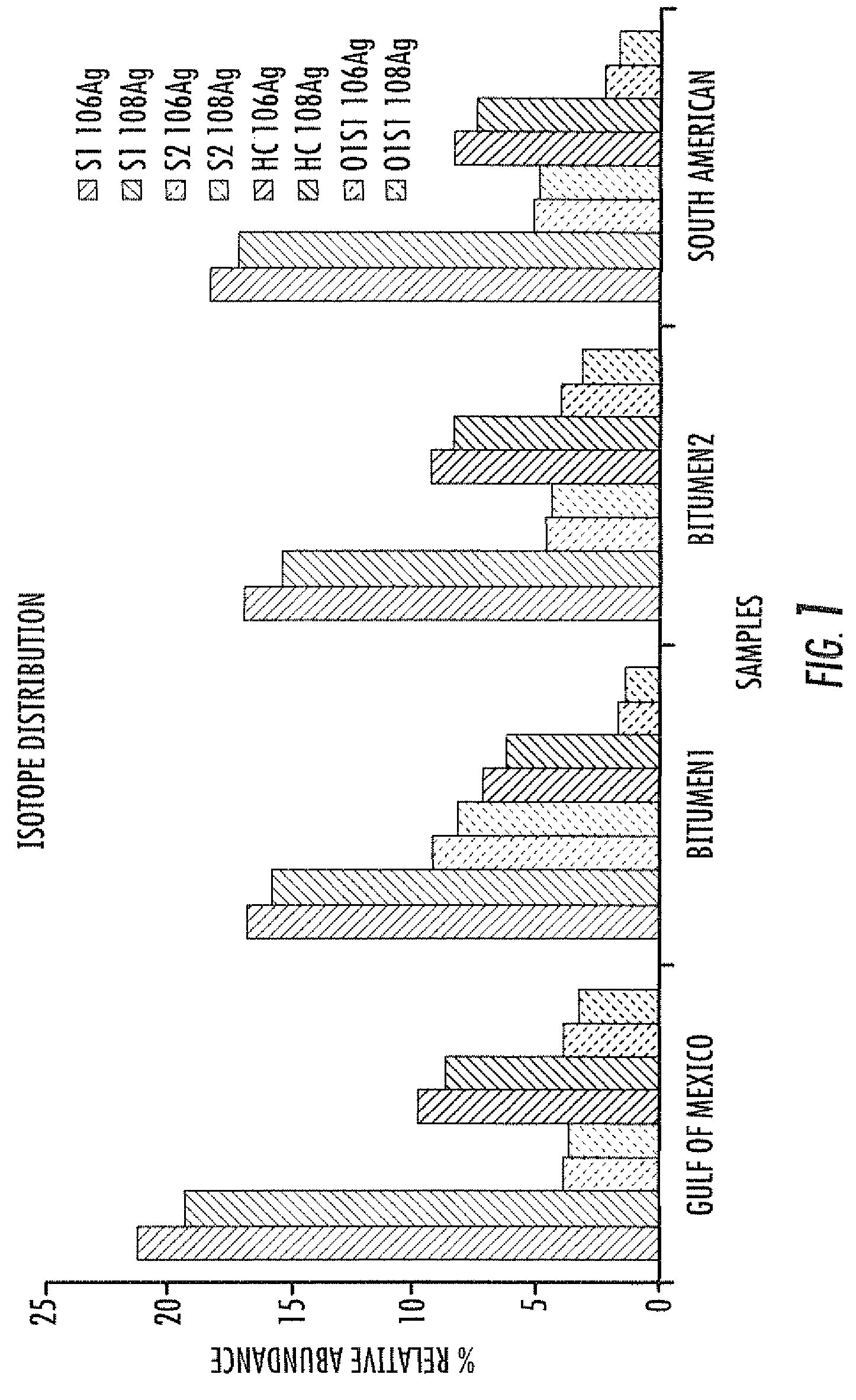
ActiveUS20100230587A1Material analysis by optical meansIsotope separationAlkaneESI mass spectrometry
A method of enhanced speciation of both positive and negatives species in an analyte is disclosed. The method can include producing a first analyte solution comprising an analyte composition and an effective amount of silver triflate, and analyzing the first analyte solution with an electrospray ionization mass spectrometer. The method can also include producing a second analyte solution comprising a portion of the analyte composition and an effective amount of a compound of formula I, and analyzing the second analyte solution with an electrospray ionization mass spectrometer. The compound of formula I is [NX+][OH−], where X is a linear, branched, or cyclic C1-C10 alkane; an aryl; a heterocyclic aromatic; or a heterocyclic moiety.
Owner:FLORIDA STATE UNIV RES FOUND INC
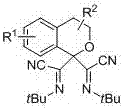
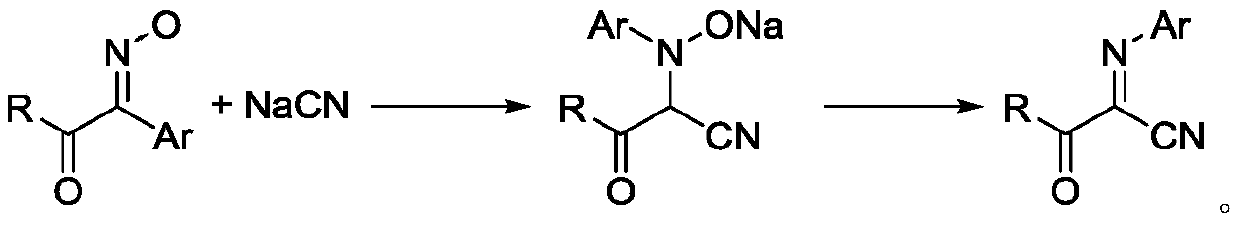
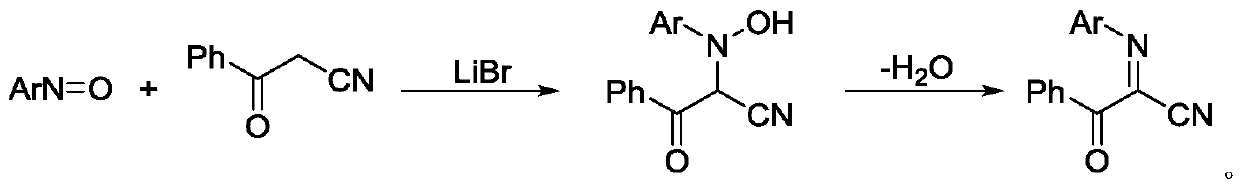
Bis alpha-cyanoimino substituted isochroman compound and synthetic method thereof
InactiveCN107082771AEasy to operateRaw materials are easy to obtainOrganic chemistryStructural formulaMedicinal chemistry
The invention relates to a bis alpha-cyanoimino substituted isochroman compound. The structural formula of the compound is as shown in the specification. Raw materials used in the method are simple and easily available. Under the action of DDQ, tert-butyl isocyanide is used as a cyano source of the reaction and shows high reaction activity under the catalysis of silver trifluoromethanesulfonate. During the reaction process, the operation is simple, conditions are mild, the method is environment-friendly, and yield is generally medium. The generated product has a special bis alpha-cyanoimino substituted skeleton. A series of chemical conversion can be carried out to generate various substituted isochroman derivatives. Therefore, the compound of the invention has a good development prospect in industrial production.
Owner:SHANGHAI UNIV
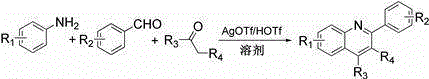
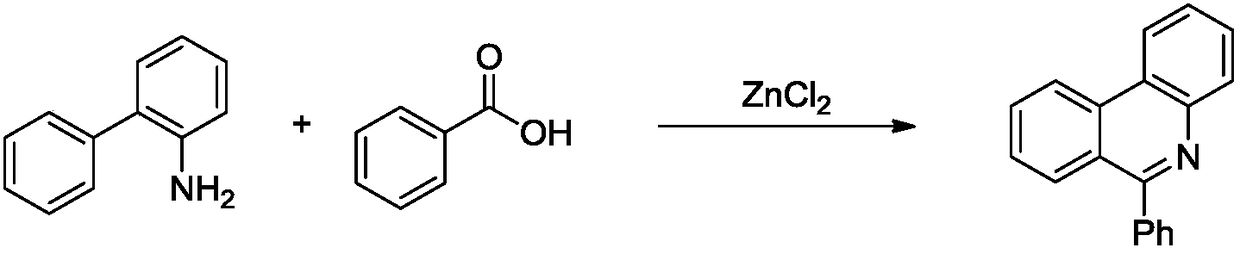
Method for synthesizing quinoline derivative by utilizing arylamine, aromatic aldehyde and ketone
InactiveCN105175328AWide variety of sourcesThere are many ways to obtainOrganic chemistryState of artTriflic acid
The invention provides a method for synthesizing a quinoline derivative by utilizing arylamine, aromatic aldehyde and ketone, and belongs to the technical field of the synthesis of the quinoline derivative. According to the method for synthesizing a quinoline derivative by utilizing arylamine, aromatic aldehyde and ketone, in the existence of silver trifluoromethanesulfonate and trifluoromethanesulfonic acid, the quinoline derivative is synthesized by virtue of the reaction of an arylamine compound, an aromatic aldehyde compound and ketone compound. A reaction general formula is as shown in the specification. Compared with the prior art, the method is not only applicable to a great amount of functional groups, but also is simple in operation, high in yield, single in product structure, convenient to separate and purify, safe, low in price and small in pollution.
Owner:NANYANG NORMAL UNIV
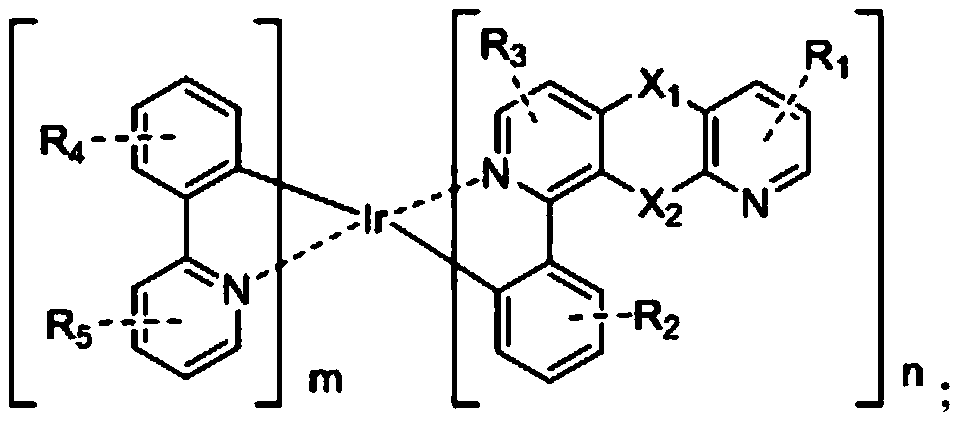
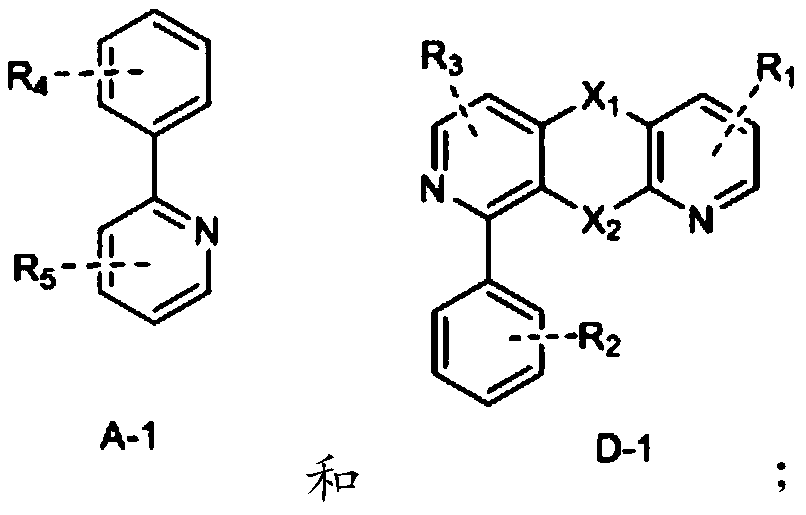
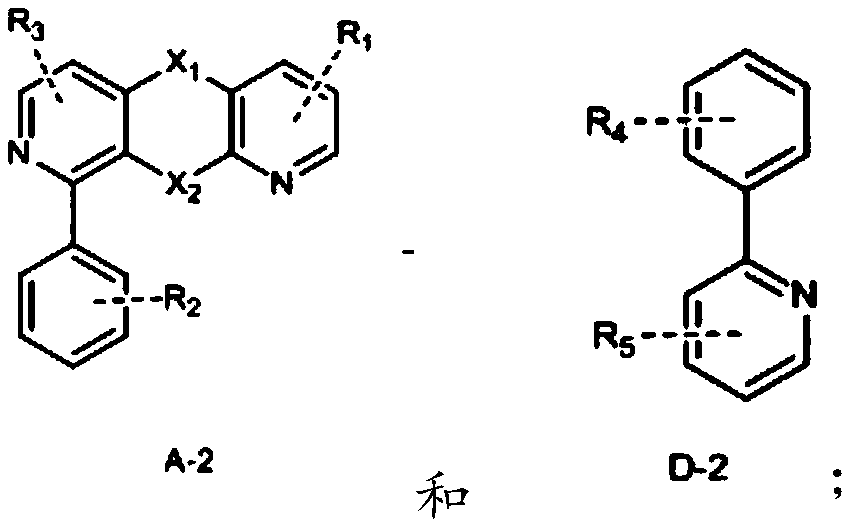
Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method
InactiveCN102268250AReduce generationEnhance proton acidityGroup 8/9/10/18 element organic compoundsLuminescent compositionsIridiumCoordination complex
The invention relates to the synthesis technology of new luminescent materials, and is a new-type electrically neutral tridentate iridium [III] complex red light material and a preparation method thereof. It is a 6-[5-trifluoromethylpyrazole]-2,2'-bipyridine·2,6-diphenylpyridine iridium[III] complex. Under the protection of nitrogen, the trihydrate iridium trichloride [III] metal salt reagent and 6-[5-trifluoromethylpyridine]-2,2'-bipyridine tridentate chelate ligand were heated in ethanol, and the reaction The liquid was pumped dry for recrystallization, filtered, washed, and vacuum-dried to obtain an orange-yellow solid intermediate; in a nitrogen glove box, the orange-yellow intermediate, silver trifluoromethanesulfonate, and 2,6-diphenylpyridine ligand were uniformly mixed , ground into powder, heated, after the reaction solution was cooled to normal temperature, the filtrate was collected and dried to obtain the crude product, the crude product was separated, the second orange-red eluate was collected and dried, and vacuum-dried to obtain the final product, the yield was up to 30-68%.
Owner:JIANGXI UNIV OF SCI & TECH
Method for preparing halogenated benzo [alfa] fluorenol
InactiveCN102659512AHigh yieldFunction increaseOrganic compound preparationPreparation by halogen introductionReaction temperatureSide reaction
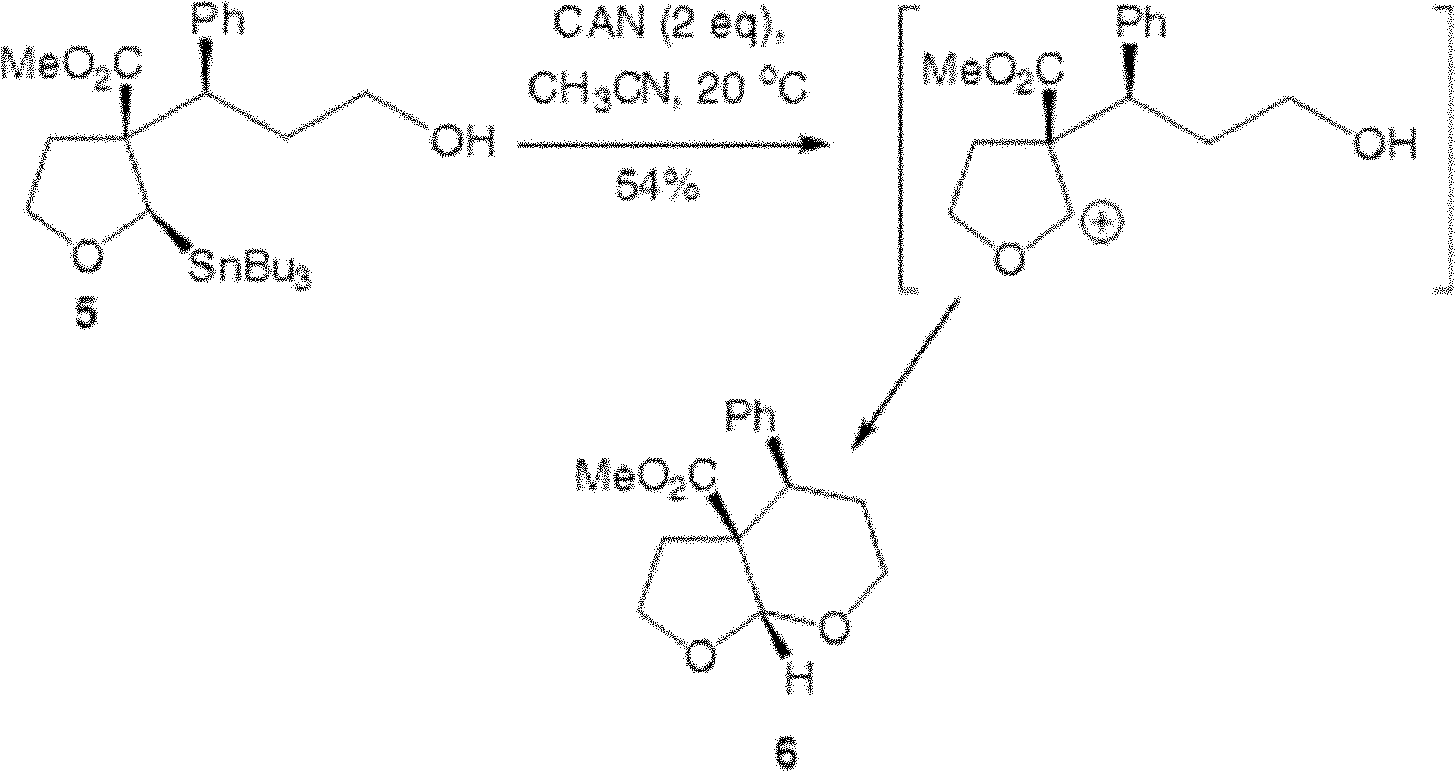
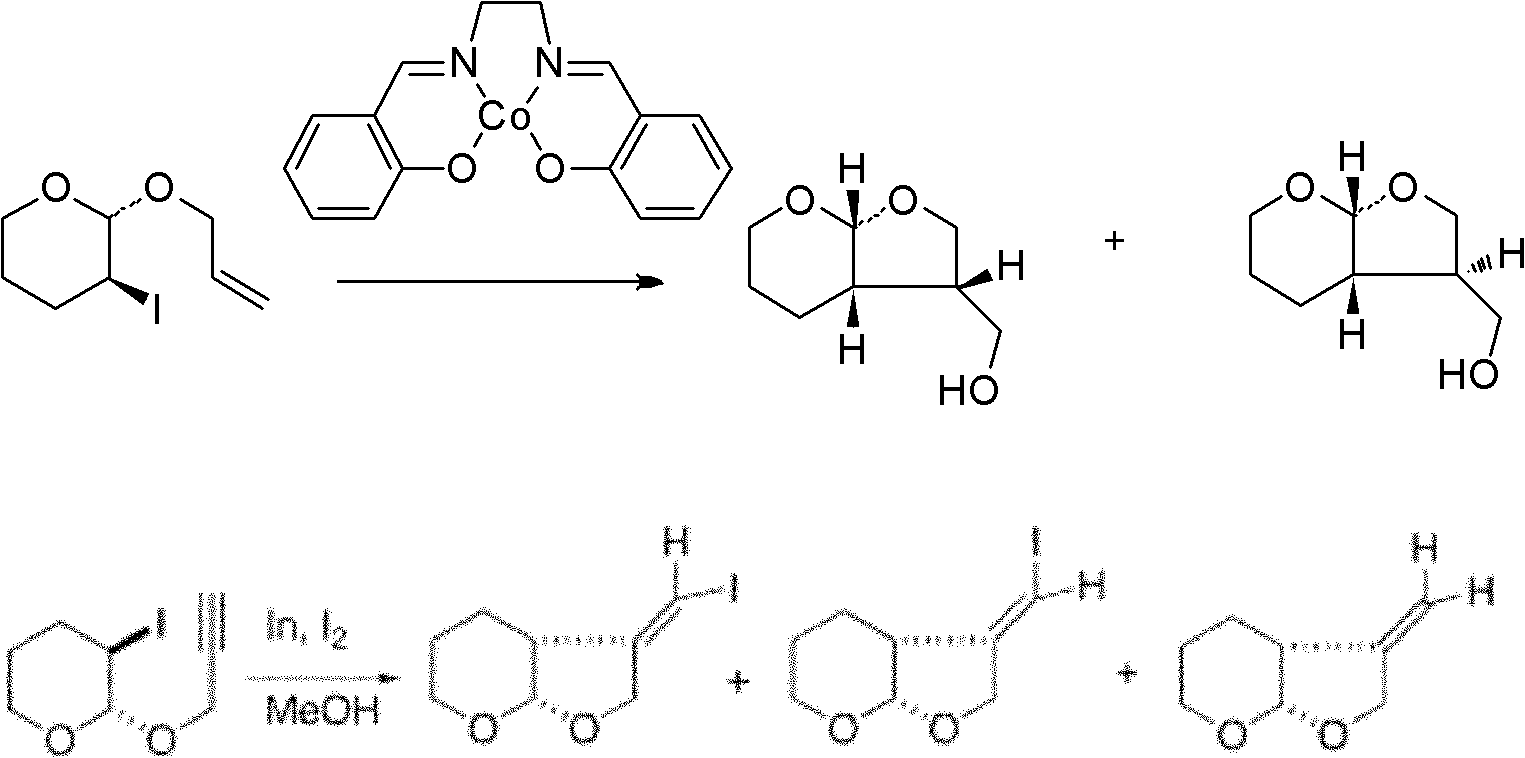
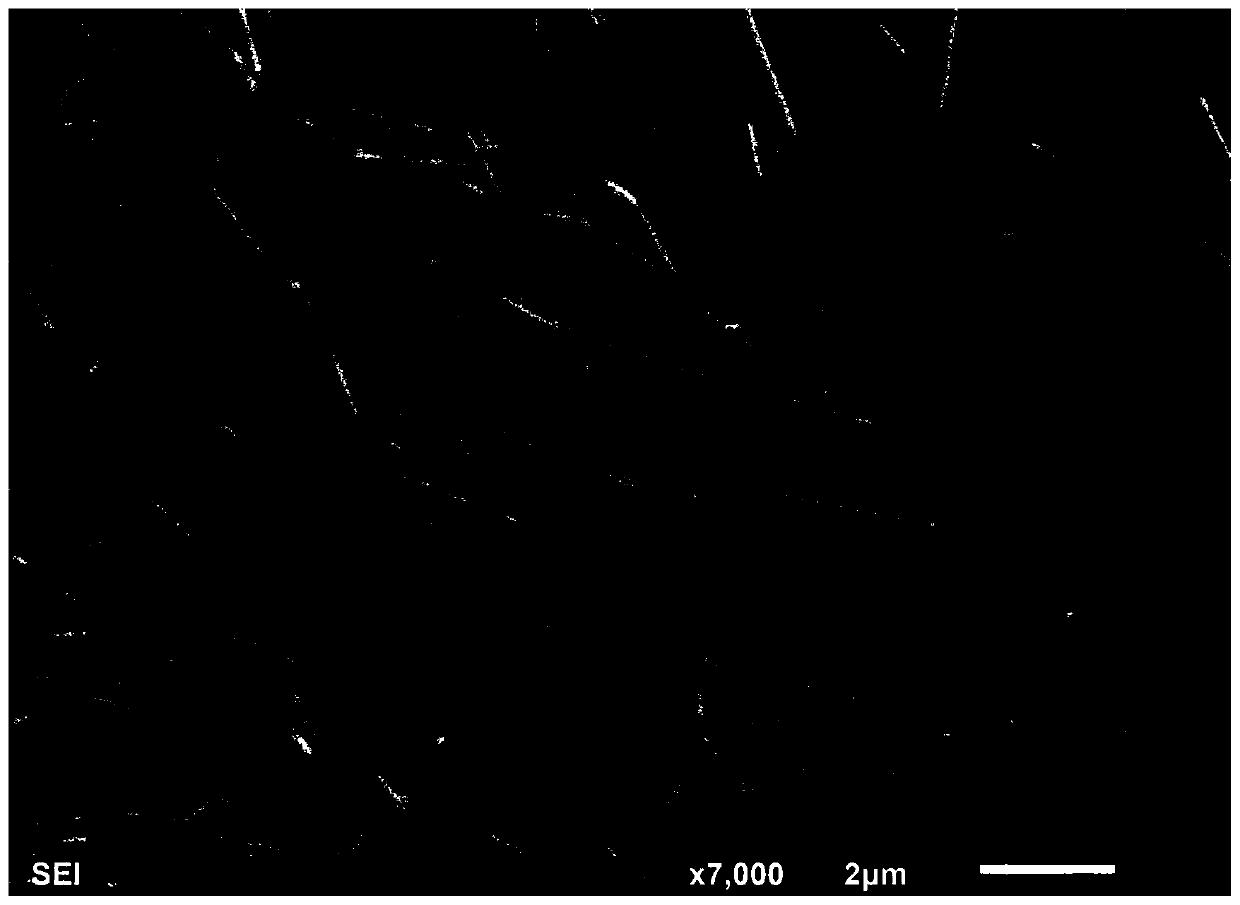
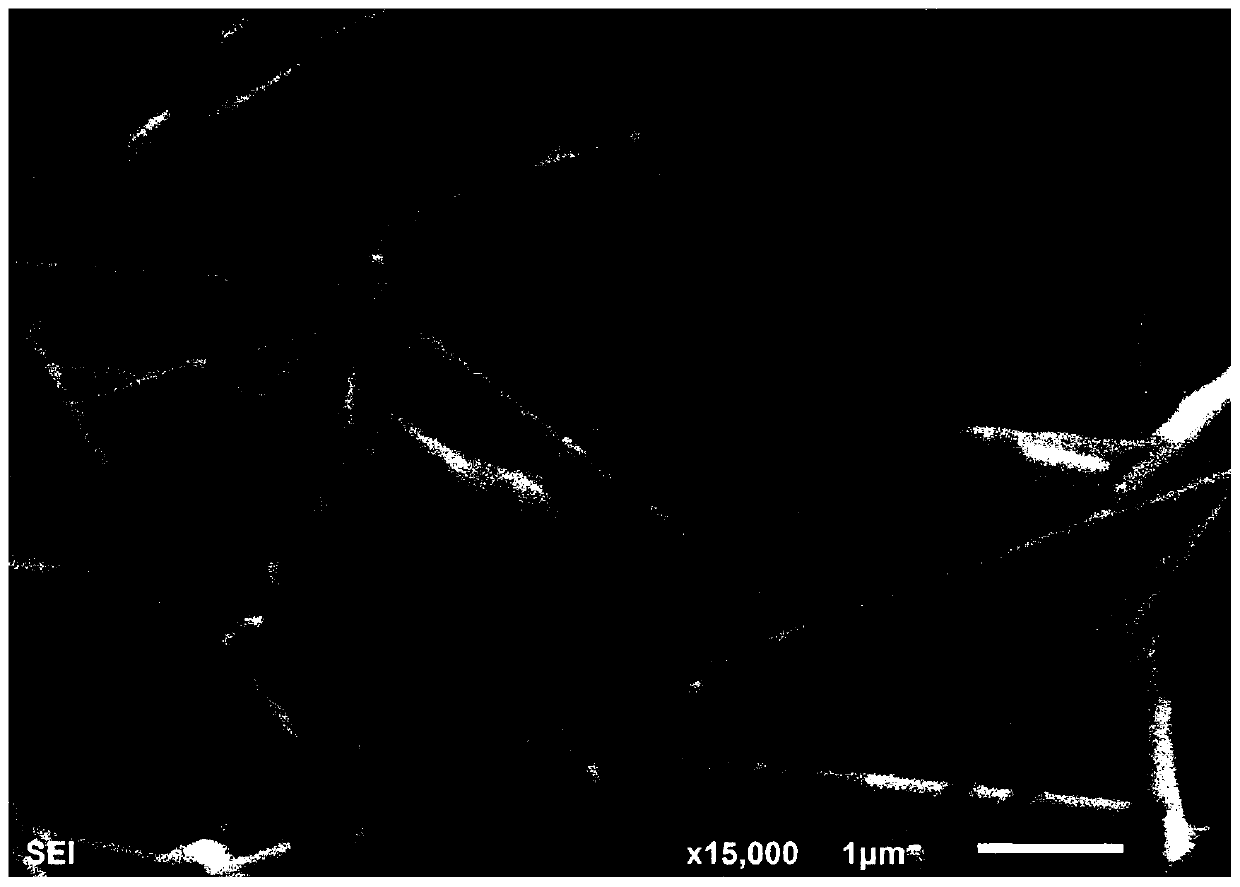
The invention provides a method for preparing halogenated benzo [alfa] fluorenol. The method comprises the following step of performing series electrophilic cyclization reaction on 3-aryl-1-(2-(2-aryl ethinyl) phenyl) propargyl-2-alcohol serving as a reaction substrate and various electrophilic reagents such as halogenated succinimide (NXS, X=I, Br and Cl) or simple substance iodine (I2), simple substance bromine (Br2) or iodine chloride (ICl) at the temperature of 0 and 15 DEG C for 10 and 15 hours under the catalysis of AgOTf, and is a 'one-pot method' for efficiently preparing the Halogenated benzo [alfa] fluorenol. The method has the advantages of mild reaction conditions, low cost, less side reaction, high product purity, is easy to operate and can be applied to mass production of the halogenated benzo [alfa] fluorenol; and moreover, separation and purification can be conveniently realized.
Owner:JIANGXI NORMAL UNIV
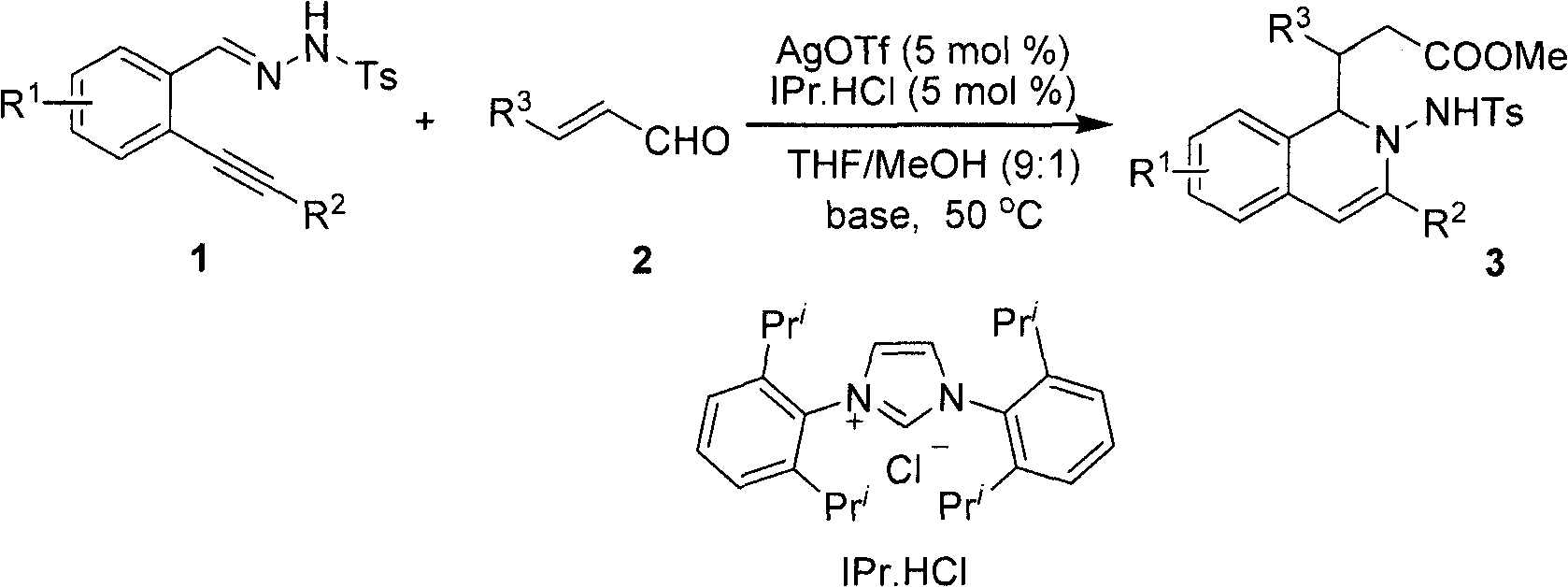
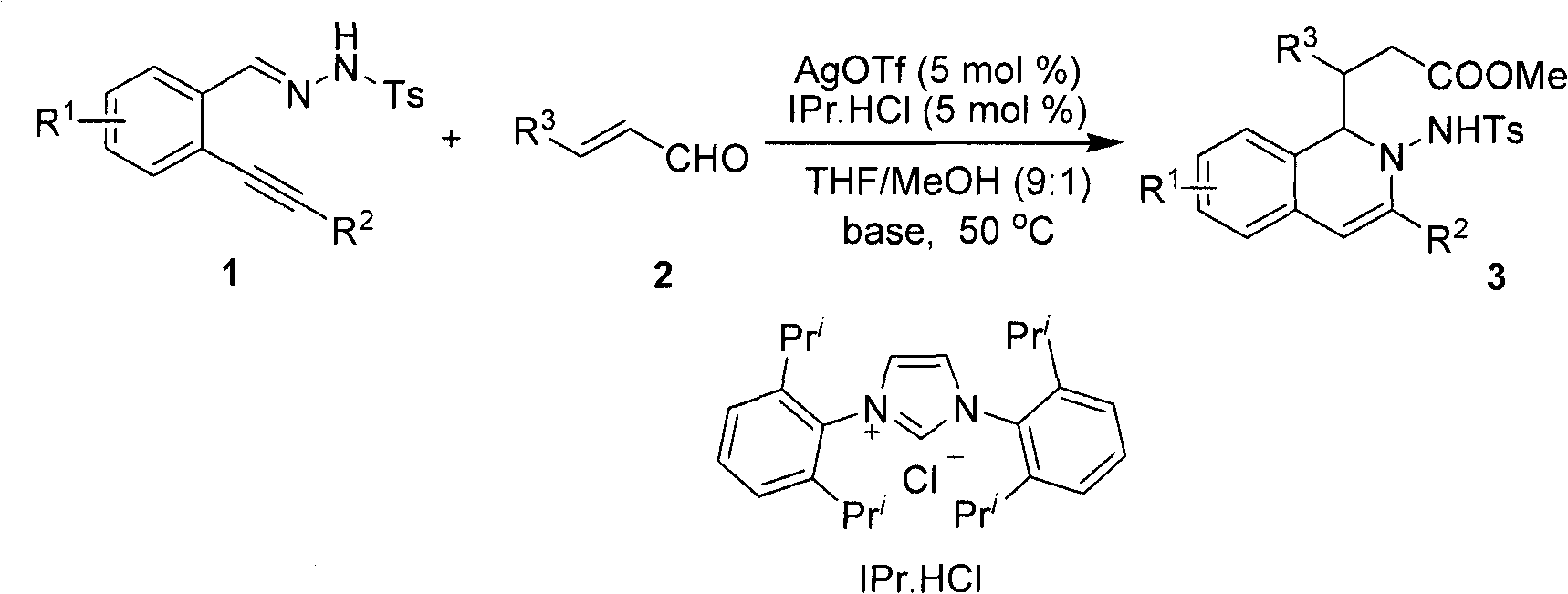
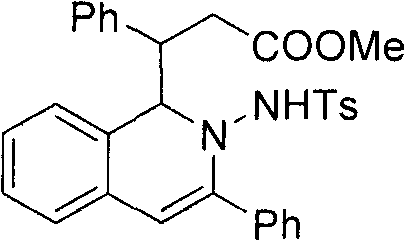
Method for preparing 2-amino-1,2-dihydro-isoquinoline compound
The invention belongs to the technical field of organic chemistry, in particular to a method for preparing a 2-amino-1,2-dihydro-isoquinoline compound. The structure of the compound is characterized and confirmed by methods including 1H-NMR, 13C-NMR, HRMS, single-crystal X diffraction, and the like. The method comprises the following steps of: under the catalytic condition of silver triflate and N-heterocyclic carbene, carrying out one kettle method of series intramolecular cyclization and polarity reversal addition reaction on o-alkynyl phenyl hydrazone and alpha, beta-unsaturated aldehyde in tetrahydrofuran, methanol and alkali to efficiently prepare the 2-amino-1,2-dihydro-isoquinoline compound- a. The method has the advantages of high reaction efficiency, mild conditions, simple operation, lower cost, high product purity, convenient separation and purification and is suitable for large-scale preparation. The skeleton structure of the product has wide spectrum biological activity, and the compound has wide application prospect in discovery of new drugs.
Owner:FUDAN UNIV
Method for stereoselective preparation of derivatives of pyrane
InactiveCN102180923AReduce pollutionEasy to operateBiocideSugar derivativesReaction temperatureSolvent
The invention belongs to the technical field of organic chemistry and pharmaceutical chemistry, and in particular relates to a method for stereoselective preparation of derivatives of pyrane. The method comprises the following steps of: under the protection of inert gases, adding derivatives of 1-p-tolylthio-2-C-glyoxyl-alpha-D-glucopyranose, derivatives of 1-thiophenyl-2-C- glyoxyl-alpha-D-glucopyranose or derivatives of 1-ethylthio p-tolyl-2-C-glyoxyl-alpha-D-glucopyranose, which serve as reactants, into a reactor, adding alcohol, phenol, trimethylsilyl azide or monosaccharide derivatives, adding a solvent such as methylene chloride to dissolve the raw materials, and performing the reaction at the reaction temperature of between 40 DEG C below zero and room temperature in the presence of a catalyst which is N-iodosuccinimide, or N-bromosuccinimide, or copper bromide and tetrabutylammonium bromide, or iodine, bromine simple substance or N-iodosuccinimide and trimethylsilyl triflate, or trifluoromethanesulfonic silver, or paratoluenesulfonic acid or trifluoromethanesulfonic acid to obtain the derivatives of pyrane. The method has the advantages of simple operation, high selectivity, low cost, light environmental pollution and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
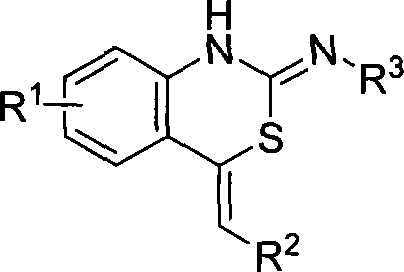
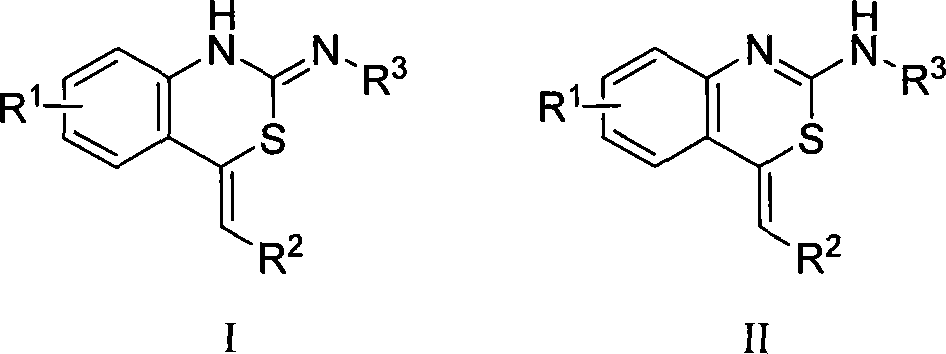
Method for producing benzo thiazides compounds
The invention belongs to the technical field of organic chemistry, in particular to a benzothiazole compounds and a preparation method for the compounds. The structures of the compounds are attributed and determined by the methods like <1>H NMR, <13>CNMR, IR, MS, elementary analysis, and a single-crystal X-ray diffraction, etc. The invention uses a silver triflate as a catalyst to prepare 4-methylene-1H-benzo[d] [1, 3] thiazine-2(4H)-imine framework compounds by mixing an ortho-alkynyl aniline and an isothiocyanate in a tetrahydrofuran to react at a room temperature. The method of the invention has the advantages of mild reaction condition, simple operation, lower cost, fewer side effects and high purity of the product; thereby having a great application prospect.
Owner:FUDAN UNIV
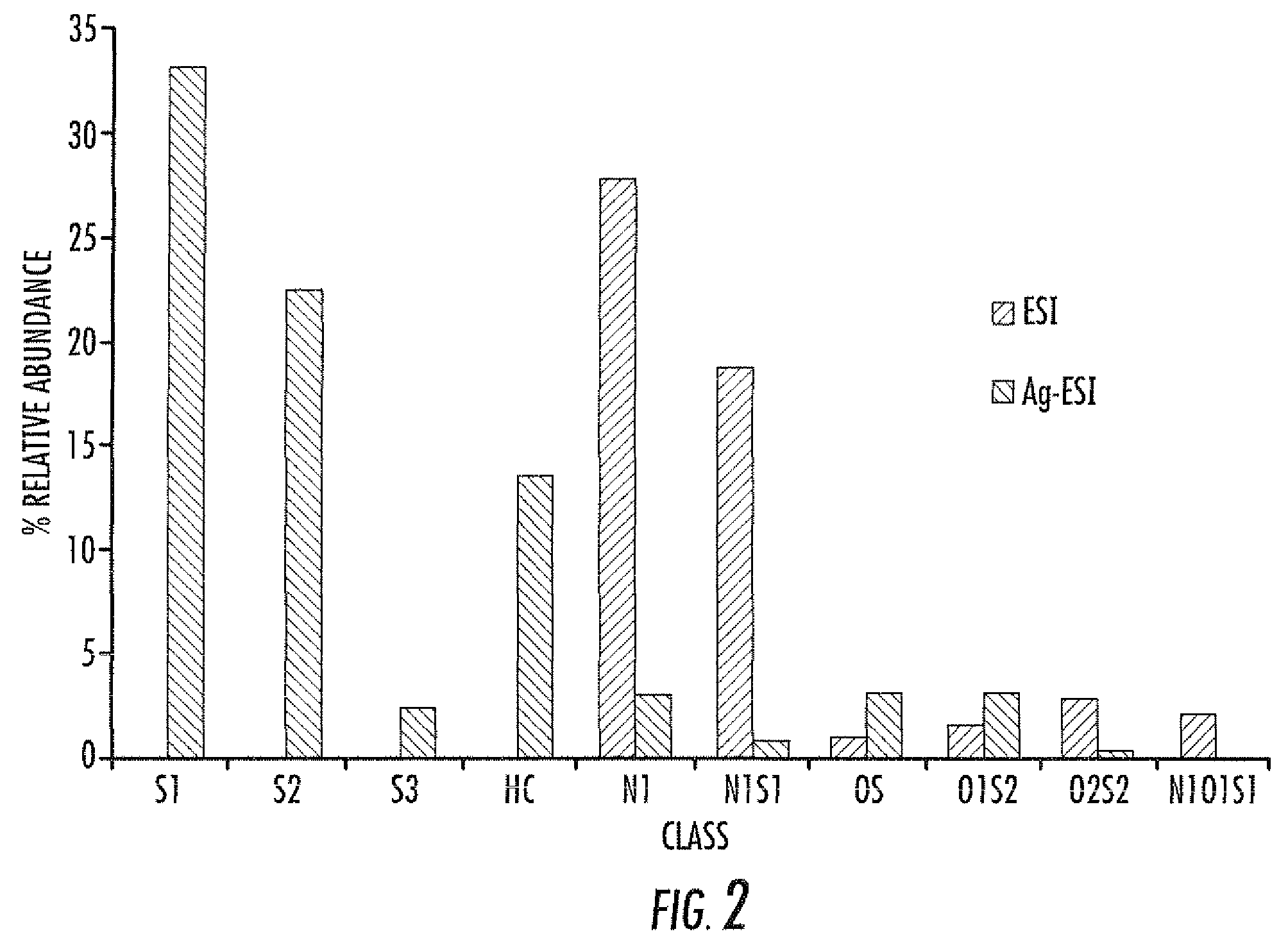
Electrospray ionization mass spectrometry methodology
A method of enhanced speciation of both positive and negatives species in an analyte is disclosed. The method can include producing a first analyte solution comprising an analyte composition and an effective amount of silver triflate, and analyzing the first analyte solution with an electrospray ionization mass spectrometer. The method can also include producing a second analyte solution comprising a portion of the analyte composition and an effective amount of a compound of formula I, and analyzing the second analyte solution with an electrospray ionization mass spectrometer. The compound of formula I is [NX+][OH−], where X is a linear, branched, or cyclic C1-C10 alkane; an aryl; a heterocyclic aromatic; or a heterocyclic moiety.
Owner:FLORIDA STATE UNIV RES FOUND INC
Method for synthesizing quinoline by aromatic amine and diketone
InactiveCN105198806AWide variety of sourcesThere are many ways to obtainOrganic chemistryState of artDiketone
The invention provides a method for synthesizing quinoline by aromatic amine and diketone and belongs to the technical field of synthesis of the quinoline. According to the method for synthesizing the quinoline by the aromatic amine and the diketone, under the existence of silver trifluoromethanesulfonate and trifluoromethanesulfonic acid, the quinoline is synthesized by reaction between the aromatic amine compound and the diketone compound; the reaction general formula is shown in the specification. Compared with the prior art, the method not only is suitable for a large number of functional groups but also is simple in operation, high in yield, single in product structure, convenient for separation and purification, safe, low in cost and low in pollution.
Owner:NANYANG NORMAL UNIV
Method for synthesizing quinoline derivative
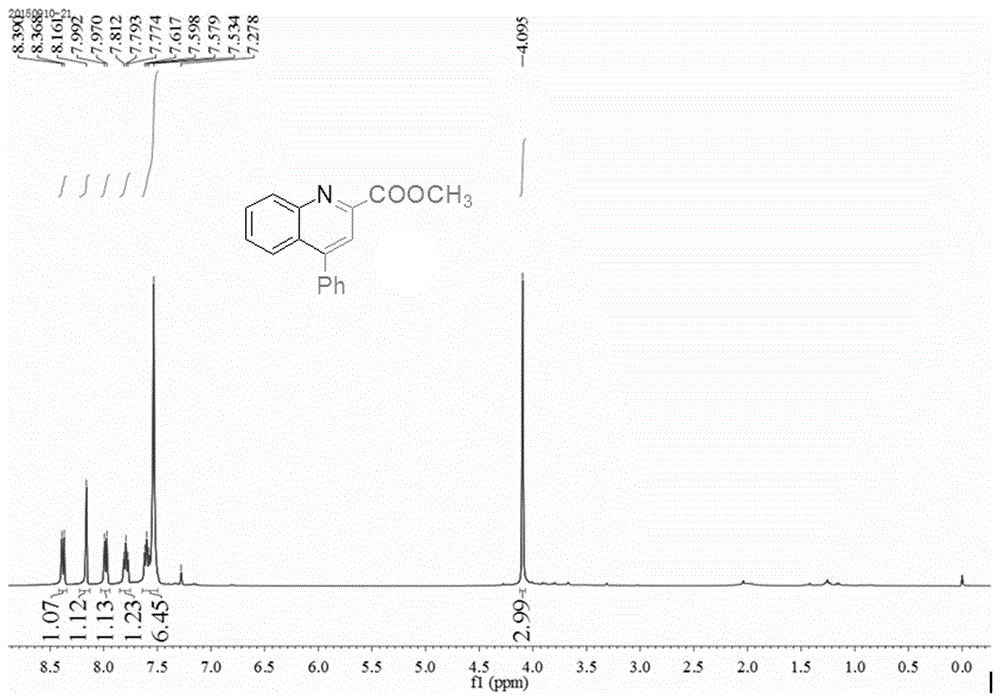
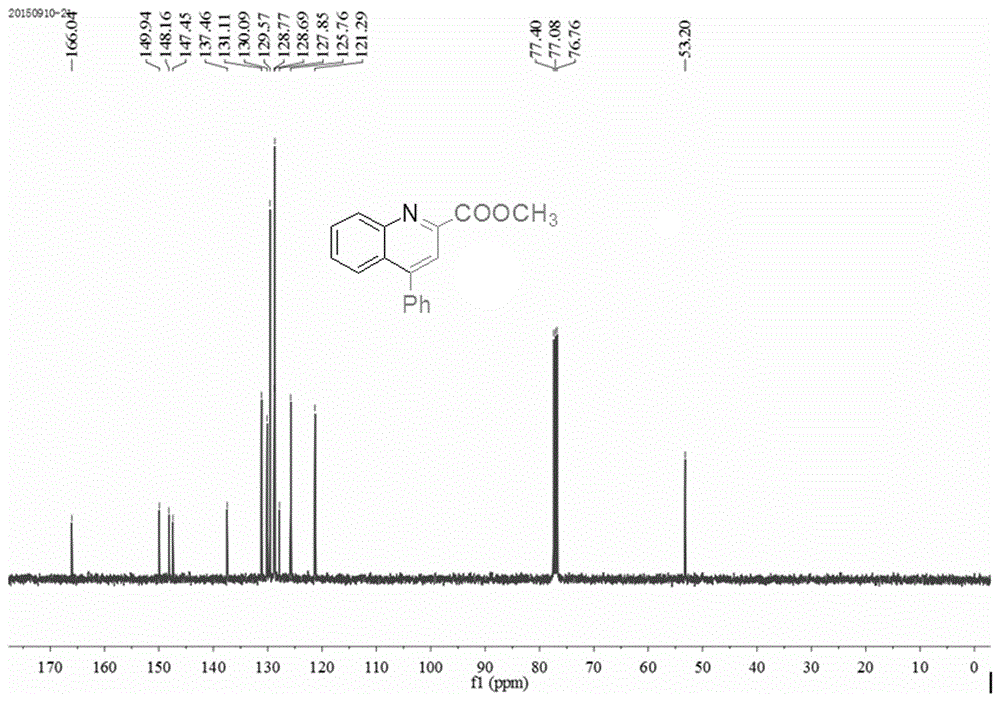
The invention provides a method for synthesizing a quinoline derivative. Aromatic amine, electrophilic alkyne and alkyne are sequentially added into a reaction vessel according to the molar ratio of 1:1:(1.2-4), solvent is added according to the proportion that 2-4 mL of solvent is added into 1 mmol of aromatic amine, then catalyst silver trifluoromethanesulfonate (AgOTf) with the molar weight being 0.8-5% that of aromatic amine and additive trifluoromethanesulfonic acid (HOTf) with the molar weight being 1.8-10% that of aromatic amine are added, reaction is conducted for 8-24 h at 100-120 DEG C in oil bath, the product is cooled to room temperature, water is added, the product is extracted three times with ethyl acetate, organic layers are combined, decompressive condensing is conducted, the product is subjected to column chromatography purification, and the product, namely the quinoline derivative, is obtained. The quinoline derivative has the advantages that reaction substrates are low in price, the yield is high, selectivity is good, separation and purification are easy, pollution is little, and steps are simple.
Owner:NANYANG NORMAL UNIV
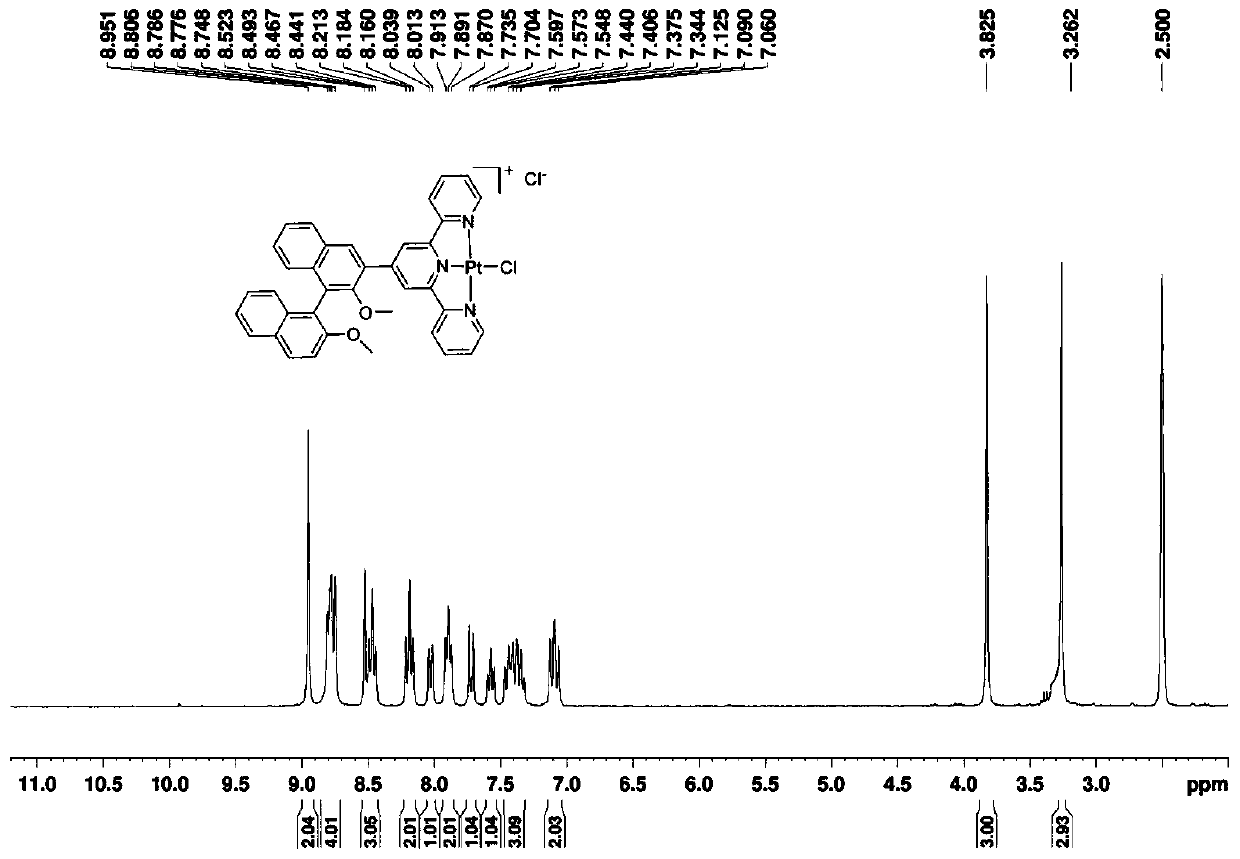
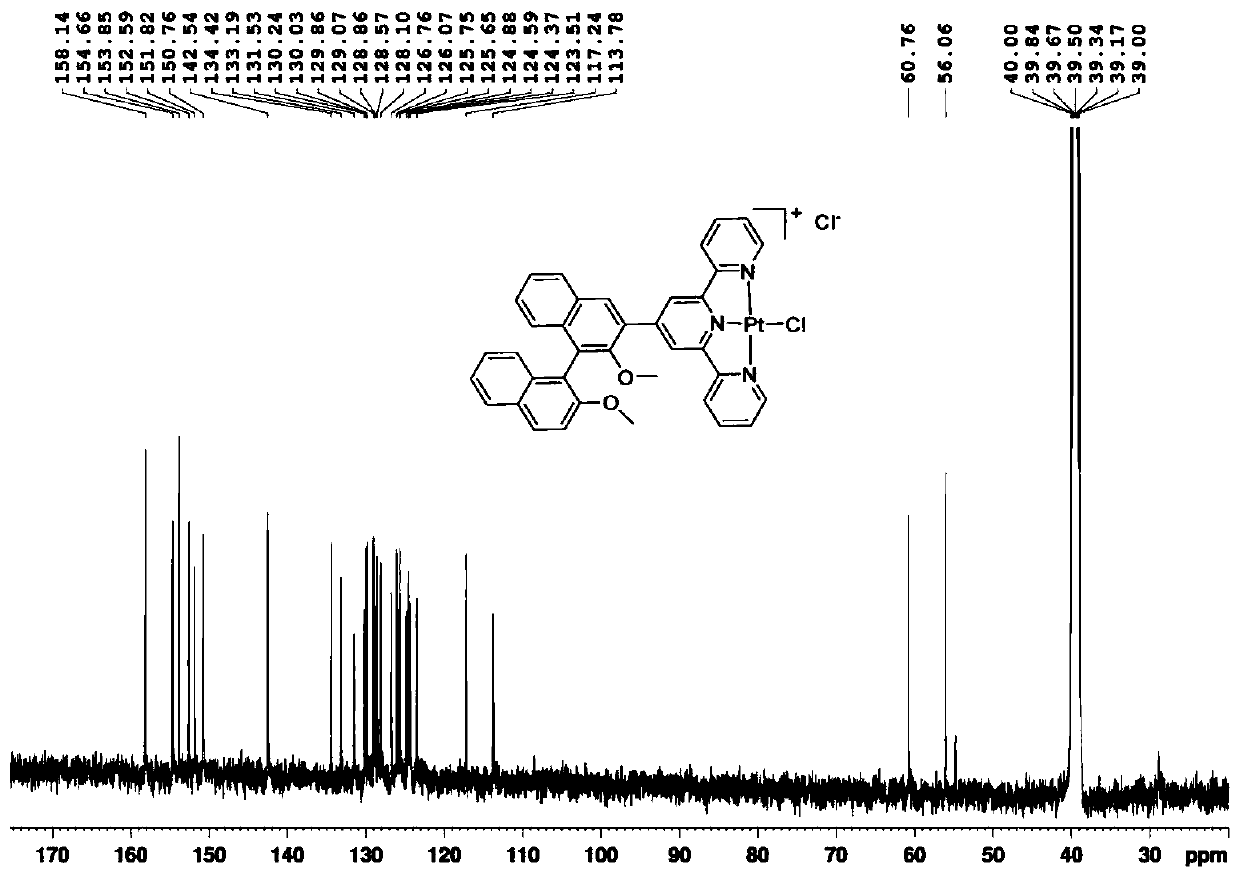
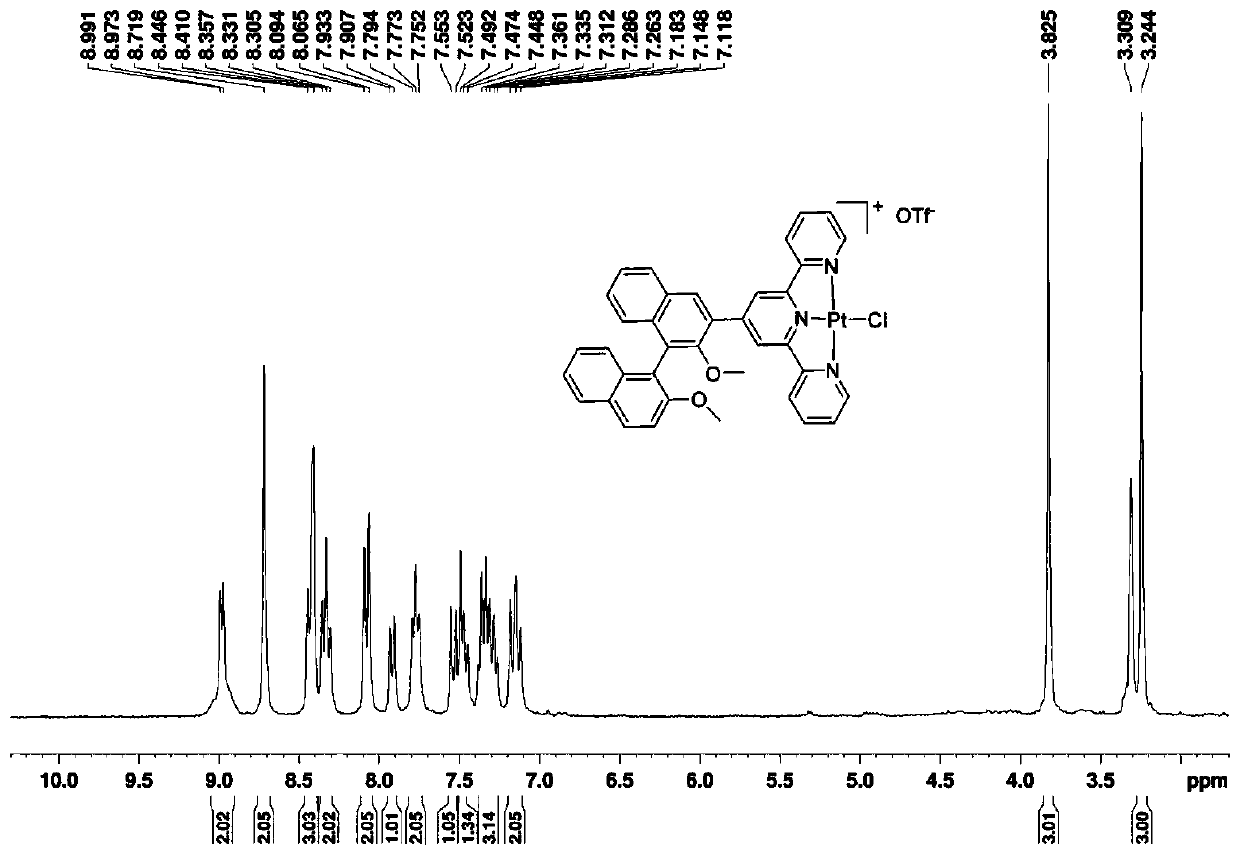
Compound based on chiral binaphthol-terpyridyl platinum and preparation method of compound
ActiveCN110655537AHas near-infrared circularly polarized luminescent propertiesEasy to purifyOrganic chemistry methodsPlatinum organic compoundsPotassium tetrachloroplatinateAlpha-naphthol
The invention discloses a compound based on chiral binaphthol-terpyridyl platinum and a preparation method of the compound. The preparation method comprises the steps: dissolving a compound BT in a first solvent and stirring to form a first solution; dissolving potassium chloroplatinite in a second solvent, and stirring to form a second solution; adding the second solution into the first solution,and carrying out heating reflux to obtain BTPt-Cl; dissolving silver trifluoromethanesulfonate and the BTPt-Cl in a third solvent, and carrying out heating reflux to obtain BTPt-OTf. The invention discloses the compound based on chiral binaphthol and terpyridyl platinum and a preparation method of the compound; the compound based on chiral binaphthol and terpyridyl platinum, having an aggregation-induced near-infrared circular polarization luminescence property and having a highest luminescence asymmetry factor up to 10<-1> is prepared through two-step synthesis. The method has the advantagesof low synthesis cost, simple synthesis route and easiness in product purification, and the synthesized target compound has the axial chiral structure of binaphthol and the fluorescence characteristic of terpyridyl platinum, and has the aggregation-induced luminescence property.
Owner:NANJING FORESTRY UNIV
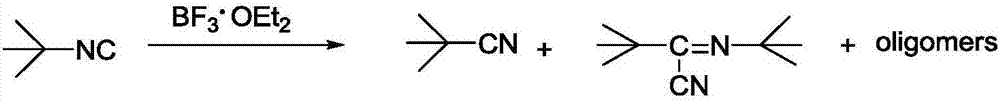
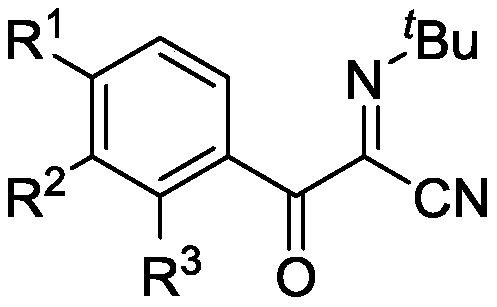
Beta-carbonyl-(alpha-cyanoimine) compound and synthesis method thereof
InactiveCN110183352ADiverse reactivityEasy to operateGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationSynthesis methodsOrganic synthesis
The invention relates to a beta-carbonyl-(alpha-cyanoimine) compound and a synthesis method thereof, wherein the compound has a structural formula defined in the specification, R<1> is hydrogen, methyl, methoxy, ester, fluorine, chlorine, bromine, alkenyl or alkynyl (C2-C3), R<2> is hydrogen or methyl, and R<3> is hydrogen or methyl. According to the present invention, the beta-carbonyl-(alpha-cyanoimine) compound is a valuable organic synthesis intermediate, wherein the carbonyl cyanoimine skeleton exhibits various reaction activities, such that the beta-carbonyl-(alpha-cyanoimine) compound can be converted into other corresponding active compounds; the raw materials used in the method are simple and easy to obtain, and t-butyl isocyanide is used as the cyano source of the reaction, and has the best reactivity and the best substrate adaptability under the promotion of silver trifluoromethanesulfonate; and the conventional reaction solvent is used during the reaction process, and the method has characteristics of simple operation, mild condition, environmental protection and general-to-excellent yield, and has good development prospect in industrial production.
Owner:SHANGHAI UNIV
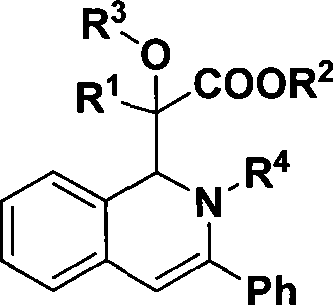
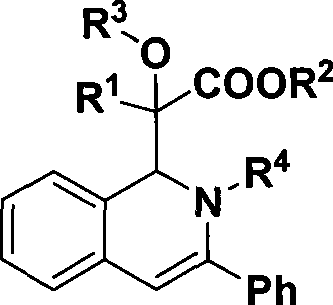
1,2-dihydroisoquinoline derivative and preparation thereof
The invention discloses a 1,2-dihydroisoquinolin derivative and a preparation method thereof, relating to a midbody which can be used for synthesizing natural alkaloid and a preparation method thereof. The 1,2-dihydro-isoquinolin derivative has a structure of formula (I). In the formula, R1 is meta-position or ortho-position substituted electron donating or weak electron-withdrawing aromatic group or H; R2 is methyl or ethyl; R3 is hydrogen or alkyl; and R4 is meta-position or ortho-position substituted aromatic group. The mol ratio of diazonium, alcohol (or water), imine, rhodium acetate and silver trifloromethanesulfonate is 1.2:1.2:1:0.01:0.05. A solvent, the alcohol (or water), the imine and a catalyst are used for preparing a mixed solution. Then, the diazonium is evenly mixed with another solvent to prepare diazonium solution which is then infused into the mixed solution. The solvent is removed by reduced pressure distillation at a temperatre of 40-70 DEG C. Column chromatographic separation is carried out to obtain the pure product of the 1,2-dihydro-isoquinolin derivative. When the 1,2-dihydro-isoquinolin undergoes reduction reaction, a tetrahydroisoquinoline derivative with pharmaceutical activity can be generated. The 1,2-dihydroisoquinolin derivative is an important structural unit of a great number of natural products and can be used as a midbody which can be used for synthesizing natural alkaloid.
Owner:EAST CHINA NORMAL UNIV
A kind of method utilizing aromatic amine, aromatic aldehyde, ketone to synthesize quinoline derivative
InactiveCN105175328BWide variety of sourcesThere are many ways to obtainOrganic chemistryState of artTriflic acid
Owner:NANYANG NORMAL UNIV
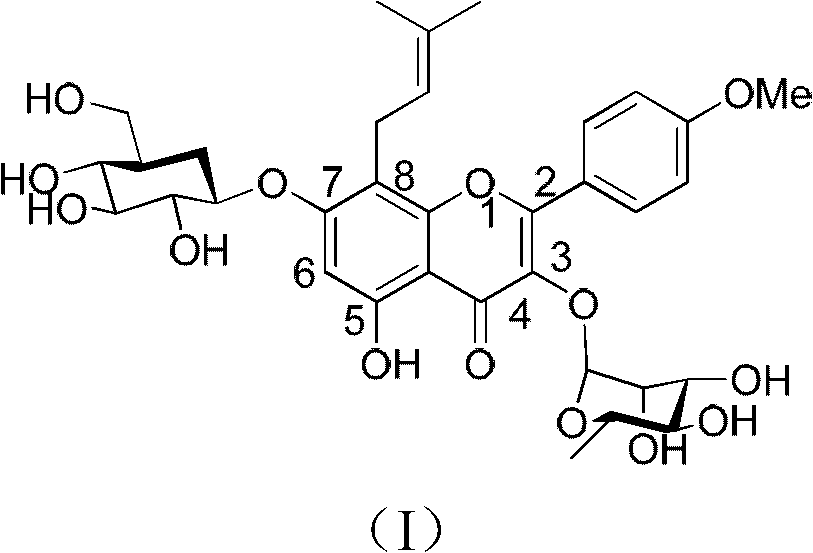
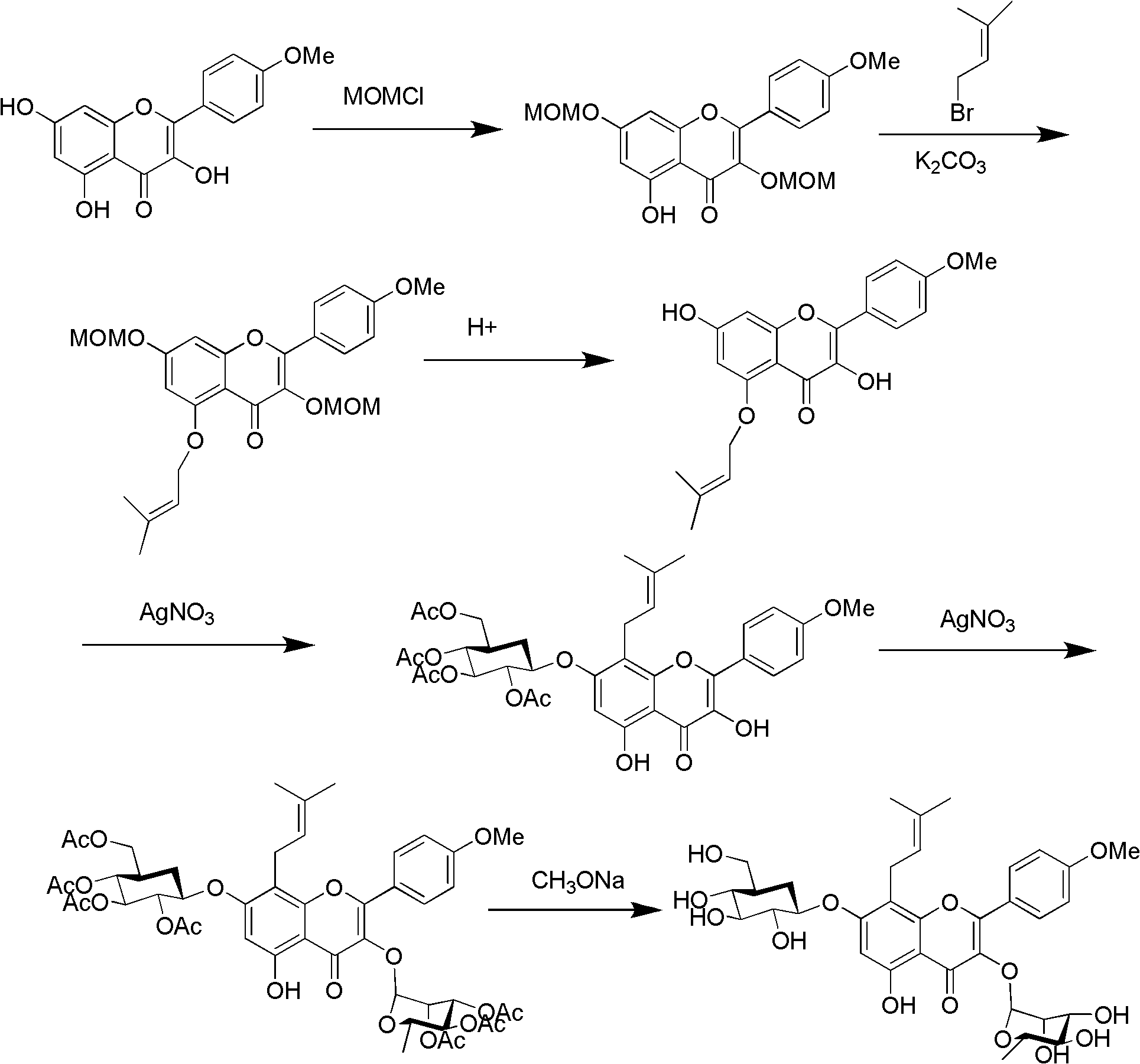
Method for synthesizing icariin by glucosidation of dehydrated epimedium herb
ActiveCN102093450AHigh selectivityHigh yieldSugar derivativesSugar derivatives preparationStrontium carbonateEpimedium
The invention relates to a method for synthesizing icariin by glucosidation of dehydrated epimedium herb. The conventional method for synthesizing icariin by glucosidation of dehydrated epimedium herb is low in selectivity. For solving the problem, the method uses strontium carbonate as a catalyst and comprises the following steps: dissolving dehydrated icaritin in an organic solvent; reacting the icaritin with alpha-bromotetraacetyl-D-glucopyranose in the presence of strontium carbonate to perform glucosidation at position-3; reacting the product of the glucosidation with alpha-bromotriacetyl-L-rhamnopyranoside in the presence of a silver trifluoromethanesulfonate catalyst to perform glucosidation at position-7; and performing deacetylation reaction to obtain icariin. The method is suitable to synthesize icariin.
Owner:内蒙古源宏精细化工有限公司
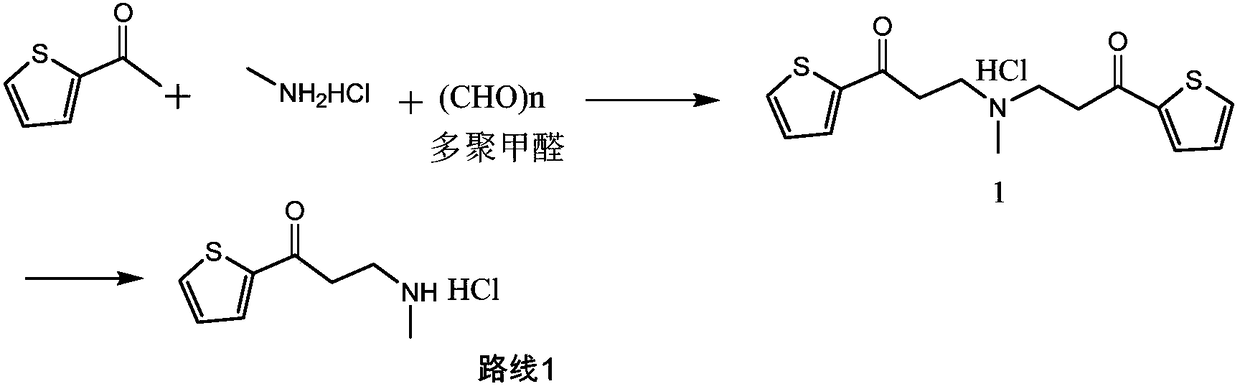
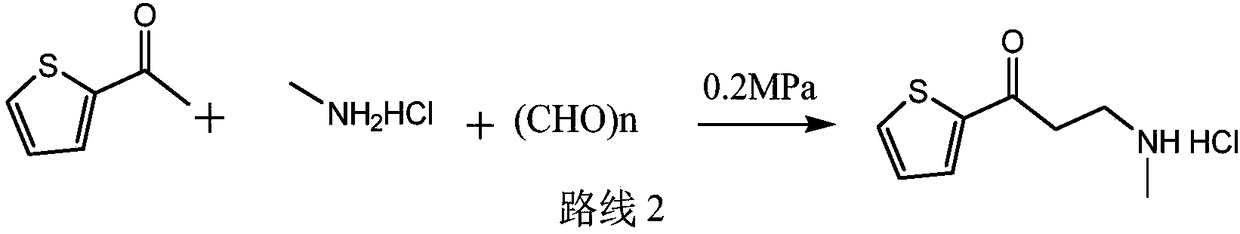
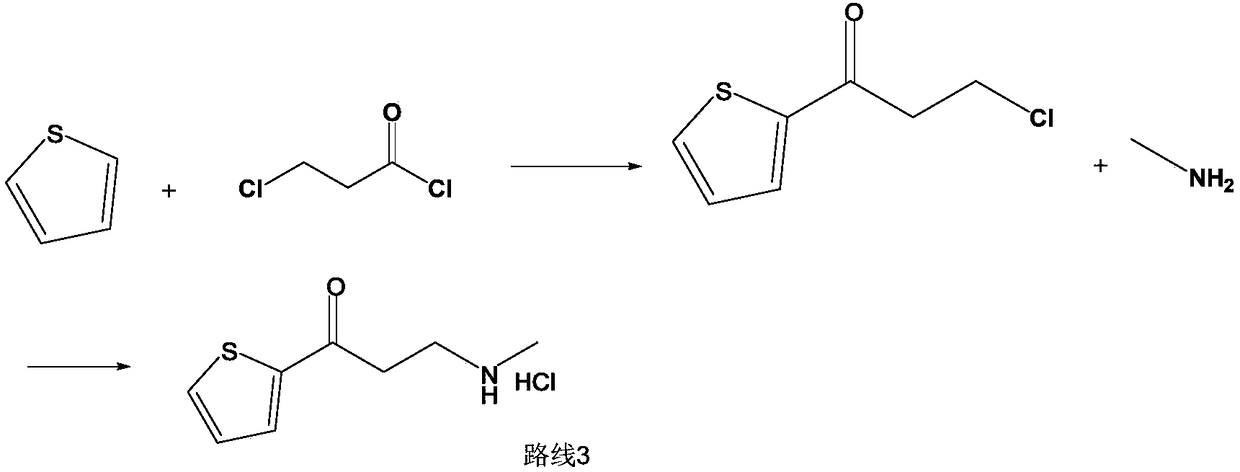
Synthetic method of 3-methylamino-1-(2-thienyl)-1-acetone hydrochloride
ActiveCN109134427AReduce pressure on environmental protectionNo irritating smellOrganic chemistryEnvironmental resistanceHigh pressure
The invention discloses a synthetic method of a compound 3-methylamino-1-(2-thienyl)-1-acetone hydrochloride, namely an intermediate of duloxetine hydrochloride, and relates to the field of drug synthesis. According to the method, a compound II, a compound III and a compound IV are taken as raw materials, and the compound I is obtained through the effect of a catalyst in a polar solvent. The usedcatalyst is one or more of silver trifluoromethanesulfonate and indium chloride. The maximum improvement characteristic of the synthetic method lies in that the product can be obtained at high yield without the conditions of high pressure and high temperature. Compared with the prior art, the raw materials used in the synthetic method are relatively low in environmental protection pressure and free of pungent smell; and moreover, the synthetic method is simple in technology, relatively low in requirement for equipment, simple and convenient in aftertreatment and high in yield.
Owner:ZHEJIANG LEPU PHARMA CO LTD
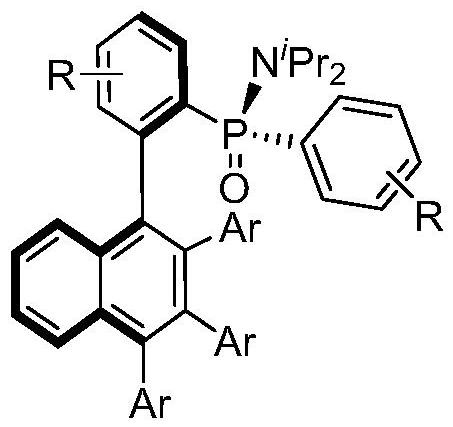
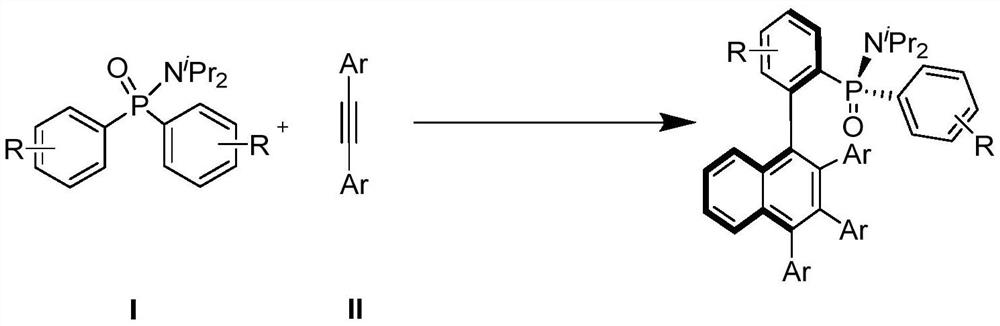
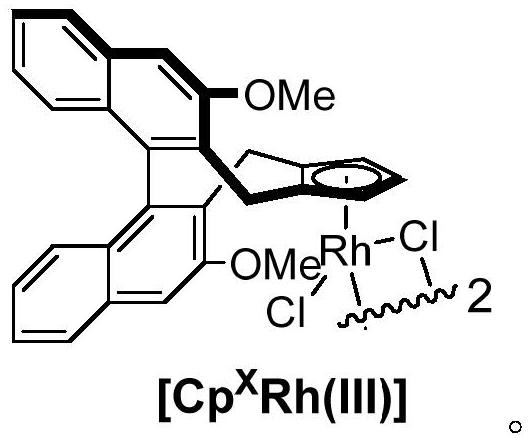
Axially chiral biaryl compound with P-stereo center and synthesis method and application thereof
InactiveCN113292598AEasy to manufactureHigh catalytic activityOrganic compound preparationGroup 5/15 element organic compoundsBoronic acidEthylic acid
The invention discloses an axially chiral biaryl compound with a P-stereo center and a synthesis method and application thereof. The structural formula of the compound is shown in the specification, chiral trivalent rhodium [CpXRh(III)] is used as a catalyst, diaryl phosphonamide and diaryl acetylene are used as raw materials, and enantioselective coupling is conducted under the assistance of silver trifluoromethanesulfonate or silver hexafluoroantimonate and silver acetate to obtain the compound. Diarylacetylene is taken as an initial raw material, the compound is stable in property and easy to prepare, but the compound is seldom applied to arylation reaction. In the prior art, aryl arylation is mainly carried out by using brominated aromatic hydrocarbon, arylboronic acid and the like. The simple diarylacetylene is adopted as an arylation reagent, the axially chiral biaryl and the P-center chiral compound are stereoscopically and specifically synthesized through double activation of C-H bonds in the aryl phosphonamide and the diarylacetylene, and the method has the advantages of being mild in reaction condition, high in enantioselectivity, good in diastereoselectivity and the like.
Owner:SHAANXI NORMAL UNIV
Synthetic method of nitrogen-containing spiro compound
ActiveCN107586298AImprove conversion efficiencyGood atom economyOrganic chemistryVacuum pumpingOrganic solvent
The invention discloses a synthetic method of a nitrogen-containing spiro compound. The method comprises adding an imine derivative, a maleimide compound, diiodide pentamethyl cyclopentadiene cobalt carbonyl, silver trifluoromethanesulfonate and nitrobenzene in an organic solvent, performing heating for a reaction in a vacuum-pumping condition, and after the reaction is completed, performing post-treatment to obtain the nitrogen-containing spiro compound. According to the method, simply-available raw materials are adopted to synthesize the nitrogen-containing spiro compound in one step. The method is high in conversion efficiency and good in atom economy. The synthetic method is simple to operate, high in reaction yield and wide in substrate adaptability.
Owner:ZHEJIANG UNIV
6-(alpha-cyanoimine) based phenidine compound and synthesis method thereof
InactiveCN109438349AEasy to operateRaw materials are easy to obtainSilicon organic compoundsSynthesis methodsOrganic synthesis
The invention relates to an alpha-cyanoimine substituted phenidine compound. A structural formula of the compound is shown as follows. The compound is an organic synthesis intermediate of great value,a phenidine framework already shows diverse bioactivity, so that the compound can be converted into corresponding other active compounds. The method is simple and easy-to-get in raw material, t-butylisonitrile is adopted as a cyano source of reaction, and high reaction activity is presented under catalysis of silver trifluoromethanesulfonate; the reaction process is simple in operation, mild in condition, environment-friendly and common to moderate in yield, therefore, the compound has great development prospect in industrial production.
Owner:SHANGHAI UNIV
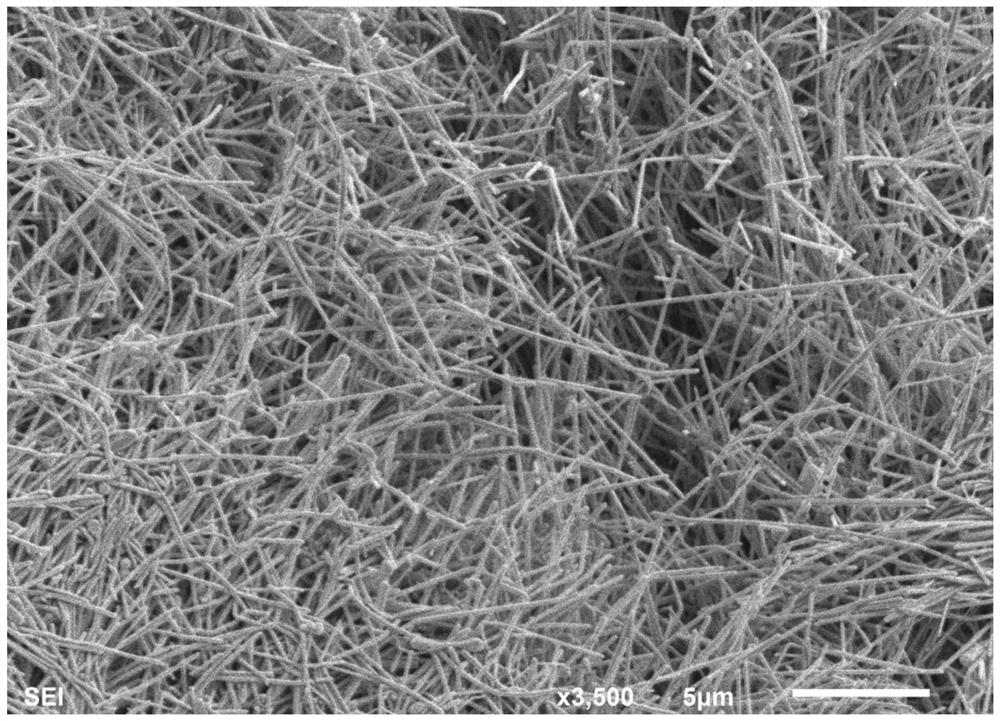
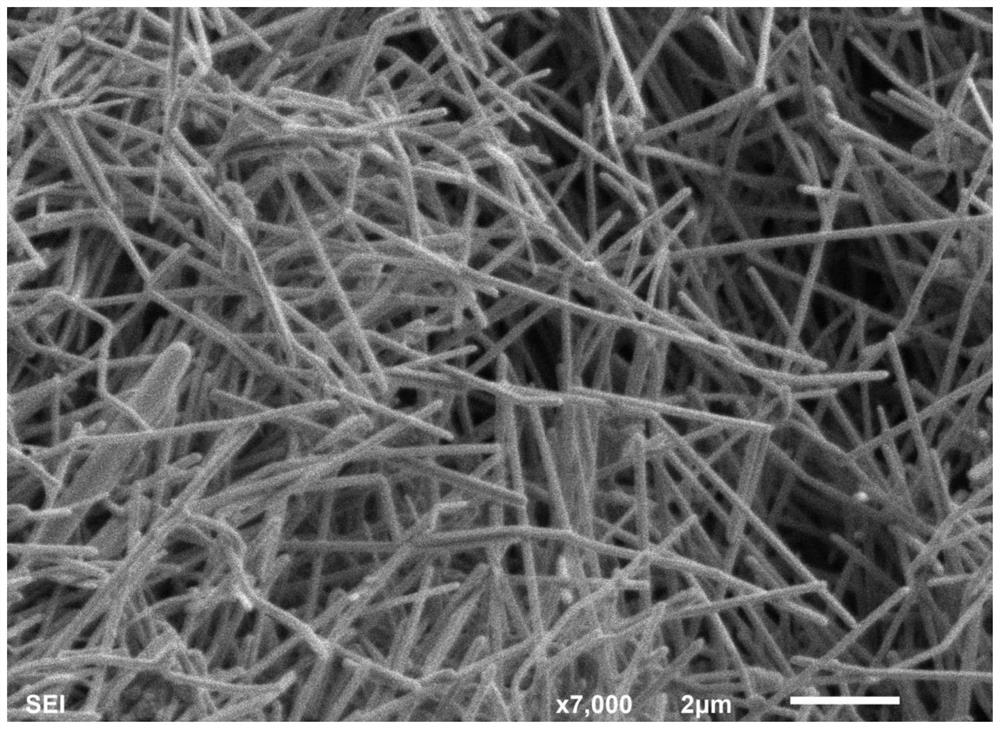
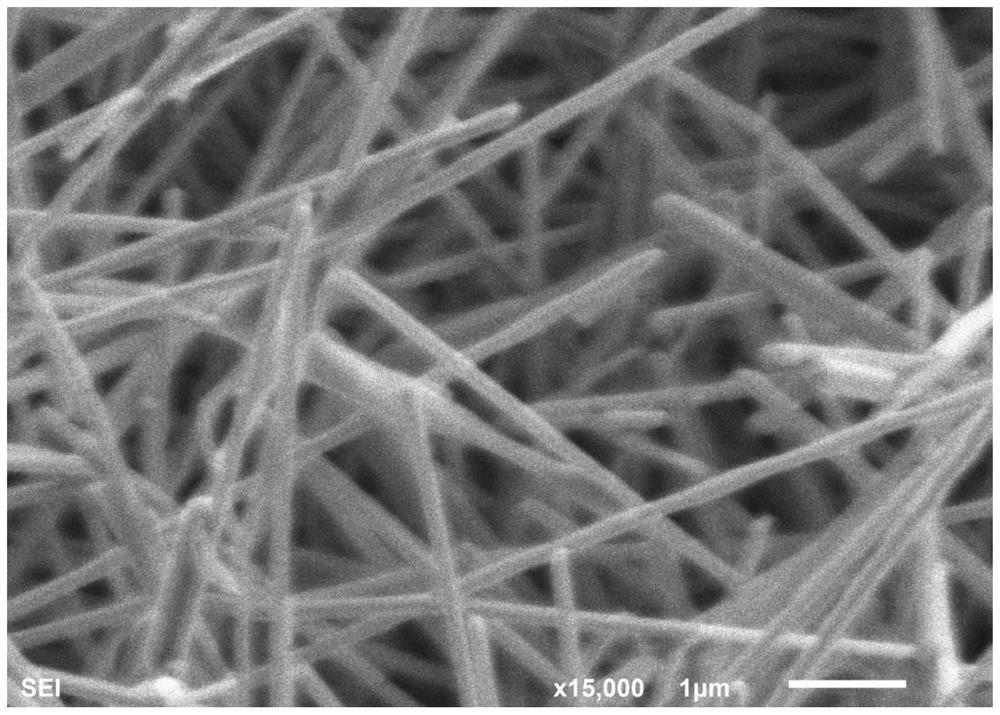
Silver nanowire and preparation method and application thereof
ActiveCN110722174ALarge aspect ratioImprove bending resistanceMaterial nanotechnologyTransportation and packagingSolventSeed crystal
The invention relates to a silver nanowire and a preparation method and application thereof. The preparation method of the silver nanowire comprises the following steps that silver salt is dissolved in a first solvent, a first mixture is obtained, the silver salt is selected from at least one of silver trifluoromethanesulfonate, silver diethyl dithiocarbamate and succinimide silver salt, and the first solvent is water; a seed crystal, a reducing agent and a dispersing agent are dissolved in a second solvent at the temperature of 48-72 DEG C, a second mixture is obtained, the seed crystal is selected from at least one of tri-silver citrate or silver tetrafluoroborate, and the second solvent is water; and the first mixture and the second mixture are mixed in a dropwise adding manner, the pHis adjusted to 8-8.5, solid-liquid separation is carried out, and the silver nanowire is obtained. By means of the preparation method, the silver nanowire with the good bending resistance and the longlength-diameter ratio can be obtained.
Owner:GUANGDONG OPPO MOBILE TELECOMM CORP LTD
Isosteviol metal gel and preparation method and application thereof
ActiveCN110681875AAchieve in situ gelationEasy to prepareMaterial nanotechnologyTransportation and packagingAlkaneNatural product
The invention discloses an isosteviol sulfoacid silver supramolecular gel and a preparation method and an application thereof in preparation of nano silver, and belongs to the field of functional materials. The gel is prepared by esterification, sulfonation and salt-forming of the natural product isosteviol and is provided with a silver trifluoromethanesulfonate structural segment. The structureis shown in Formula 1, wherein R is an alkane group and an aromatic hydrocarbon group. The gel has good gel properties, the lowest gel concentration is as low as 0.2%w / v, and the nano silver can beprepared in situ through the green and convenient photoluminescence reduction process. The gel system has the advantages of a simple preparation method, low cost, and recoverable and reusable solvent.The prepared nano silver particle size is uniform and the method is simple, so that a convenient template for the preparation of the nano silver is provided. Please see the Formula 1 in the description.
Owner:XINXIANG MEDICAL UNIV
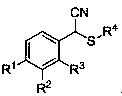
Alpha-thioether aryl acetonitrile compound and synthesis method thereof
The invention relates to a synthesis method of an alpha-thioether aryl acetonitrile compound. A structural formula of the compound is as shown in the specification, wherein R1 = hydrogen, methyl, isopropyl, phenyl, fluorine or bromine; R2 = hydrogen, methyl or chlorine; R3 = hydrogen, methyl or bromine; and R4 = tert-butyl or 1-adamantyl. According to the method, raw materials are simple and easy to obtain, t-butyl isocyanide is used as a cyano source of a reaction, and relatively high reactivity is exhibited under catalysis of silver trifluoromethanesulfonate. In a reaction process, the operation is simple, the condition is mild, the environment is friendly, and the yield is general to moderate. New compounds can be synthesized by applying the method, and the new compounds have potential medicinal values, so that the method has good application prospects in industrial production.
Owner:SHANGHAI UNIV
Iridium complex electroluminescent material, preparation method thereof and electroluminescent device thereof
ActiveCN111116671AImprove luminous efficiencyIndium organic compoundsSolid-state devicesOrganic electroluminescenceOrganic matter
The invention discloses an iridium complex electroluminescent material, and the structural formula is shown in the specification. A preparation method comprises the following steps: under the protection of nitrogen, carrying out a heating reaction on a compound A and iridium trichloride trihydrate to generate B, carrying out a reaction on the B and silver trifluoromethanesulfonate to generate C, and finally, carrying out a reaction on the C and a compound D to obtain the iridium complex electroluminescent material. The invention also discloses an organic electroluminescent device containing the iridium complex electroluminescent material, the organic electroluminescent device comprises a first electrode, a second electrode and one or more organic matter layers arranged between the first electrode and the second electrode, and the organic matter layers contain the iridium complex electroluminescent material. According to the iridium complex electroluminescent material, a specific heterocyclic ligand is selected for combination, the wavelength of the compound is adjusted, and after the obtained iridium complex electroluminescent material is used for an organic electroluminescent device, the luminous efficiency of the device is improved, and the service life is prolonged.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Silver nanowires and their preparation methods and applications
ActiveCN110722174BFlexibleBendableMaterial nanotechnologyTransportation and packagingSolventSeed crystal
Owner:GUANGDONG OPPO MOBILE TELECOMM CORP LTD
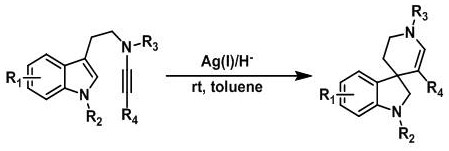
A kind of synthetic method of hydrogenated pyridine spiroindoline ring catalyzed by monovalent silver
The present invention provides a kind of synthetic method that under the action of monovalent silver and reducing agent, the tryptamine alkyne amide substrate containing indole can generate hydrogenated pyridine spiroindoline ring at room temperature, the general reaction formula is as follows , where R 1 , R 2 , R 3 , R 4 As described in the claims and description. The silver catalyst required for the reaction is one of silver trifluoromethanesulfonate, silver hexafluoroantimonate, silver tetrafluoroborate, and silver bistrifluoromethanesulfonimide. The reducing agent required for the reaction is Hans ester. The medium required for the reaction is: toluene. The implementation of the reaction is stirring at room temperature. The method of the invention has easy-to-obtain raw materials, simple operation, wide application range, good atom economy, green reaction and low price.
Owner:SHENYANG PHARMA UNIVERSITY
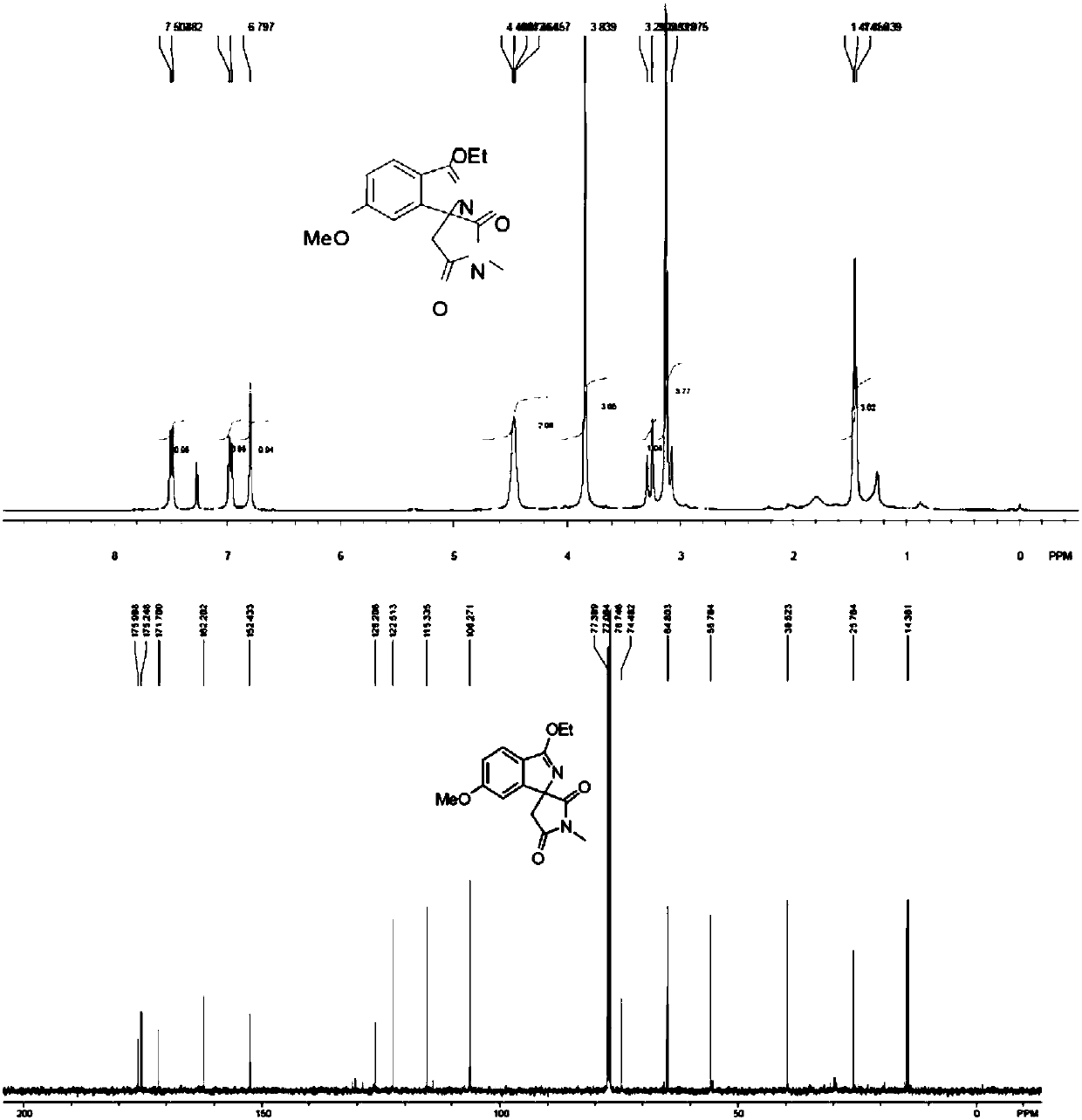
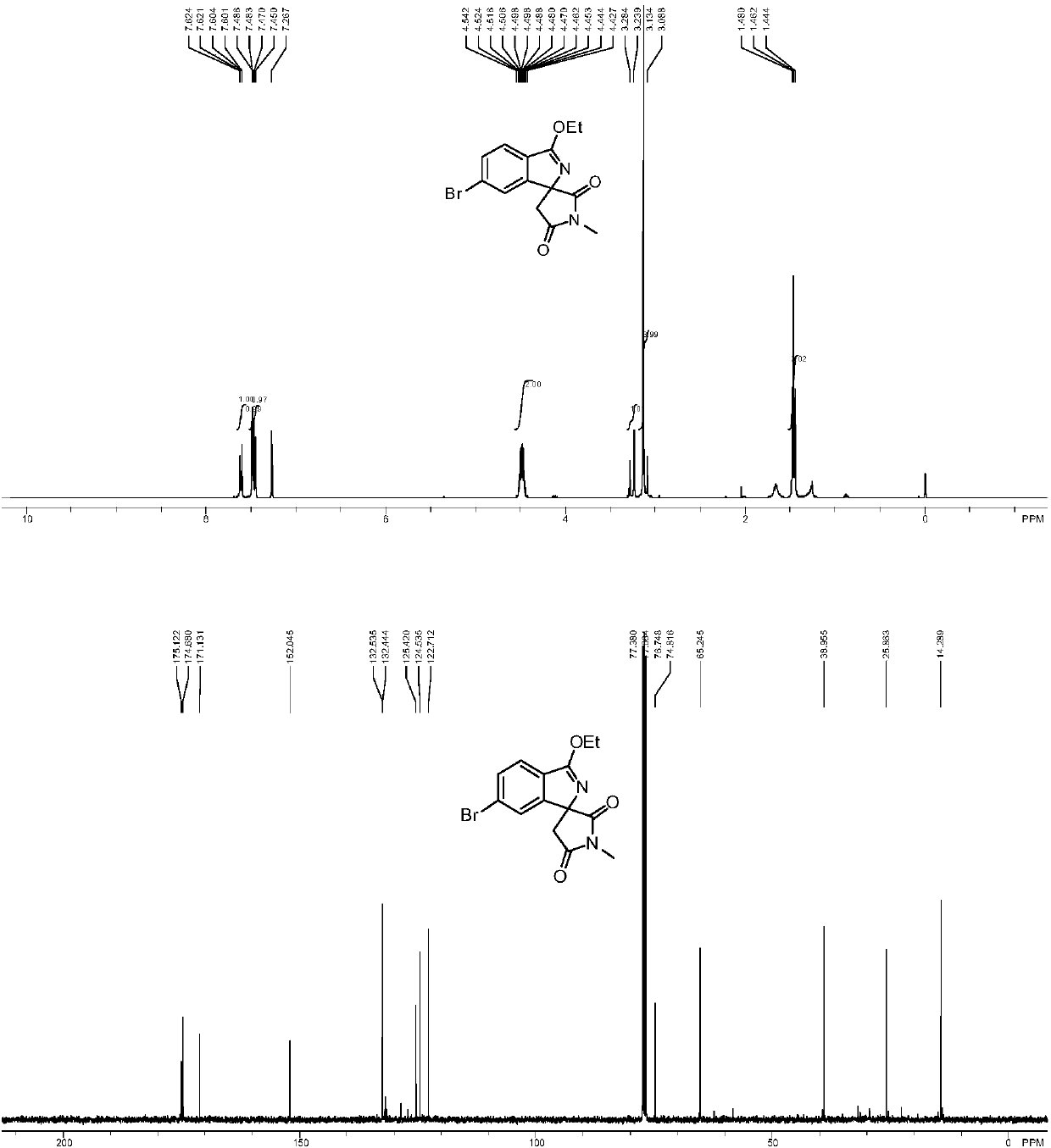
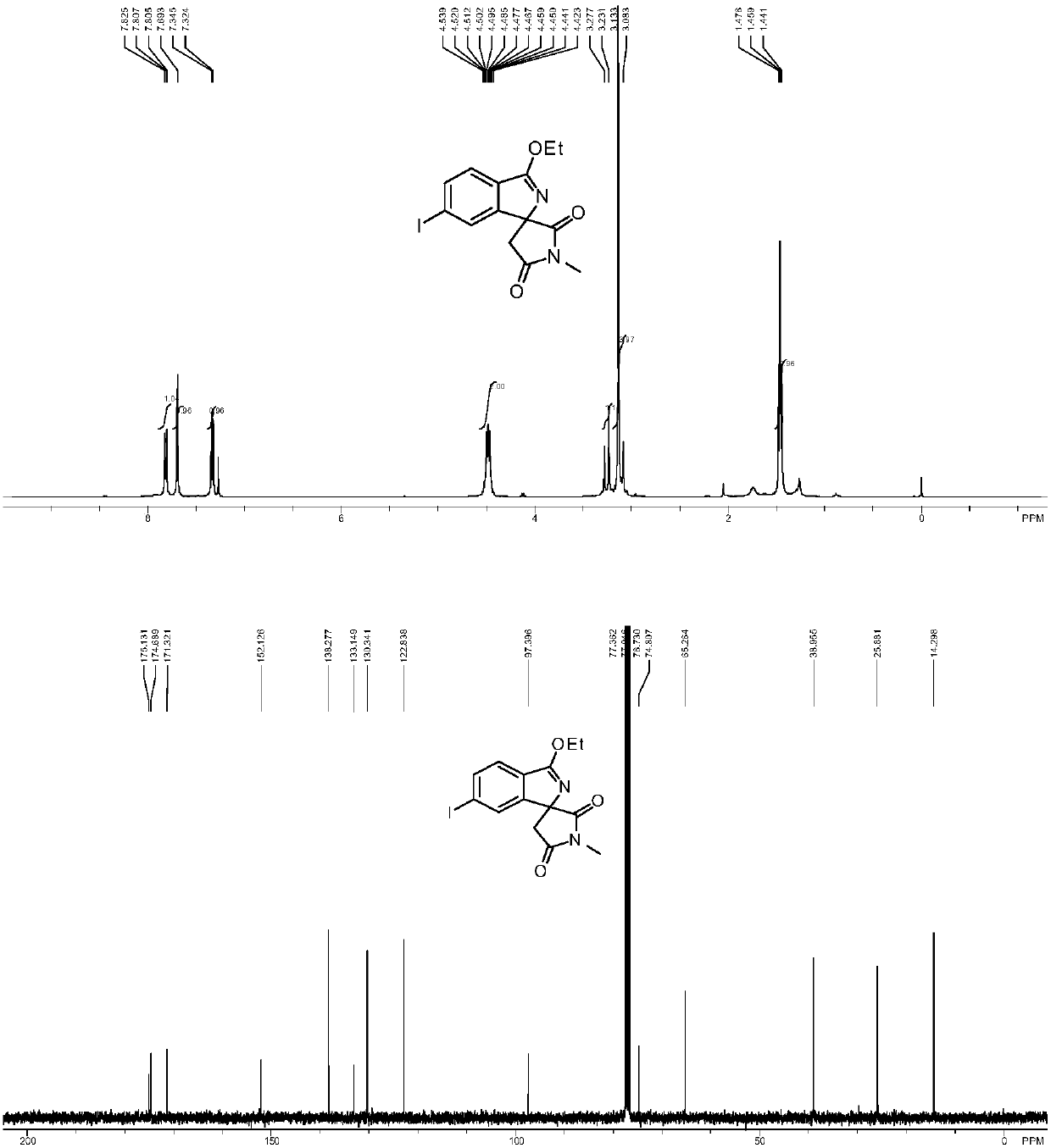
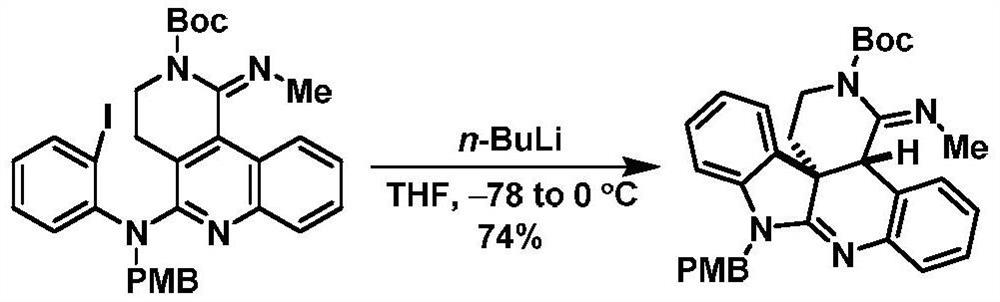
A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives
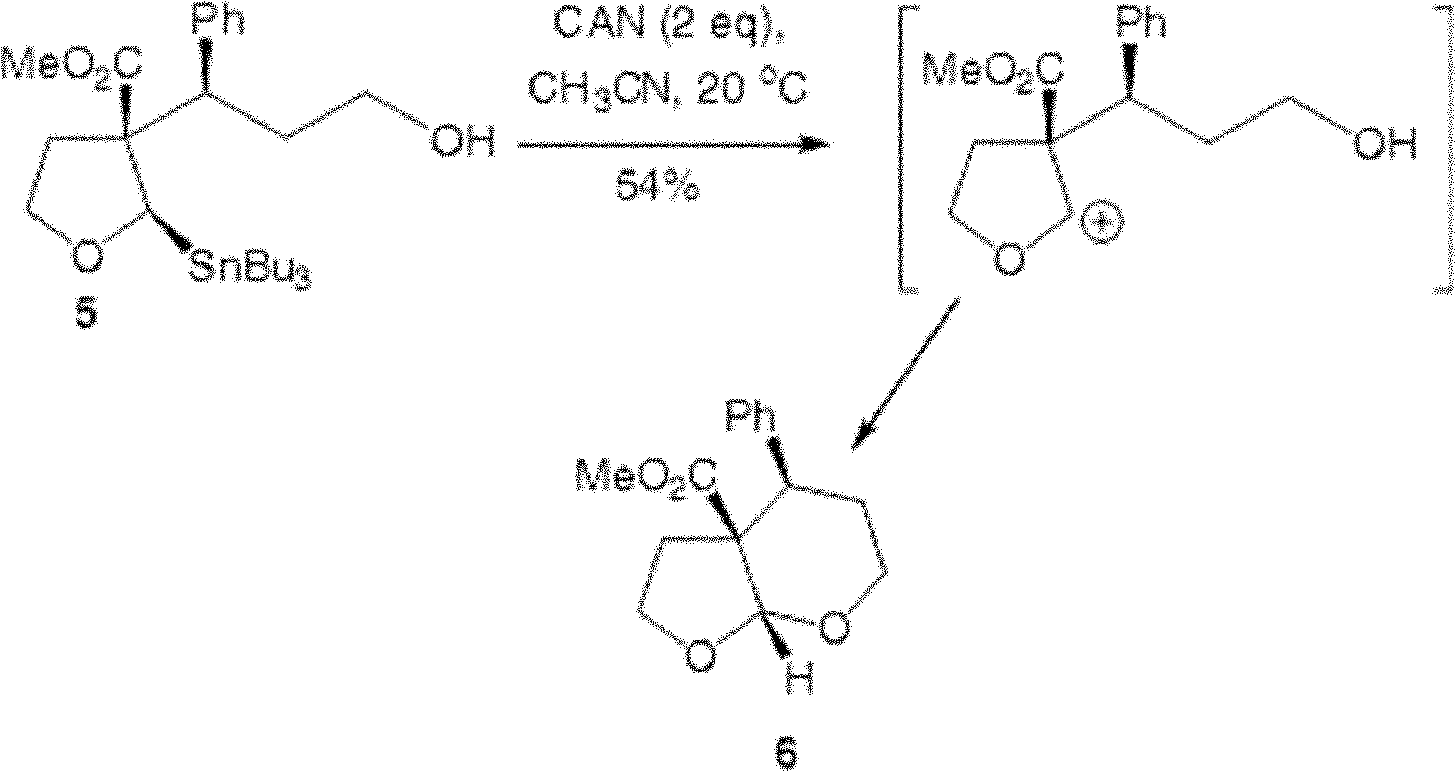
The invention provides a preparation method of a cyclopentyl[f]pyrrolo[2,1,5-cd]indolizine derivative and belongs to the technical field of organic synthesis chemistry, aiming at solving the problemsthat a molecular structure and a spatial configuration of a pyrrolo[2,1,5-cd]indolizine derivative are complicated so that the cyclopentyl[f]pyrrolo[2,1,5-cd]indolizine derivative is difficult to prepare. The cyclopentyl[f]pyrrolo[2,1,5-cd]indolizine derivative is prepared by taking the pyrrolo[2,1,5-cd]indolizine derivative containing iodine atoms and chlorine atoms as a raw material, convertingthe pyrrolo[2,1,5-cd]indolizine derivative into an ether derivative and further treating under a catalysis effect of ruthenium trichloride and silver trifluoromethanesulfonate. According to the preparation method provided by the invention, the cyclopentyl[f]pyrrolo[2,1,5-cd]indolizine derivative is efficiently and stereoselectively through two-step reaction; the reaction yield is high and no byproducts are generated.
Owner:TAIYUAN UNIV OF TECH
A method for efficiently synthesizing quinoline derivatives
The invention provides a method for efficiently synthesizing quinoline. The quinoline is synthesized by phenylamine and olefin ketone with substituents catalyzed by silver trifluoromethanesulfonate, as well as an olefine aldehyde derivative. The method is simple to operate, is applicable to many functional groups, has high yield and a single product, facilitates separation and purification, and is safe, cheap and low in pollution.
Owner:NANYANG NORMAL UNIV
Application of palladium source catalyst in alkyne polymerization
The invention relates to an application of a palladium source catalyst in catalyzing alkyne polymerization, and belongs to the field of alkyne polymerization. The application is the application of thepalladium source catalyst in catalysis of a polymerization reaction for alkyne polymerization as a catalyst, and catalysis of disubstituted alkyne polymerization as a catalyst. The catalyst is different from known palladium catalysts, the palladium source can be directly purchased, polymerization of disubstituted alkyne can be catalyzed without coordination with various ligands, in the polymerization process, no cocatalyst such as silver trifluoromethanesulfonate (AgOTf) and sodium tetrakis(3,5-bis(trifluoromethyl)phenyl) borate (NaBAF) needs to be added, a polymer with high molecular weightcan be obtained, and cis-selective poly(1-chloro-2-phenylacetylene) (PCPAs) with high molecular weight and narrow molecular weight distribution is obtained. The method solves the problems of complex synthesis of a disubstituted alkyne polymerization catalyst, participation of the cocatalyst in the polymerization process, high cost and the like, is simpler, more economical and more efficient, and provides a new idea for polymerization of disubstituted alkyne.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Method for stereoselective preparation of derivatives of pyrane
InactiveCN102180923BReduce pollutionEasy to operateBiocideSugar derivativesReaction temperaturePyran
The invention belongs to the technical field of organic chemistry and pharmaceutical chemistry, and in particular relates to a method for stereoselective preparation of derivatives of pyrane. The method comprises the following steps of: under the protection of inert gases, adding derivatives of 1-p-tolylthio-2-C-glyoxyl-alpha-D-glucopyranose, derivatives of 1-thiophenyl-2-C- glyoxyl-alpha-D-glucopyranose or derivatives of 1-ethylthio p-tolyl-2-C-glyoxyl-alpha-D-glucopyranose, which serve as reactants, into a reactor, adding alcohol, phenol, trimethylsilyl azide or monosaccharide derivatives, adding a solvent such as methylene chloride to dissolve the raw materials, and performing the reaction at the reaction temperature of between 40 DEG C below zero and room temperature in the presence of a catalyst which is N-iodosuccinimide, or N-bromosuccinimide, or copper bromide and tetrabutylammonium bromide, or iodine, bromine simple substance or N-iodosuccinimide and trimethylsilyl triflate, or trifluoromethanesulfonic silver, or paratoluenesulfonic acid or trifluoromethanesulfonic acid to obtain the derivatives of pyrane. The method has the advantages of simple operation, high selectivity, low cost, light environmental pollution and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method](https://images-eureka.patsnap.com/patent_img/164985d5-8176-43e7-989e-b8f2afc13456/BDA0000065013020000011.PNG)
![Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method](https://images-eureka.patsnap.com/patent_img/164985d5-8176-43e7-989e-b8f2afc13456/BDA0000065013020000021.PNG)
![Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method Novel electrically neutral tridentate iridium [iii] complex red light material and preparation method](https://images-eureka.patsnap.com/patent_img/164985d5-8176-43e7-989e-b8f2afc13456/FDA0000065013010000011.PNG)
![Method for preparing halogenated benzo [alfa] fluorenol Method for preparing halogenated benzo [alfa] fluorenol](https://images-eureka.patsnap.com/patent_img/730ecbfb-2118-4186-9b35-a5d7820d2da6/HSA00000715058900011.PNG)
![Method for preparing halogenated benzo [alfa] fluorenol Method for preparing halogenated benzo [alfa] fluorenol](https://images-eureka.patsnap.com/patent_img/730ecbfb-2118-4186-9b35-a5d7820d2da6/HSA00000715058900012.PNG)
![Method for preparing halogenated benzo [alfa] fluorenol Method for preparing halogenated benzo [alfa] fluorenol](https://images-eureka.patsnap.com/patent_img/730ecbfb-2118-4186-9b35-a5d7820d2da6/BSA00000715058800041.PNG)
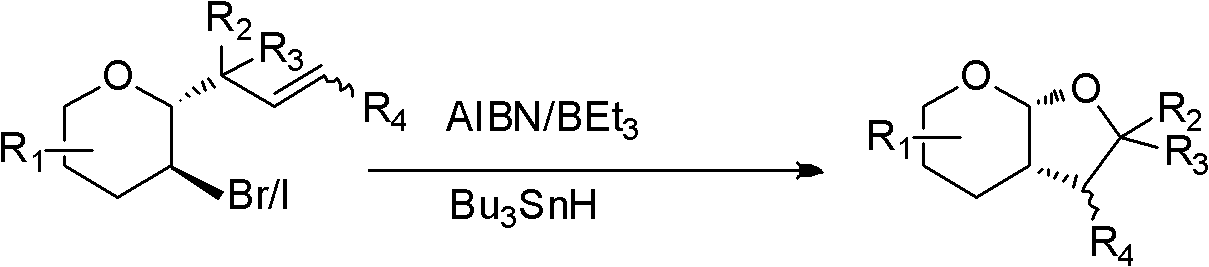
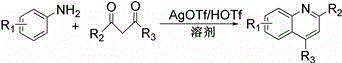
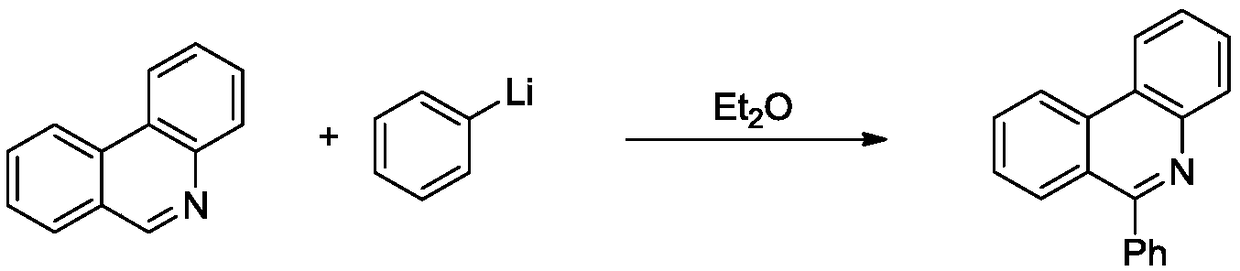
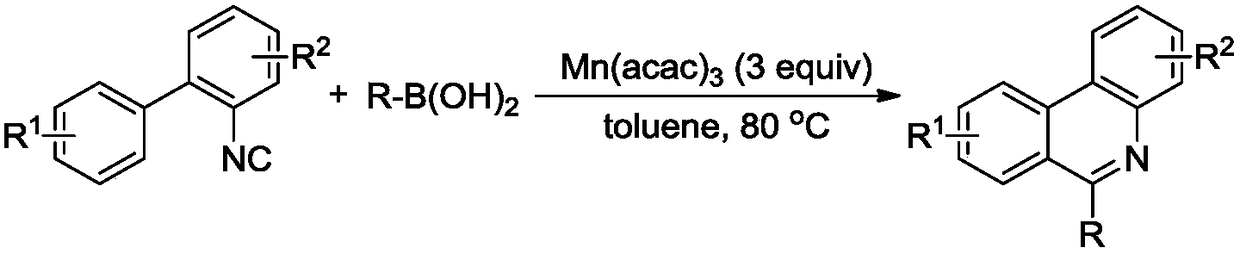


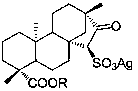
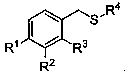
![A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives](https://images-eureka.patsnap.com/patent_img/0f71161c-d4e6-4c00-8065-f7e3d3fb035b/171219093659.png)
![A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives](https://images-eureka.patsnap.com/patent_img/0f71161c-d4e6-4c00-8065-f7e3d3fb035b/DEST_PATH_180316135320.png)
![A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives A preparation method of cyclopentyl[f]pyrrolo[2,1,5-cd]indazine derivatives](https://images-eureka.patsnap.com/patent_img/0f71161c-d4e6-4c00-8065-f7e3d3fb035b/DEST_PATH_180316135324.png)