Synthetic method of nitrogen-containing spiro compound
A synthesis method and compound technology are applied in the synthesis field of nitrogen-containing spirocyclic compounds, which can solve problems such as reaction conditions of multiple reaction steps, and achieve the effects of wide substrate adaptability, simple experimental conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~15
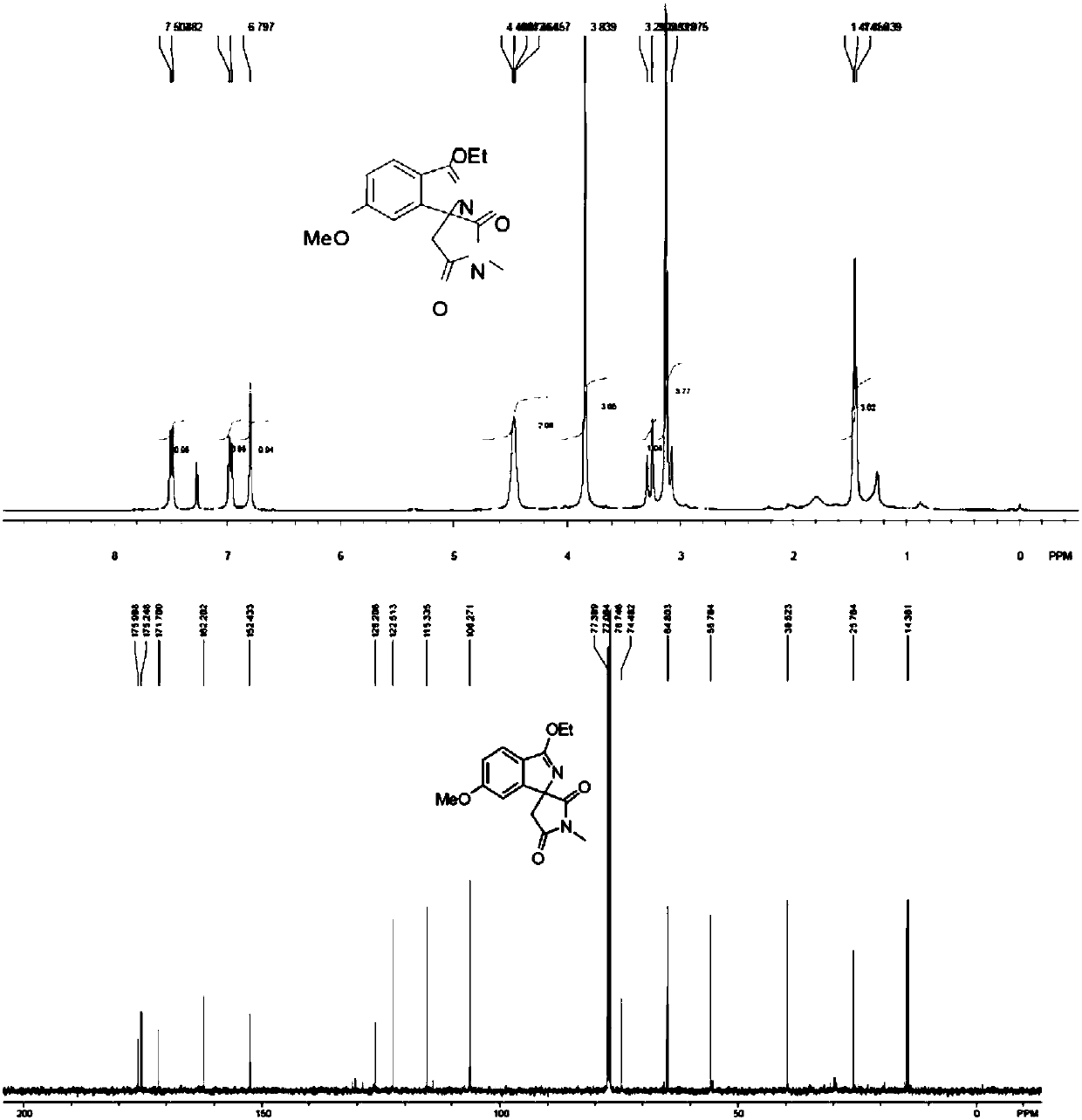
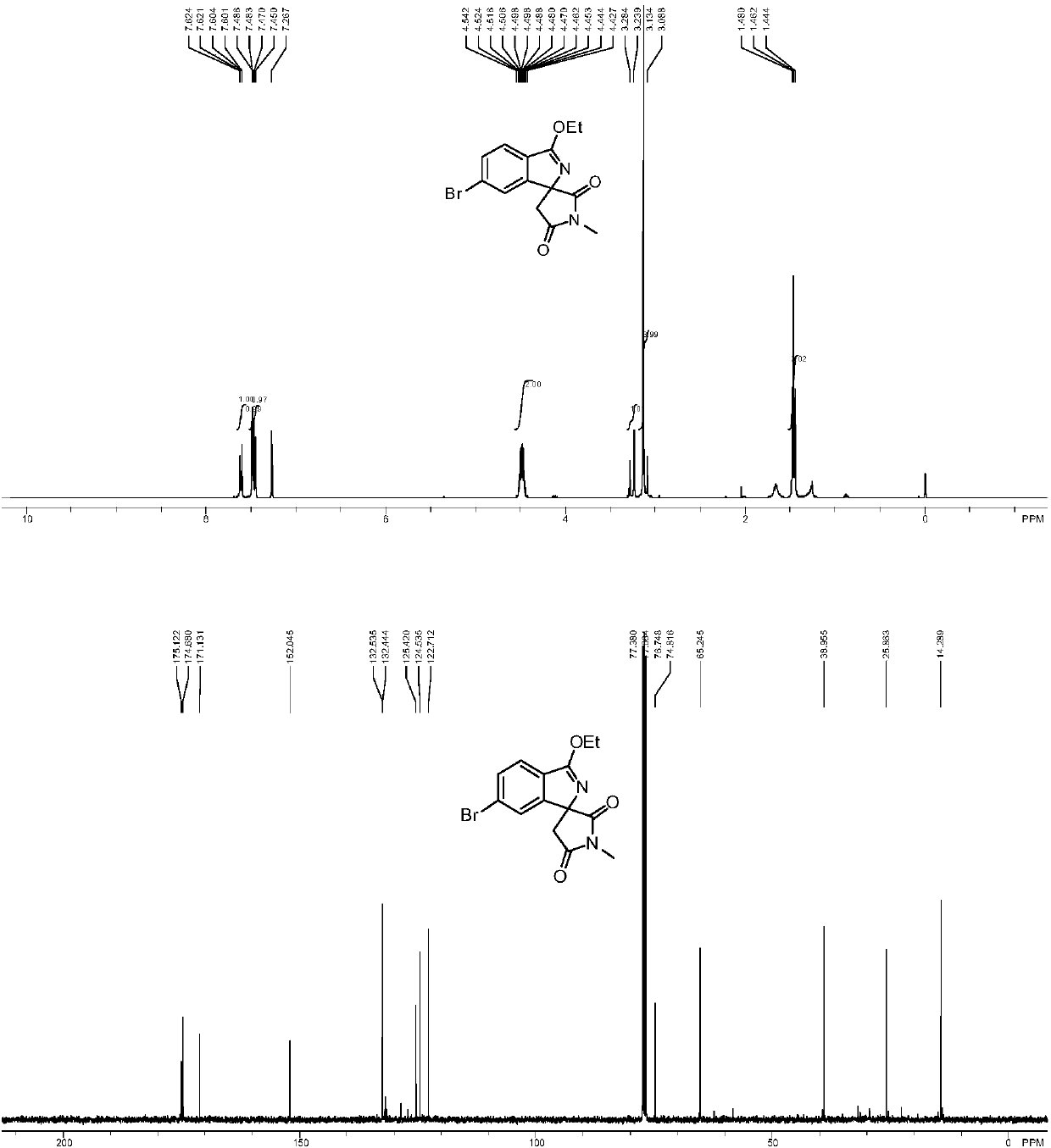
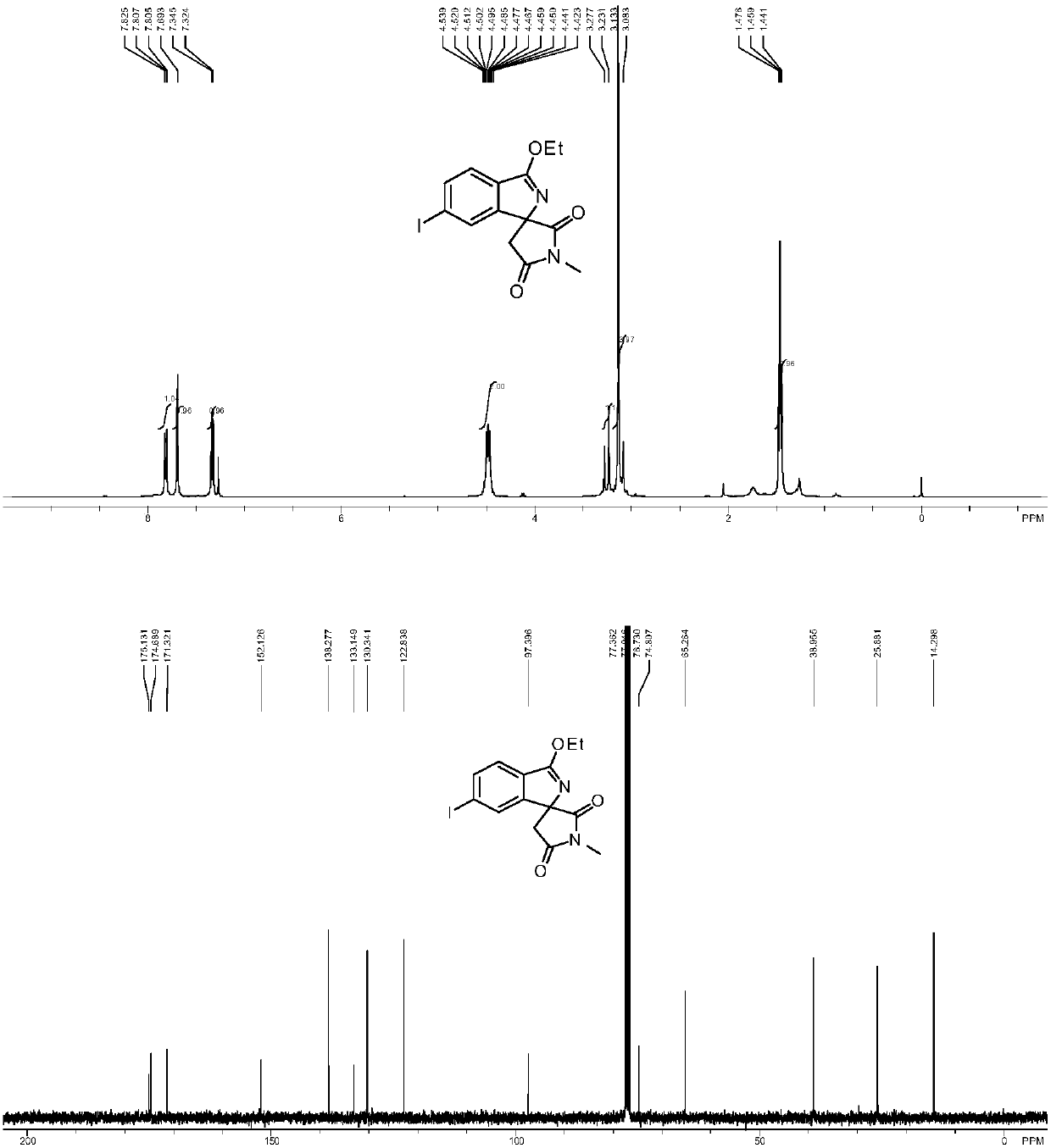
[0040] Add imine (0.3mmol), maleimide compound (0.2mmol), diiodide pentamethylcyclopentadiene-cobalt carbonyl (0.02mmol), trifluoro Silver methanesulfonate (0.04mmol), nitrobenzene (0.2mmol) and 1mL of organic solvent were mixed and stirred evenly. After the reaction was completed according to the reaction conditions in Table 2, it was cooled, filtered with suction, mixed with silica gel, and purified by column chromatography to obtain Corresponding nitrogen-containing spiro compound (I), the reaction process is shown in the following formula:
[0041]
[0042] The raw material proportioning of table 1 embodiment 1~15
[0043]
[0044] The reaction condition and reaction result of table 2 embodiment 1~15
[0045]
[0046]
[0047] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, 2-naphthyl is 2-substituted naphthyl, CF 3 is trifluoromethyl, Ph is phenyl, and 2-thiophene is 2-substituted thiophene.
[0048] a with AgSbF 6 Instead o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com