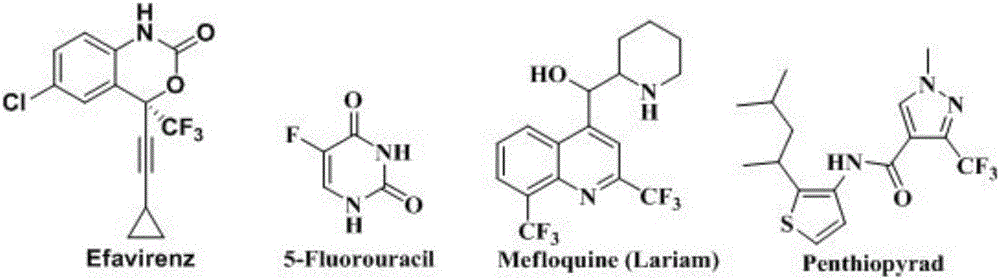
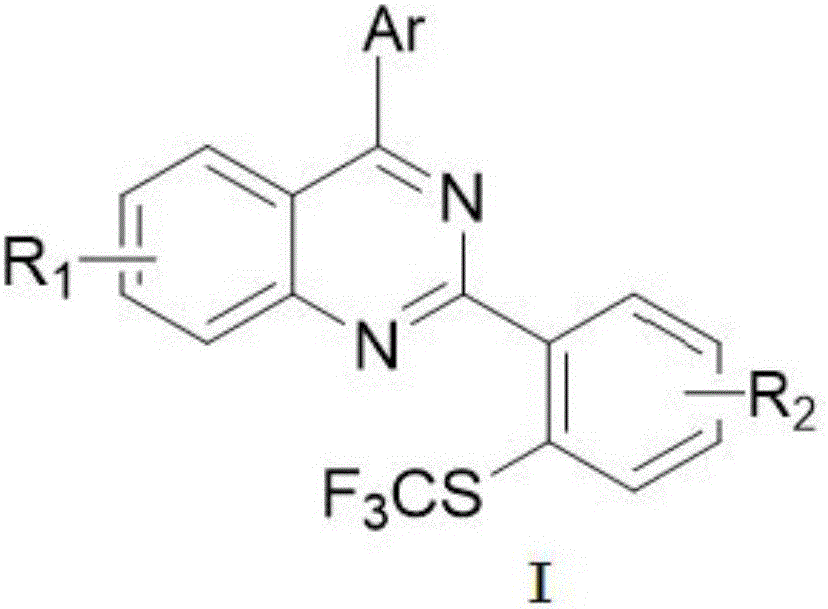
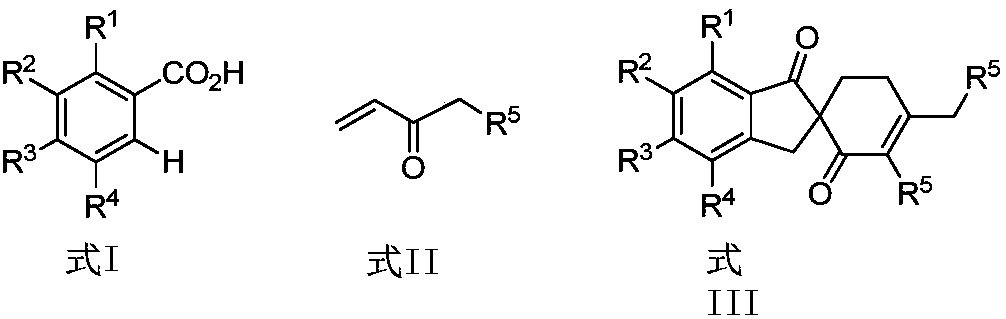
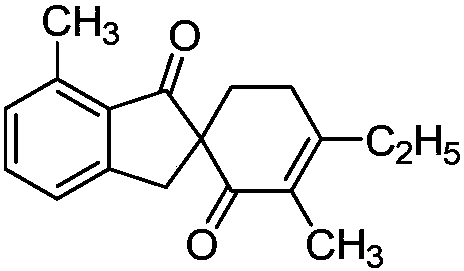
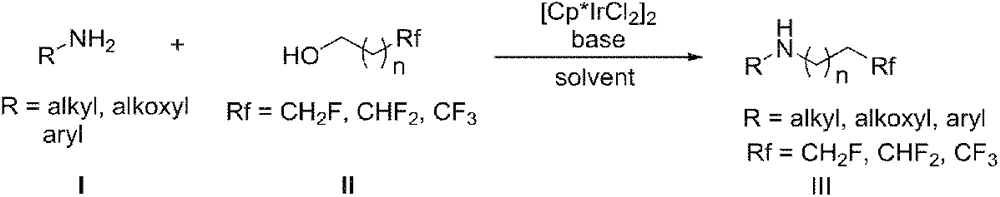
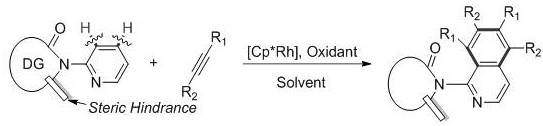
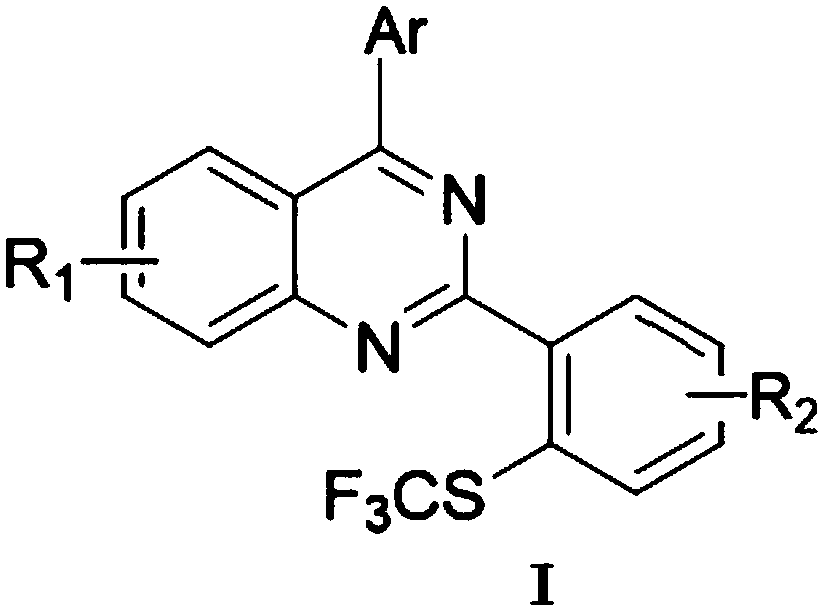
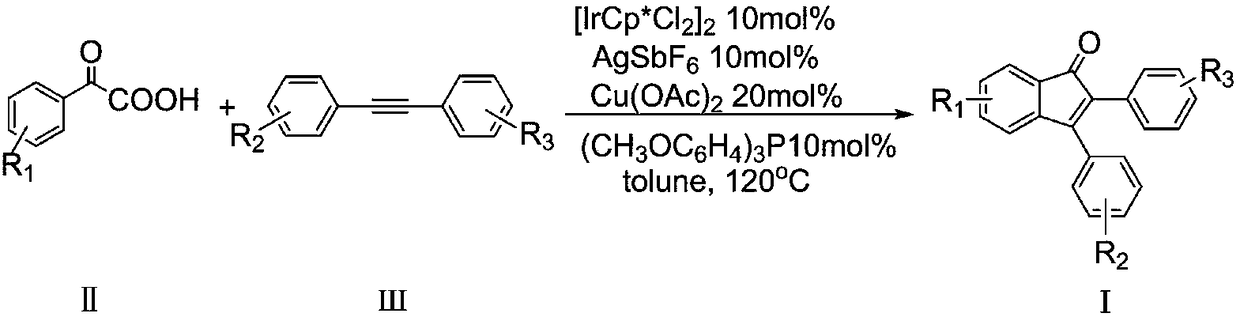
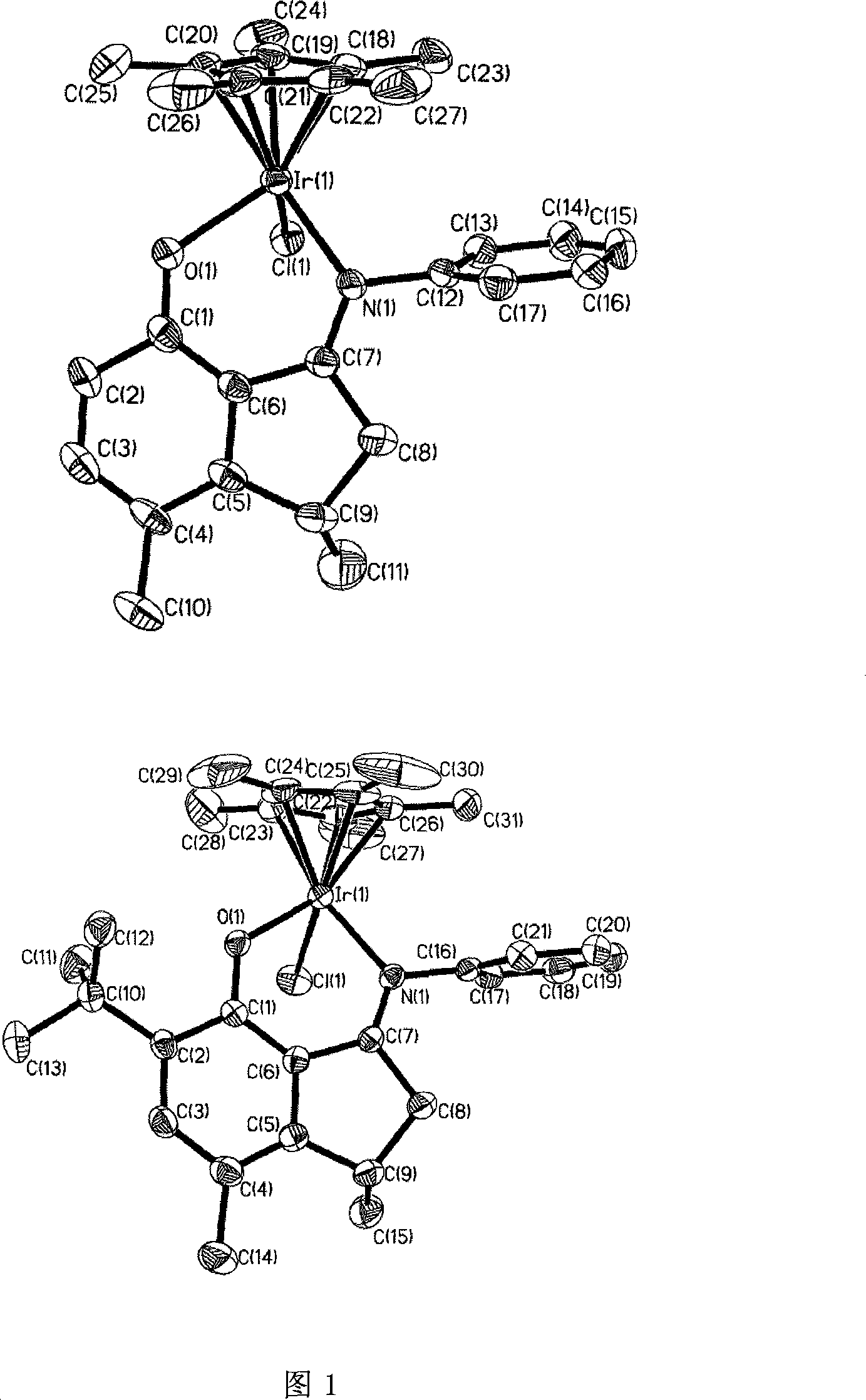
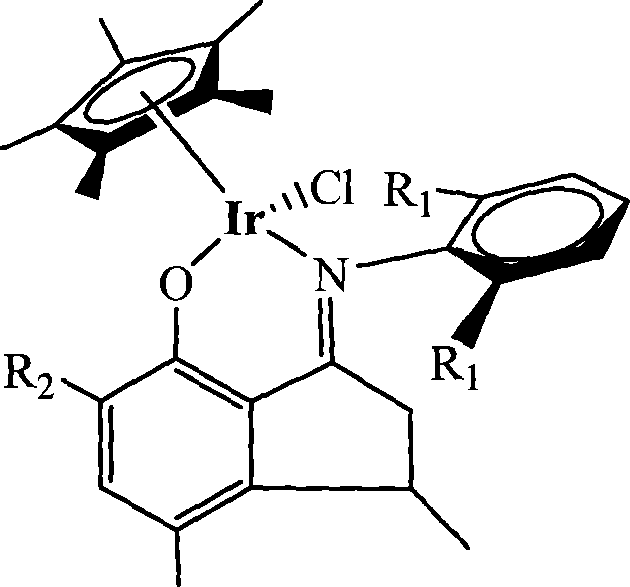
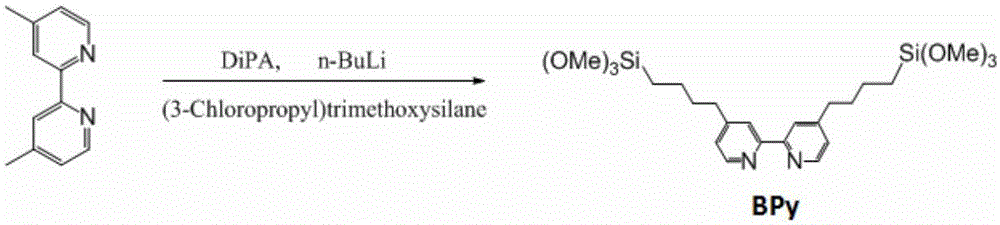
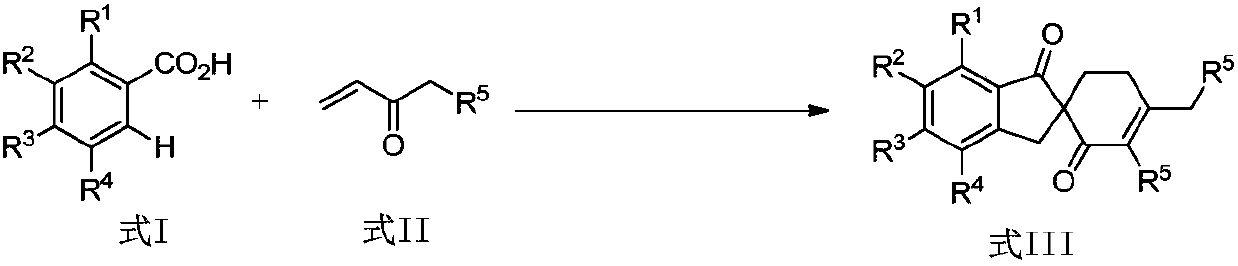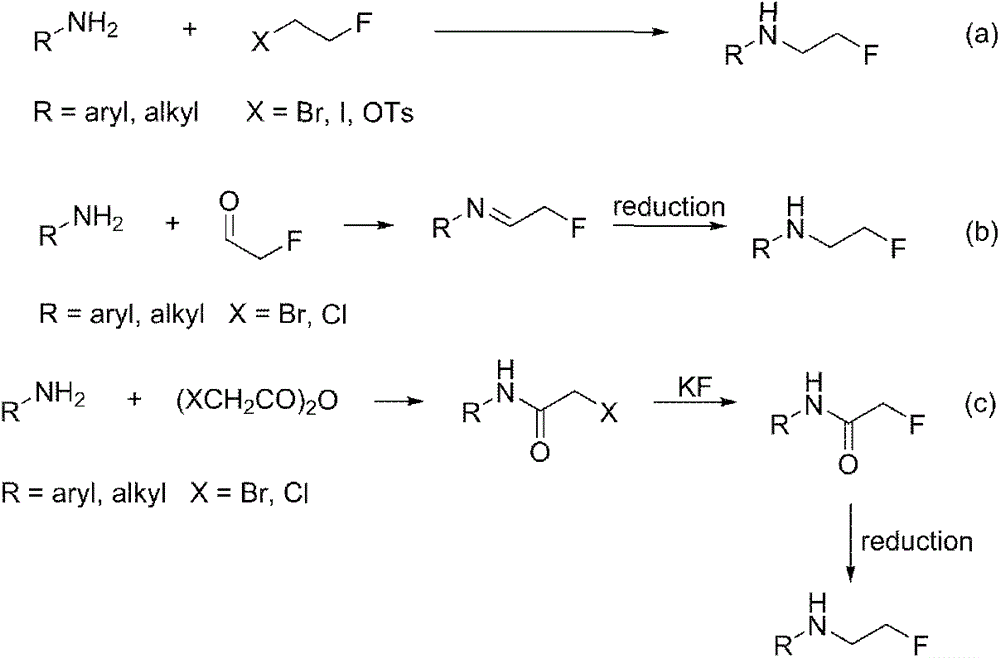Patents
Literature
111 results about "Pentamethylcyclopentadiene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic dialkene with the formula C₅Me₅H (Me = CH₃). 1,2,3,4,5-Pentamethylcyclopentadiene is the precursor to the ligand 1,2,3,4,5-pentamethylcyclopentadienyl, which is often denoted Cp* (to signify the five methyl groups radiating from the periphery of this ligand as in a five-pointed star). In contrast to less-substituted cyclopentadiene derivatives, Cp*H is not prone to dimerization.
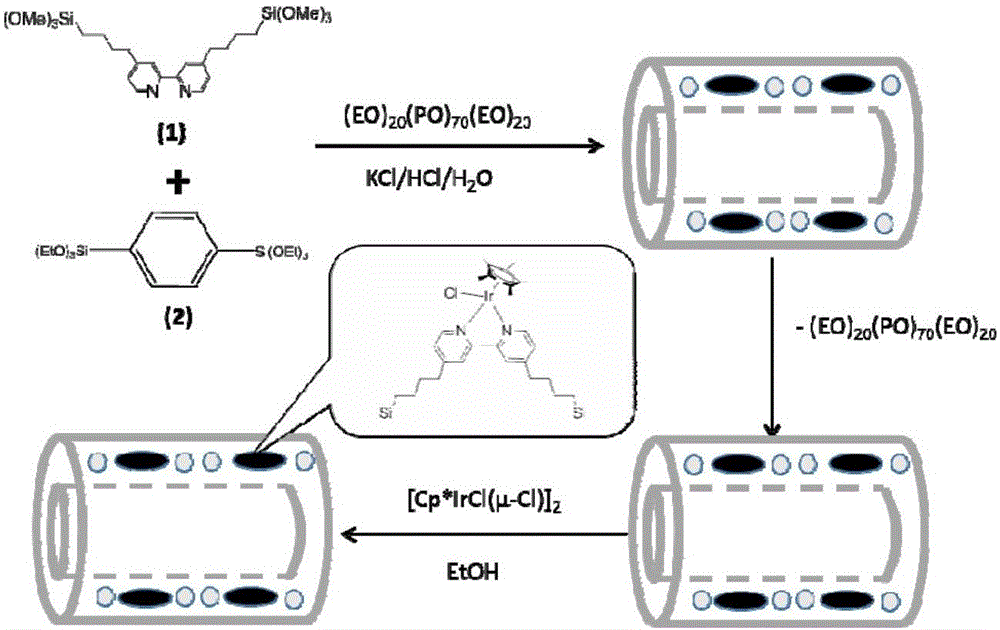
Iridium complex as well as preparation method and application thereof
InactiveCN104402939AGood activityInhibitory activityAntiparasitic agentsAntineoplastic agentsPentamethylcyclopentadieneIridium
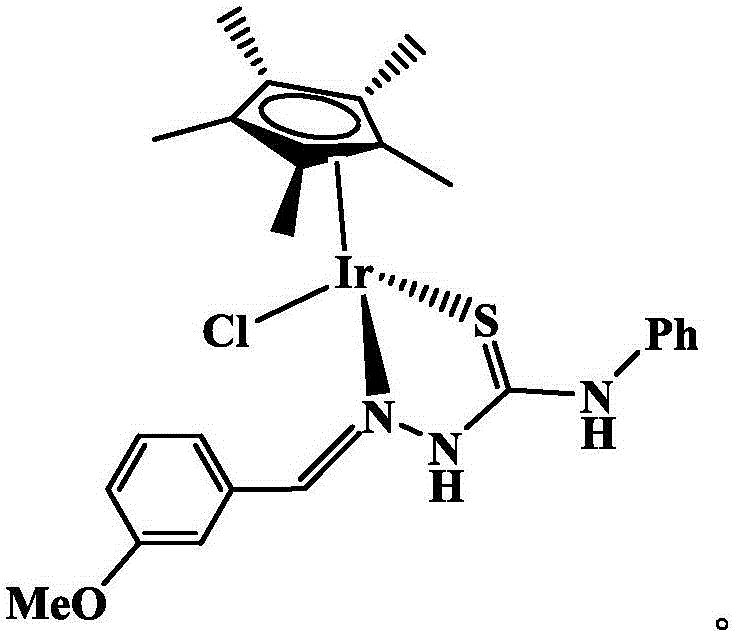
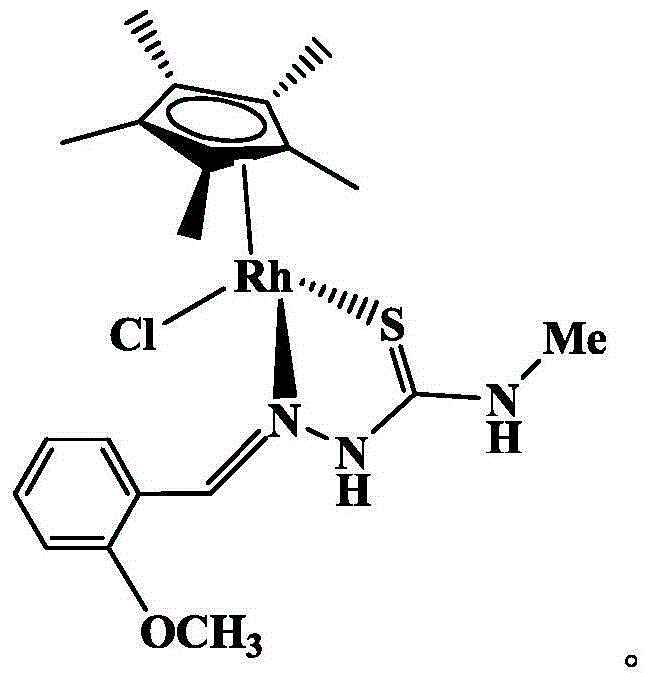
The invention discloses an iridium complex as well as a preparation method and an application thereof. The iridium complex is monochloro-mono-meta-methoxybenzaldehyde-4-phenyl-3-thiosemicarbazide-pentamethyl cyclopentadienyl iridium (III) and can be applied to preparation of medicines for treating cancers, and the structural formula of the complex is shown in the specification.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Iridium complexes for electrocatalysis
ActiveUS20150021194A1Improve stabilityConvenient bindingPlatinum group organic compoundsFrom normal temperature solutionsIridiumPentamethylcyclopentadiene
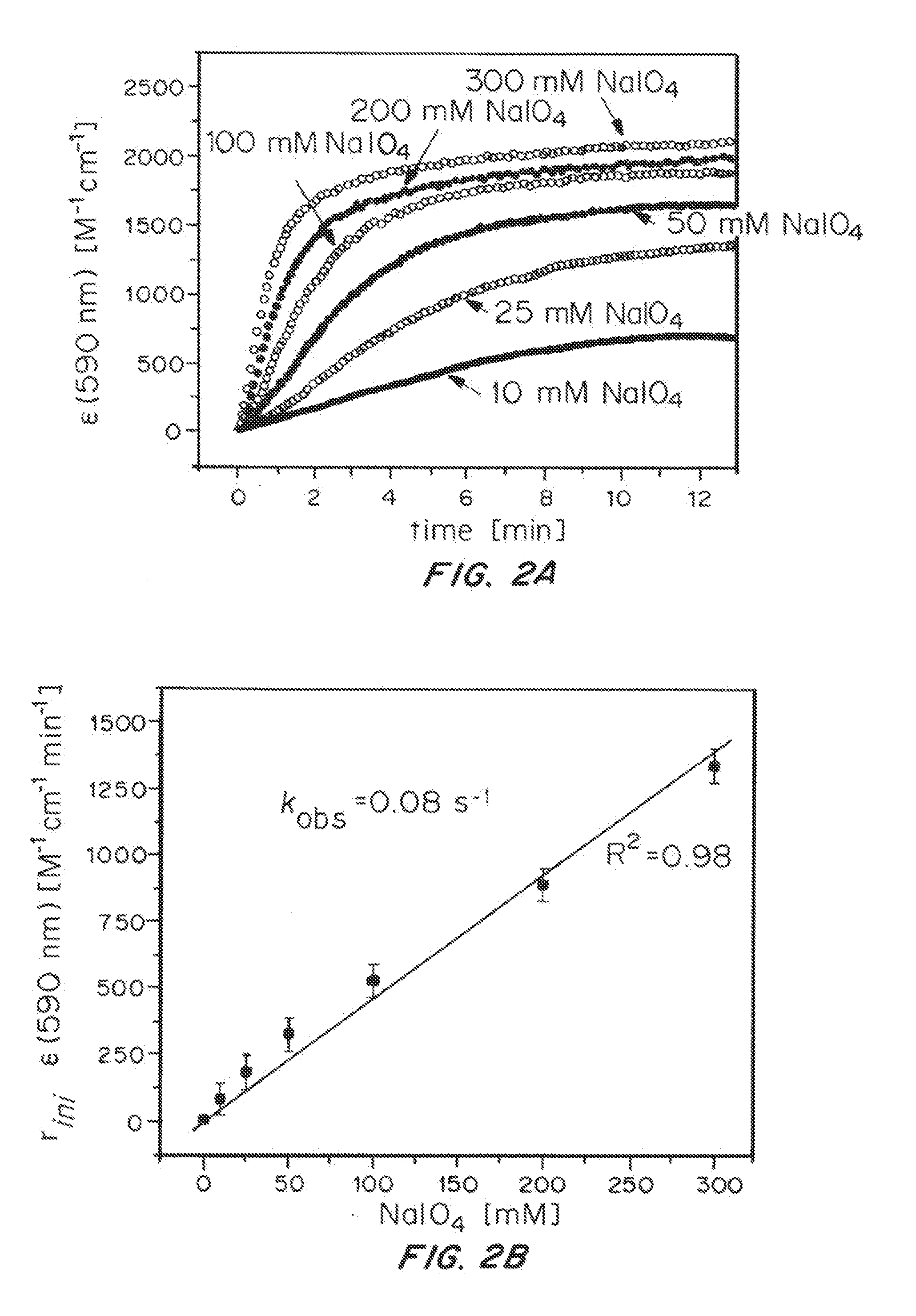
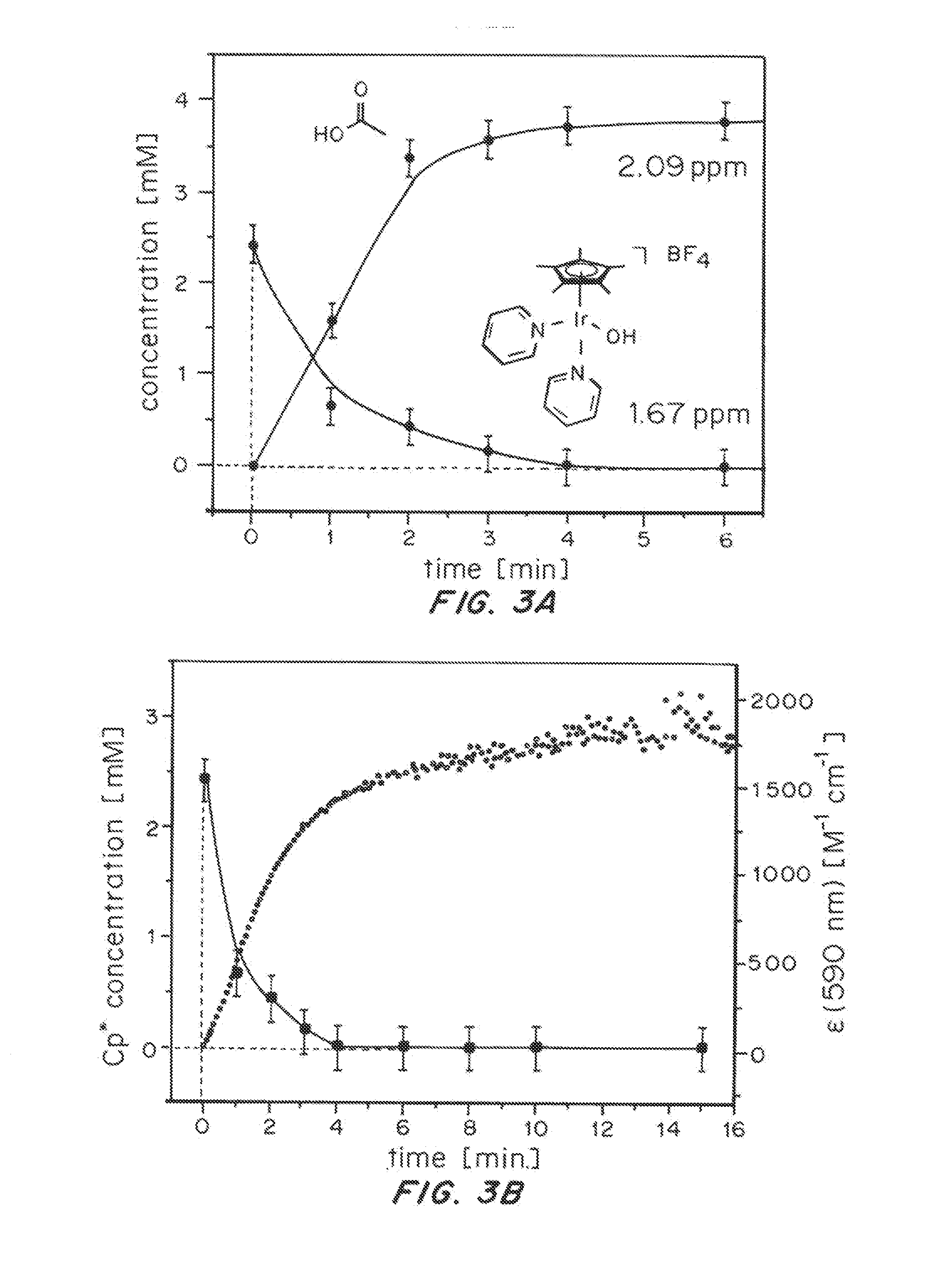
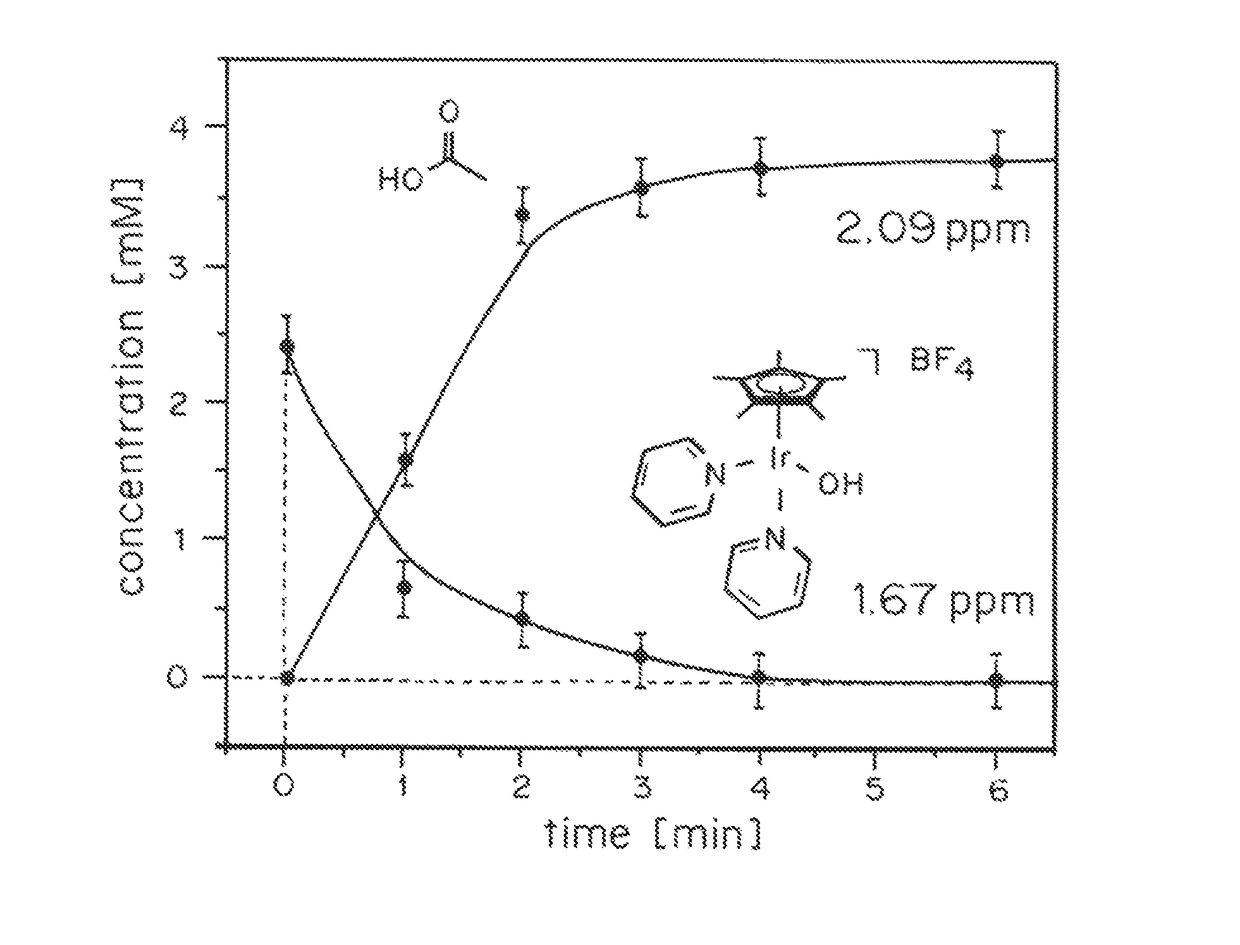
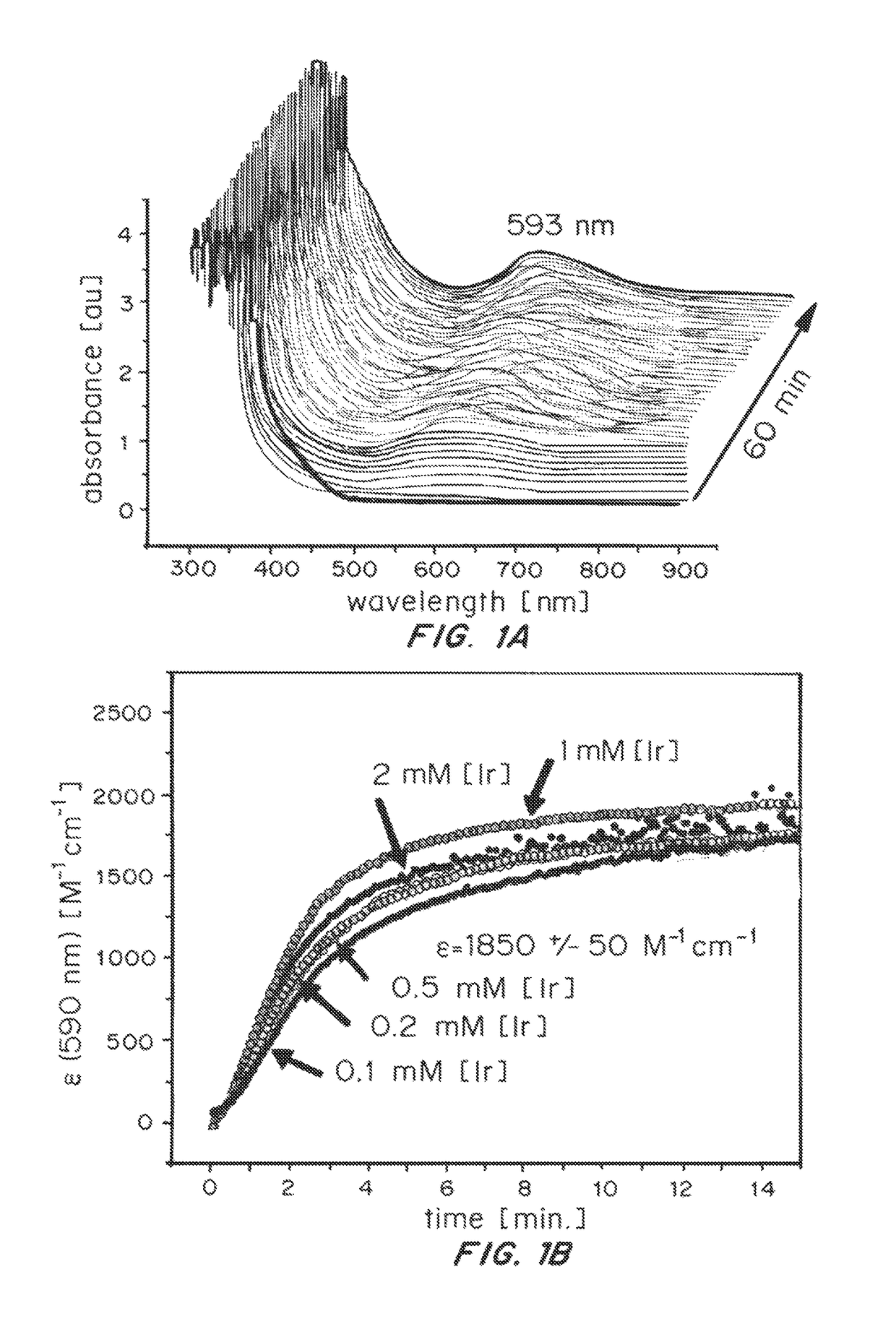
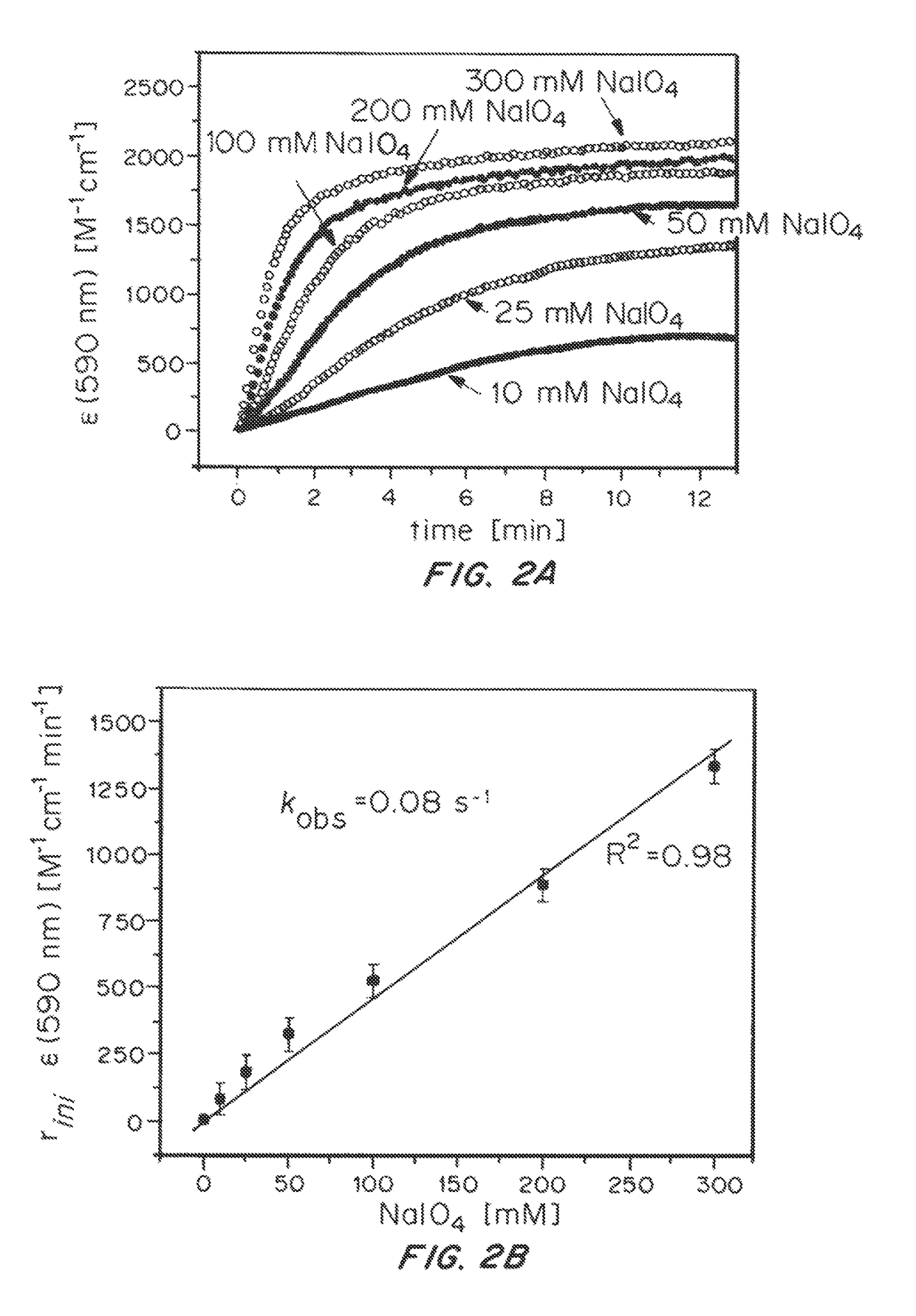
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
Rhodium complex as well as preparation method and application thereof
InactiveCN104402940AGood activityImprove the bactericidal effectAntibacterial agentsAntimycoticsPentamethylcyclopentadieneBenzaldehyde
The invention discloses a rhodium complex as well as a preparation method and application thereof. The rhodium complex is monochloride o-mono-methoxy benzaldehyde acetal 4-methyl-3-thiosemicarbazide-pentamethylcyclopetadienyl-rhodium (III), and the rhodium complex can serve as the application for preparing drugs for treating cancers. The structural formula of the rhodium complex is shown in the Specification.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Norbornene, hexafluoropropylene and acrylonitrile ternary polymerization catalyst and ternary polymerization method
ActiveCN105693928AHigh yieldImprove corrosion resistancePentamethylcyclopentadieneHexafluoropropylene
The invention relates to a norbornene, hexafluoropropylene and acrylonitrile ternary polymerization catalyst. The preparation method of the catalyst comprises the steps that (pentamethyl cyclopentadiene) cerium chloride and a ligand are dissolved into a first solvent in a dry three-mouth flask in the inertia atmosphere, titanium tetrachloride is dropped into the solution while stirring is performed, stirring is performed at the constant temperature of 30-60 DEG C for 30-60 min after dropping is performed, and a cerium-titanium complex catalyst is obtained. The polymerization method comprises the steps that a norbornene monomer, a hexafluoropropylene monomer and an acrylonitrile monomer are taken and added into a reaction kettle where repeated vacuumizing and nitrogen charging are performed, and a second solvent is added for dissolution; then, the cerium-titanium complex catalyst is added, a reaction is performed under the certain temperature and pressure, and the product is washed to obtain the terpolymer. Catalyst raw materials are low in price and easy to obtain, catalytic activity can be generated at low temperature, norbornene, hexafluoropropylene and acrylonitrile ternary polymerization is catalyzed, the reaction temperature is low, the catalysis efficiency is high, the copolymer yield is high, and the terpolymer is good in photoetching performance.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
Norbornene, styrene and nikasol ternary polymerization catalyst and ternary polymerization method
The invention relates to a norbornene, styrene and nikasol ternary polymerization catalyst and a ternary polymerization method. The preparation method of the catalyst comprises the steps that (pentamethyl cyclopentadiene) iridium chloride, aluminum alkyl and a ligand are dissolved into a first solvent in a dry three-mouth flask in the inertia atmosphere, stirring is performed at the constant temperature of 20-50 DEG C at the speed of 110-130 rpm for 20-50 min, and an iridium-aluminum complex catalyst is obtained. The polymerization method comprises the steps that a norbornene monomer, a styrene monomer and a nikasol monomer are taken and added into a reaction kettle, and a second solvent is added for dissolution; then, the iridium-aluminum complex catalyst is added, a reaction is performed under the certain stable pressure, and the product is washed to obtain the terpolymer. Catalytic activity can be generated at low temperature, norbornene, styrene and nikasol ternary polymerization is catalyzed, the reaction temperature is low, the catalysis efficiency is high, the copolymer yield is high, and the terpolymer is good in photoetching performance.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
3-amino indanone compound synthesis method
ActiveCN107033016AImprove conversion efficiencyGood atom economyOrganic compound preparationGroup 5/15 element organic compoundsMANGANESE ACETATEPentamethylcyclopentadiene
The invention discloses a 3-amino indanone compound synthesis method which includes the steps: adding an imine derivative, an olefin compound, pentamethyl dichloride rhodium cyclopentadiene and manganese acetate into an organic solvent; performing heating reaction under air conditions; performing post-treatment after complete reaction to obtain a 3-amino indanone compound. According to the method, simple and easily obtained raw materials are synthesized at one step to obtain the 3-amino indanone compound, conversion efficiency is high, and atom economy is good. Besides, the synthesis method is simple in operation and high in reaction yield, and a substrate is wide in adaptability.
Owner:ZHEJIANG UNIV
Novel polysubstitution isoquinoline derivative and synthesis method thereof
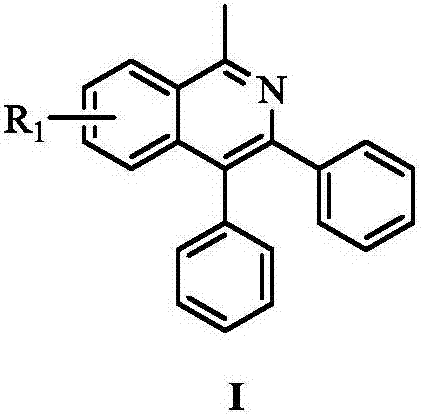
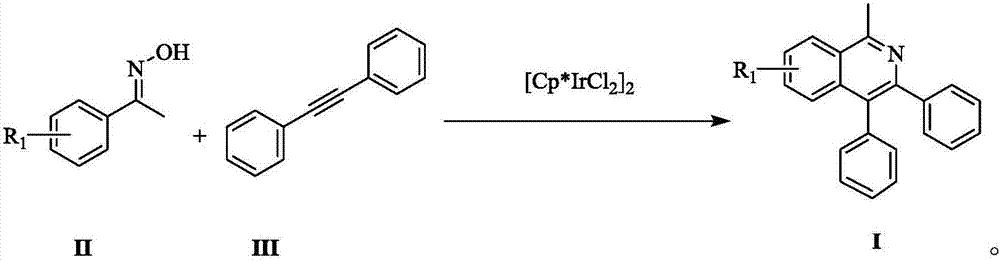
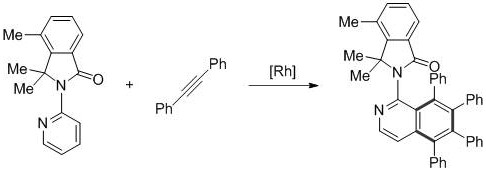
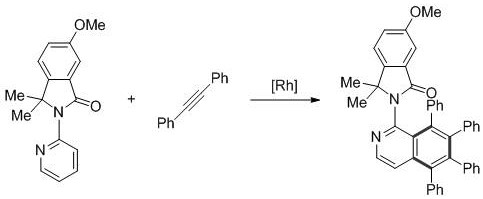
ActiveCN107382856AReduce dosageFew reaction stepsOrganic chemistryAntipyreticPentamethylcyclopentadieneIsoquinoline
The invention provides a novel polysubstitution isoquinoline derivative shown as a formula (I) and a synthesis method thereof. The formula I is shown in the specification. A C-H activation / cyclization method is used; ketoxime and diphenylacetypene are used as raw material; pentamethyl cyclopentadiene iridochloride is used as a catalyst for synthesizing the isoquinoline derivative. The preparation method is simple; the catalyst consumption is low; the reaction conditions are mild; the reaction steps are few; the reaction time is short; the yield is high. The isoquinoline derivative provided by the invention belongs to a kind of alkaloid compounds with important medicine activity, and can be widely applied to the study in aspects of fields of medicine and agriculture.
Owner:JIANGSU UNIV OF TECH
Method for preparing 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline
InactiveCN106632087AHigh yieldHigh purityOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsPentamethylcyclopentadieneTrifluoromethylation
The invention discloses a 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline compound and a preparation method thereof. The preparation method comprises the following steps: taking 2,4-diarylquinazoline as a reaction substrate, carrying out a reaction with NIS under the catalytic action of pentamethylcyclopentadienylrhodium (III) chloride dimer / silver hexafluoroantimonate under a condition of 80 DEG C, carrying out a reaction with a sulfur trifluoromethylation reagent, and taking copper iodide as a catalyst, wherein the reaction temperatire is 85 DEG C, and the reaction time is 7-10 hours; performing carbon hydrogen bond activation process, thereby obtaining the 4-aryl-2-(2-(trifluoromethyl)aryl)quinazoline compound. The preparation method disclosed by the invention is mild in reaction conditions, easy and convenient to operate, low in cost, few in side reactions, high in product purity and convenient for separation and purification and can be suitable for large-scale preparation, and the obtained product has high drug activity and excellent potential application prospects.
Owner:JIANGXI NORMAL UNIV
Catalyst for catalyzing formaldehyde or derivative thereof to prepare hydrogen, and synthesis method and application thereof
ActiveCN108499604AHigh catalytic activityAvoid poisoningHydrogenOrganic-compounds/hydrides/coordination-complexes catalystsHigh concentrationPentamethylcyclopentadiene
The invention discloses a catalyst for catalyzing formaldehyde or a derivative thereof to prepare hydrogen, and a synthesis method and application thereof. The chemical formula of the catalyst is Ru(Y)BnXm, wherein Y comprises any one selected from the group consisting of 4-methylisopropylphenyl, pentamethylcyclopentadienyl, phenyl, tolyl, trimethylphenyl, 1,2,4, 5-tetramethylphenyl and hexamethylphenyl; B comprises H2O or NH3; and X comprises any one selected from the group consisting of PO4, NO3, BF4, SO4 and PF6, or a mixture of two or more selected from the group. The synthesis method forthe catalyst comprises the following steps: providing [Ru(Y)]nZm, dispersing [Ru(Y)]nZm in water to form a suspension, controlling the temperature of the suspension to be in a range of 0 and 100 DEG C, then adding a silver salt, and carrying out a reaction, wherein Ru(Y)BnXm is obtained after completion of the reaction. The catalyst of the invention has high catalytic activity, is easily soluble in water, does not need strong alkali; the concentration of carbon monoxide in generated gas is less than 10 ppm; and the catalyst can be directly applied to a hydrogen-oxygen fuel cell system and eradicates the problem of fuel cell poisoning caused by high concentration of carbon monoxide.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Iridium complexes for electrocatalysis
ActiveUS9790605B2Improve stabilityConvenient bindingIndium organic compoundsCell electrodesIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
Synthetic method of spirocyclic compound containing 1-indanone skeleton
ActiveCN108047007AAtom utilization is highEasy to operateOrganic compound preparationCarbonyl group formation/introductionPentamethylcyclopentadieneMANGANESE ACETATE
The invention discloses a synthetic method of a spirocyclic compound containing 1-indanone skeleton. The method comprises the following steps: taking aromatic carboxylic acid and alpha, beta-unsaturated ketone as raw materials, taking any of p-cymene ruthenium chloride dimer, pentamethylcyclopetadienyl rhodium chloride dimmer, tris(acetonitrile)(pentamethylcyclopentadienyl)rhodium bis(hexafluoroantimonate) as a catalyst, taking any of anhydrous manganese acetate, manganese acetate tetrahydrate, anhydrous zinc acetate and zinc acetate as an additive, and thus synthesizing the spirocyclic compound containing 1-indanone skeleton by adopting a one-step method. The reaction comprises four steps such as conjugate addition reaction of aromatic carboxylic acid ortho-position C-H bond and alpha, beta-unsaturated ketone, intramolecular dehydration, Michael addition with second molecular alpha,beta-unsaturated ketone and intramolecular aldol condensation. The synthetic method has the characteristics that the raw materials are low in price and easy to obtain, the efficiency is high, the atom utilization rate is high, the reaction operation is simple and four new C-C bonds are constructed by adopting the one-step method.
Owner:SHAANXI NORMAL UNIV
Method for converting alkyne into alcohol
InactiveCN103214344AReduce pollutionHigh yieldPreparation by oxo-reaction and reductionPentamethylcyclopentadieneIridium
The invention discloses a method for converting alkyne into alcohol. The method comprises the following steps of: under the protection of argon, reacting phenylacetylene, p-methyl phenylacetylene, p-bromo phenylacetylene, m-chloro phenylacetylene or di-phenylacetylene with formic acid under the catalysis of chloro(pentamethyl cyclopentadiene)-(5-methoxy-2-{1-[(4-methoxyphenyl)imine iridium or [N-[(1R,2R)-2-(amino-)-1,2-diphenethyl]-4-methylbenzene sulfonamide-N]chloro(pentamethyl cyclopentadiene)-ruthenium; and adjusting the pH value to 3-4 or neutrality to obtain achiral alcohol and chiral alcohol. The method disclosed by the invention has the advantages of easiness in operation, mild reaction conditions, little environmental pollution, high yield and low production cost, and can be applied to industrial production.
Owner:SHAANXI NORMAL UNIV
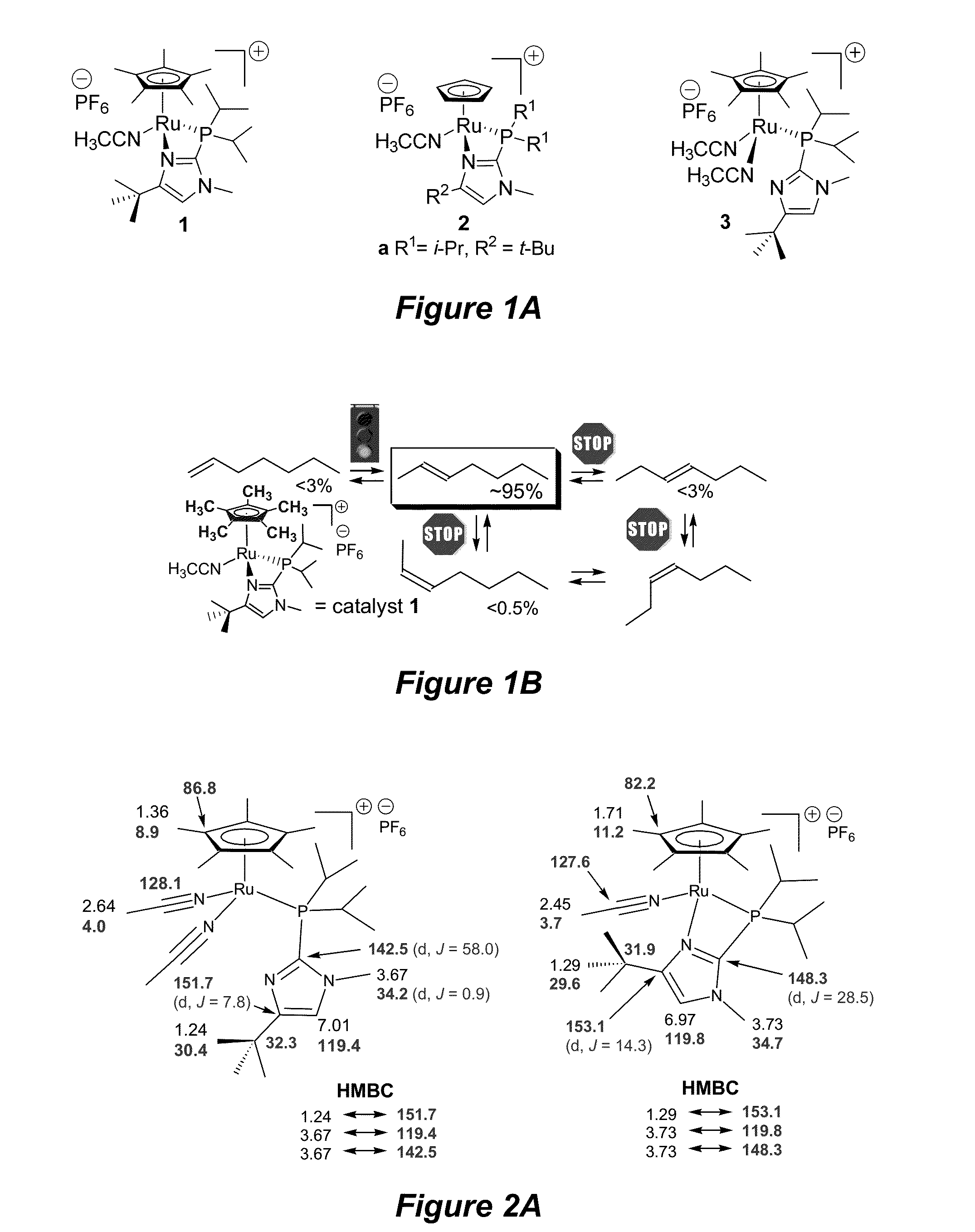
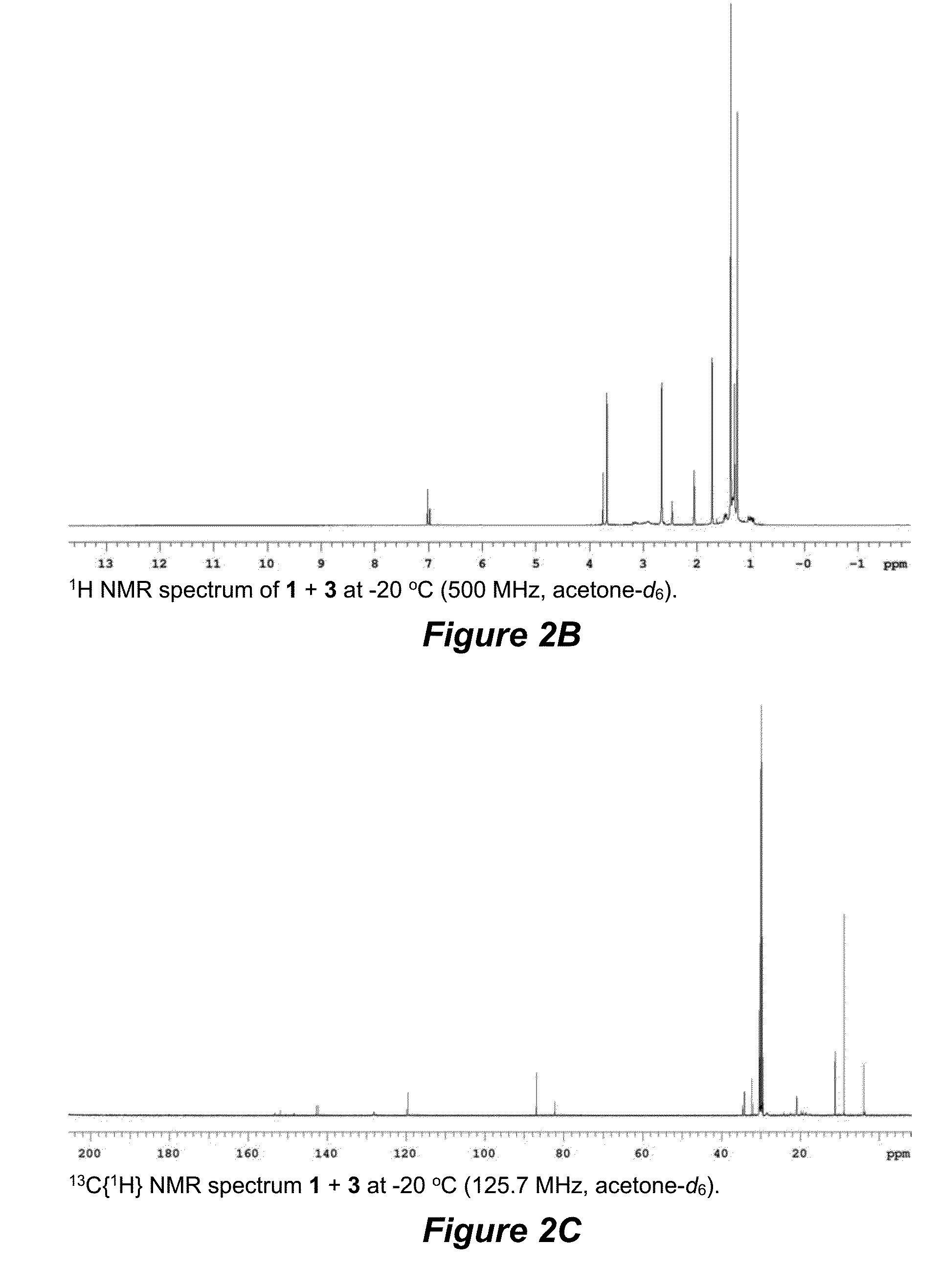
Terminal alkene monoisomerization catalysts and methods
ActiveUS20150231621A1Ruthenium organic compoundsGroup 4/14 element organic compoundsPentamethylcyclopentadieneIsomerization
The invention provides novel catalysts and methods of using catalysts for controlling the position of a double bond and cis / trans-selectivity in isomerization of terminal alkenes to their 2-isomers. Catalysts such as (pentamethylcyclopentadienyl)Ru formulas 1 and 3 having a bifunctional phosphine can be used in the methods. A catalyst loading of 1 mol % of formulas 1+3 can be employed for the production of (E)-2-alkenes at 40-70° C.; lower temperatures can be used with higher catalyst loading. Acetonitrile-free catalysts can be used at lower loadings, room temperature, and in less than a day to accomplish the same results as catalysts 1+3. The novel catalyst systems minimize thermodynamic equilibration of alkene isomers, so that the trans-2-alkenes of both non-functionalized and functionalized alkenes can be generated.
Owner:SAN DIEGO STATE UNIV RES FOUND
Norbornene, pentene and vinylene carbonate ternary copolymerized catalyst and ternary copolymerizing method
The invention relates to a norbornene, pentene and vinylene carbonate ternary copolymerized catalyst and a ternary copolymerizing method. The method for preparing the catalyst comprises the following steps: dissolving bis(pentamethylcyclopentadienyl) hafnium dichloride (iV) and ligand in a first solvent in a dried three-mouth flask at an inert gas atmosphere, dripping aluminum alkyl under 200-400rpm, stirring at constant temperature at the same stirring speed at constant temperature of 20-60 DEG C for 20-60 minutes to obtain a hafnium-aluminum complex catalyst; adding norbornene, pentene and vinylene carbonate into a reaction kettle which is vacuumized for multiple times and inflated with nitrogen, adding a second solvent and dissolving; adding the hafnium-aluminum complex catalyst, and reacting for 1-6 hours at certain temperature and pressure; and pouring the product obtain in the reaction into a hydrochloric acid-containing alcohol solution, washing the precipitate to be neutral with alcohol, and drying in vacuum to obtain the norbornene, pentene and vinylene carbonate ternary copolymerized catalyst.
Owner:NINGBO UNIVERSITY OF TECHNOLOGY
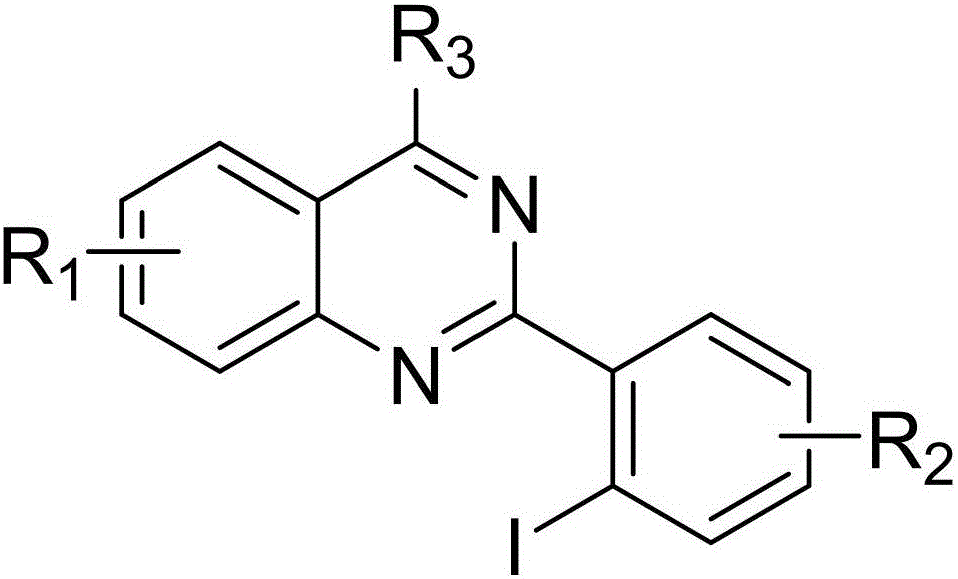
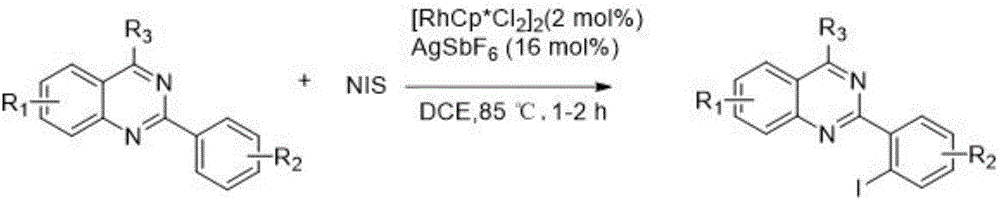
2-(2-iodoaryl)quinazoline compound and preparation method thereof
ActiveCN106632086AImprove efficiencyEasy to operateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPentamethylcyclopentadieneReaction temperature
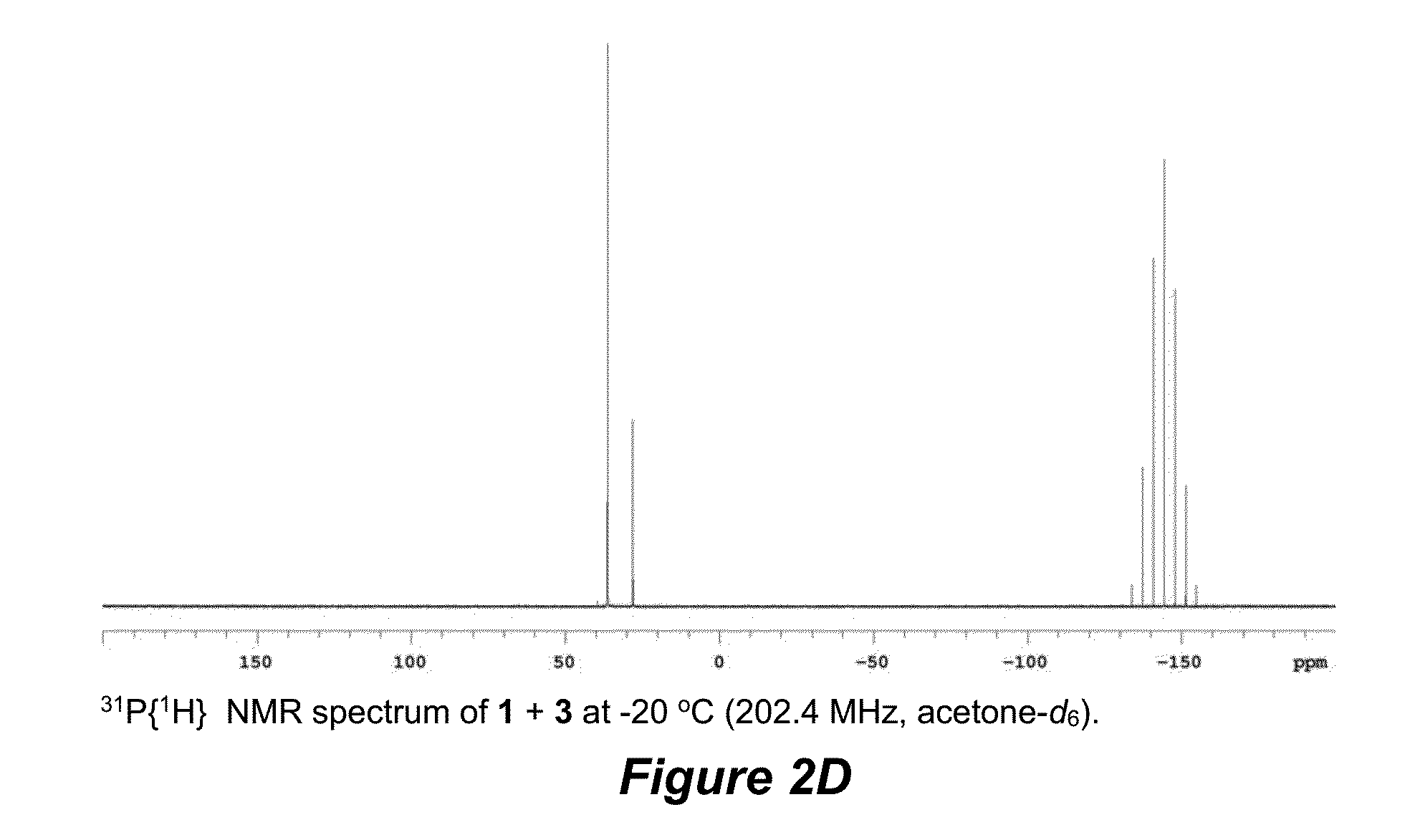
The invention discloses a 2-(2-iodoaryl)quinazoline compound and a preparation method thereof. The 2-(2-iodoaryl)quinazoline compound is prepared by using 2-arylquinazoline as a reaction substrate through the process of carbon-hydrogen bond activation iodination with iodosuccinimide under the catalytic action of dichloro(pentamethylcyclopetadienyl)rhodium (III) dimer / silver hexafluoroantimonate, wherein the reaction temperature is 85 DEG C, and the reaction time is 1 to 2 hours. The preparation method provided by the invention has the advantages of high yield, excellent reaction chemical selectivity, mild reaction conditions, simple and convenient operation, low cost, less side reaction and high product purity; and the product disclosed by the invention is a novel compound, facilitates to separation and purification, is applicable to large-scale preparation, and has good application value and potential biological activity.
Owner:JIANGXI ACAD OF FORESTRY
Method for preparing fluorine-containing secondary amine
InactiveCN106748802AHigh yieldEasy to getOrganic compound preparationAmino compound preparationPentamethylcyclopentadieneAlcohol
The invention discloses a synthetic method of a fluorine-containing secondary amine compound. Pentamethylcyclopentadienyl iridium chloride dimer is used as a metal catalyst, fluoroalkyl comes from fluorinated alcohol, alkali is used as a co-catalyst, and the fluorine-containing secondary amine is prepared via the following step: primary amine reacts with the fluorinated alcohol under the action of the transition metal iridium catalyst and the alkali in an organic solvent to obtain the fluorine-containing secondary amine compound. The method overcomes the defects in the prior art that the fluorine-containing alkylating agent is high in toxicity and instable and needs to be prepared in advance, the reaction operation is complex, the steps are fussy and the like, and can obtain considerable yield. The adopted fluorinated alcohol is readily available, stable in chemical property and low in toxicity. The method is simple in reaction, has very high atom economy and step economy, and is energy-saving, environment-friendly and simple in operation. By adopting [Cp*IrCl2]2 as the catalyst, ligands are not needed, and the system is easy to realize.
Owner:NANJING UNIV OF SCI & TECH
Method for synthesizing polysubstituted amino isoquinoline compound by cyclizing pyridine and alkyne under catalysis of rhodium
ActiveCN111808071AAvoid complexationRegulatory selectivityOrganic chemistryPentamethylcyclopentadienePtru catalyst
The invention discloses a method for synthesizing a polysubstituted aminoisoquinoline compound by cyclizing pyridine and alkyne under the catalysis of rhodium, which comprises the following steps: byusing a pentamethylcyclopentadienyl rhodium complex as a catalyst and a 2-aminopyridine derivative and an internal alkyne compound as raw materials, reacting in an organic solvent at 30-150 DEG C for1-24 hours, cooling the reaction solution after the reaction is finished, removing the organic solvent under reduced pressure; purifying the crude product through a silicon dioxide column, and elutingwith petroleum ether / ethyl acetate to obtain the pure polysubstituted aminoisoquinoline derivative. According to the method, a guide group (carbonyl) or a steric hindrance group (methyl) is introduced into the reaction to realize C-H bond activation and avoid the coordination effect of pyridine on the metal catalyst, and the regulation and control of the reaction activity and selectivity are efficiently realized through the regulation and control of the group with large steric hindrance. The obtained generation target products are high in selectivity, and the selectivity is 90% or above, byproducts are few, and the product separation cost is reduced, and the method is simple in synthesis process, mild in reaction condition and convenient for industrial production.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Rare-earth antifungal agent composition
InactiveCN106087392AGood antibacterial and antifungal effectNot easy to loseFibre treatmentAntifungalPentamethylcyclopentadiene
The invention provides a rare-earth antifungal agent composition. A certain amount of nano titanium dioxide silver antifungal agent is dispersed in ethyl alcohol, bis(pentamethylcyclopentadienyl)cobalt, cyclopentadienylrhenium tricarbonyl, benzoyl peroxide, polyvinyl alcohol and sodium dodecyl benzene sulfonate are added according to the proportions, and a nano titanium dioxide silver antifungal agent micro suspension product containing rare earth is obtained through surface polymerization.
Owner:王金明
Preparation method of 4-aryl-2-(2-(thiotrifluoromethyl)aryl)quinazoline
ActiveCN108976173AHigh yieldMild reaction conditionsOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsPentamethylcyclopentadieneIodide
The invention discloses a 4-aryl-2-(2-(thiotrifluoromethyl)aryl)quinazoline compound and a preparation method thereof. 2,4-diarylquinazoline is used as a reaction substrate to react with NIS under thecatalytic effect of dichloro(pentamethylcyclopentadienyl) rhodium(III) dipolymer / silver hexafluoroantimonate and then react with a thiotrifluoromethy reagent for 7 to 10 hours at the reaction temperature of 85 DEG C by adopting cuprous iodide as a catalyst by virtue of a carbon-hydrogen-bond activation process, so that the 4-aryl-2-(2-(thiotrifluoromethyl)aryl)quinazoline compound is obtained. The preparation method of the invention is mild in reaction condition, simple in operation, low in cost, less in side reaction, high in product purify, convenient in separation purification and suitablefor mass preparation, and the prepared product is good in drug activity and good in potential application prospect.
Owner:JIANGXI NORMAL UNIV
Synthesis method for 2,3-diphenyl-1H-indene-1-one derivatives
InactiveCN109180446AImprove conversion efficiencyGood atom economyOrganic compound preparationCarboxylic acid esters preparationIridiumPentamethylcyclopentadiene
The invention discloses a synthesis method for 2,3-diphenyl-1H-indene-1-one derivatives. The synthesis method comprises the steps of: adding benzoyl formic acid, a diphenyl acetylene compound, pentamethyl cyclopentadiene dichloride iridium, copper acetate, silver hexafluoroantimonate and a silver hexafluoroantimonate into an organic solvent; heating for reaction in the presence of air; and after the reaction, carrying out post-treatment to obtain the 2,3-diphenyl-1H-indene-1-one derivative. The method synthesizes the 2,3-diphenyl-1H-indene-1-one derivative in one step through simple and easilyavailable raw materials, so that the conversion efficiency is high and the atomic economical benefit is good. Meanwhile, the synthetic method is simple to operate, high in reaction yield and wide inprimer adaptability.
Owner:JILIN INST OF CHEM TECH
Preparation method of polytetrafluoroethylene core-shell polymer containing rare earth
InactiveCN105949405ASpecial Peripheral Electronic StructureImprove the coordination effectPentamethylcyclopentadieneRare earth
The invention relates to a preparation method of a polytetrafluoroethylene core-shell polymer containing rare earth. The polytetrafluoroethylene polymer consists of a core and a shell, wherein the shell is a polymer of a polytetrafluoroethylene emulsion, bis(tricarbonyl cyclopentadienyl molybdenum) and (3R)-1-[4,4-bis(3-methyl thiophene)-2-yl)but-3-enyl]piperidine-3-carboxylic acid, butyl acrylate and bis(pentamethylcyclopentadiene)cobalt; the shell is a polymer of styrene, 1-heptylene-1,2-hypoboric acid bis(2,3-dimethyl-2,3-butanediol)ethyl ester; the PTFE (Polytetrafluoroethylene) composite emulsion with good stability and coating property is prepared by adopting seeded emulsion polymerization.
Owner:QUZHOU ZHONGTONG CHEM
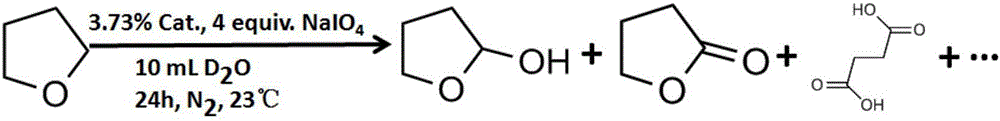
Tetrahydrofuran C-H multiphase oxidation method
InactiveCN106588829AEasy to manufactureHigh selectivityCarboxylic preparation by oxidationIridiumPentamethylcyclopentadiene
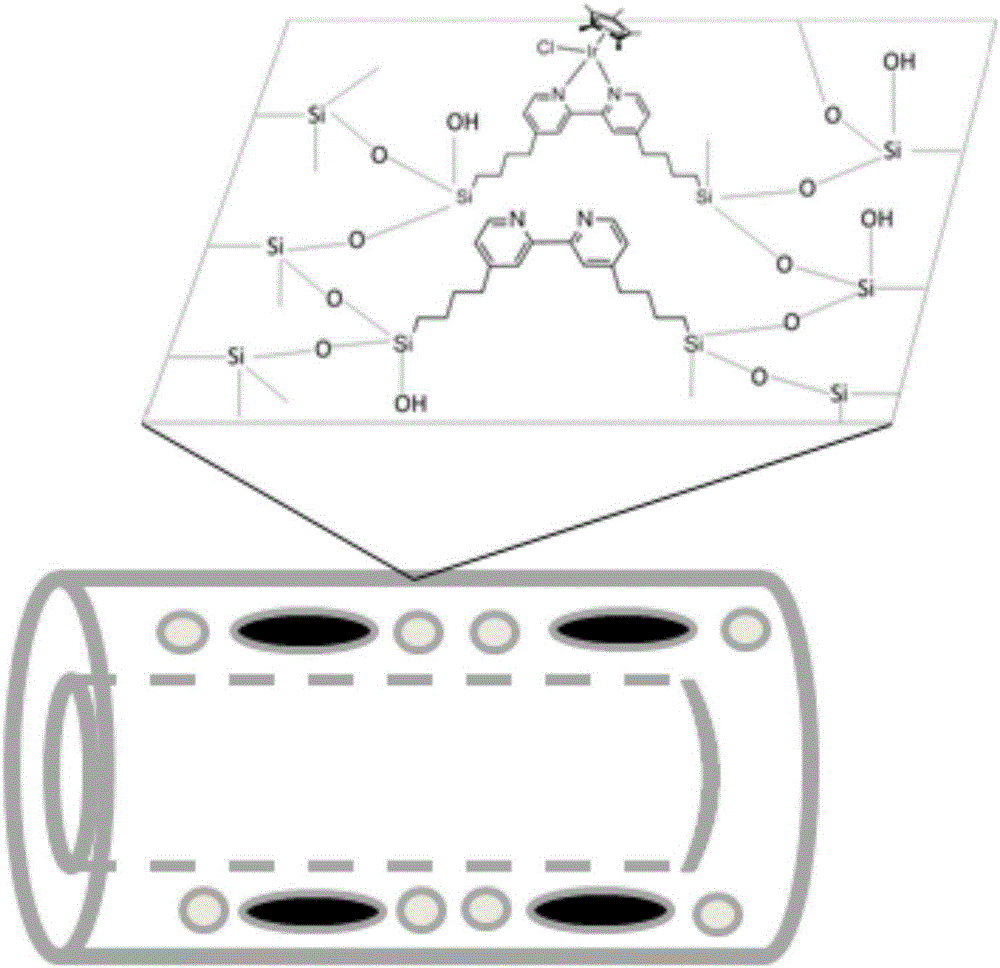
The invention relates to a tetrahydrofuran C-H multiphase oxidation method. 4,4'-[4-(trimethoxy silane) butyl]-2,2'-bipyridine is embedded into an organic silicon nanotube, and dichloro(pentamethylcyclopentadienyl) iridium dimer is coordinated into the nanotube to obtain an iridium-based bipyridine-organic silicon nanotube multiphase catalyst. The catalyst is mixed with tetrahydrofuran, and a reaction is performed at a normal temperature by taking sodium periodate as an oxidant and heavy water as a solvent. The catalyst has a clear porous structure, a large specific surface area and uniform active sites, and has a very good catalytic effect on tetrahydrofuran C-H oxidation, and because the catalyst has the characteristics of relatively high stability and easy recovery, the catalyst has a very good actual application prospect in industry.
Owner:TIANJIN UNIV
Method for synthesizing 2,4-dialkylene-substituted indole compound with one-pot process
InactiveCN106631973ASimple operating conditionsHigh reaction yieldOrganic chemistryLuminescent compositionsPentamethylcyclopentadieneBiological activation
The invention discloses a method for synthesizing a 2,4-dialkylene-substituted indole compound with a one-pot process. The method comprises steps as follows: indolecarboxylic acid, ethylene compounds, [Cp*RhCl2]2(pentamethylcyclopentadienerhodium chloridedimer), alkaline copper carbonate, potassium acetate and silver hexafluoroantimonate are added to an organic solvent and heated for a reaction, aftertreatment is performed after a complete reaction, and the 2,4-dialkylene-substituted indole compound is obtained. According to the method, with the adoption of specific catalysts and the alkaline additive, C-H bonds of indolecarboxylic acid and ethylene compounds are subjected to activation, alkylation and decarboxylation reactions at the same time, the operation conditions are simple, and the reaction yield is high.
Owner:ZHEJIANG UNIV
Preparation method of carbon tetrachloride purification adsorbent
InactiveCN106040206ALarge specific surface areaDense pore size distributionOther chemical processesHalogenated hydrocarbon preparationPentamethylcyclopentadieneSorbent
The invention provides a preparation method of carbon tetrachloride purification adsorbent. The preparation method comprises the steps of adding water and polyvinyl alcohol into a reaction kettle and evenly mixing to form a water phase; adding styrene, 1,5,9-cyclododecatriene, 3-allyloxy-2-hydroxy propane sulfonic acid, methacrylamide and pentamethyl cyclopentadienyl isopropyl titanate into a reaction kettle and evenly mixing to form an oil phase; adding an oil-phase solution into the reaction kettle where the water phase is prepared; reacting in an appropriate temperature; acetone extracting reaction mixture after reaction is finished; drying in a vacuum mode to obtain the carbon tetrachloride purification adsorbent. The carbon tetrachloride purification adsorbent is large in specific surface area, stable in property and high in product purity after adsorption.
Owner:王金明
Practical method for synthesizing novel bioactive molecule with N-methoxy-amide as nitrogen source
ActiveCN109912603ARaw materials are easy to getRich typeOrganic chemistryIridiumPentamethylcyclopentadiene
The invention discloses a practical method for synthesizing a novel bioactive molecule with N-methoxy-amide as a nitrogen source. The practical method comprises the following steps: sequentially adding an aryl-substituted nitrogen heterocyclic compound, a dichloro-(pentamethyl cyclopentadienyl) iridium (III) dimer, silver hexafluoroantimonate and N-methoxybenzamide into a glass reaction tube and performing a reaction at the temperature of 120-140 DEG C by taking 1,2-dichloroethane as a solvent to obtain the novel bioactive molecule synthesized by taking N-methoxy-amide as the nitrogen source.With adoption of the method disclosed by the invention, obtained products are diversified in type, can be directly applied to synthesis and decoration of pharmaceutical molecules and can also be usedfor other further reactions; meanwhile, the method is safe and easy in synthesis route, low in cost, simple in reaction operation and aftertreatment process and good in selectivity; methanol is an only by-product, therefore, the practical method conforms to the concept of green chemistry.
Owner:SUZHOU UNIV
Method for preparing norbornene open-loop translocation polymer or addition polymer
The invention pertains to chemical industry, particularly relates to a norbornene ring-opening metathesis polymer and preparation of addition polymer. Catalyst system is composed of two portions of A and B, A portion is trivalent iridium catalyst in half-sandwich structure, which is represented by formula Cp*IrL, wherein Cp* represents ligand of 1,2,3,4,5-Pentamethylcyclopentadiene, L represents bidentate ligand containing O, N heteroatom which is capable of coordinating with late transition metal; B portion is represented by methylaluminoxane (MAO) or modidied methylaluminoxane (MMAO). A portion of the invention is simple to prepare, stable in air. Purified norbornene ring-opening metathesis polymer and catalysate of addition polymer can be respectively obtained by regulating proportion of A and B portion.
Owner:FUDAN UNIV
Iridium based dipyridine-organic silicon nanotube heterogeneous catalyst and preparation method thereof
InactiveCN106582845ASimple and easy channel structureClear pore structureGroup 4/14 element organic compoundsCatalyst carriersIridiumPentamethylcyclopentadiene
Owner:TIANJIN UNIV
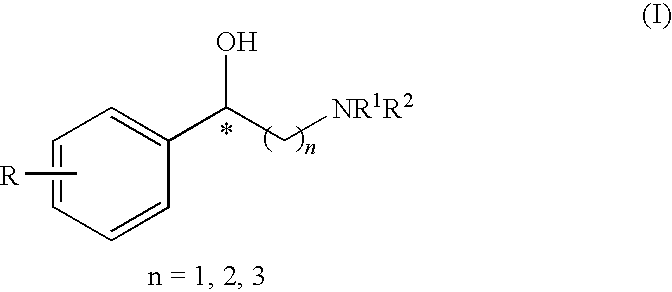
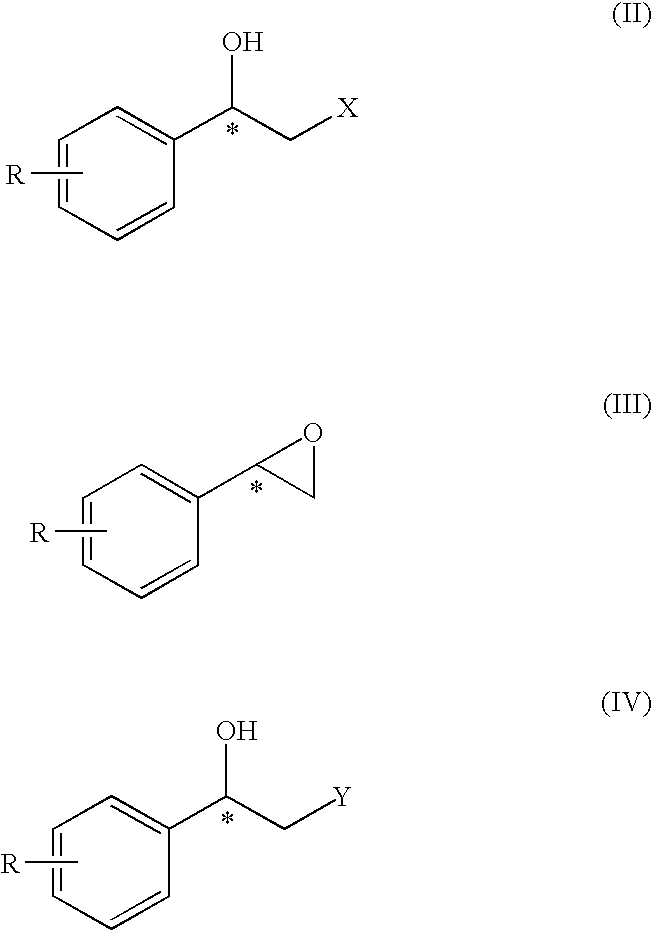
Method for the preparation of optically active 2-sulfonyloxy-1-phenylethanol derivatives
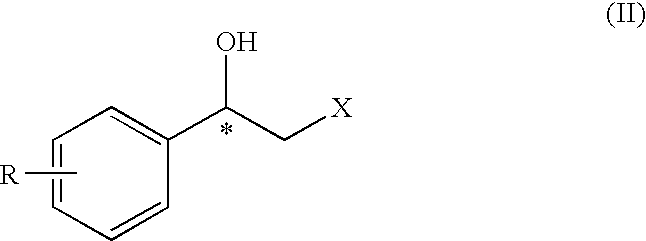
InactiveUS20100063317A1Group 4/14 element organic compoundsOrganic compound preparationHydrogenAcetophenone
Optically active 2-sulfonyloxy-1-phenylethanol derivative of formula (II) can be prepared easily and selectively by the method of the present invention using an asymmetric reduction of an α-sulfonyloxy acetophenone compound with a rhodium catalyst having petamethylcyclopentadienyl group and a hydrogen donor, and the compound of formula (II) obtained in the inventive method exhibits a higher e.e. (enantiomer excess) value than that of the products in the conventional methods.
Owner:KOREA RES INST OF CHEM TECH
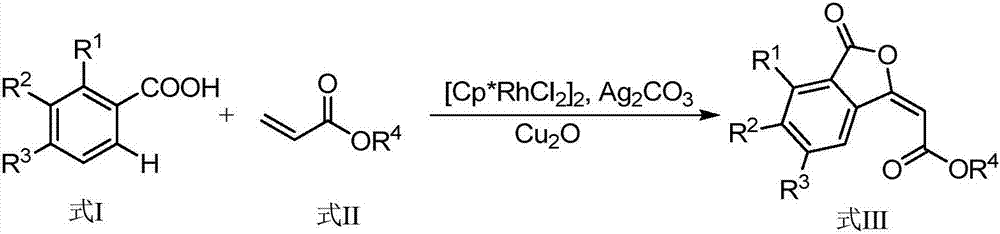
Synthesis method of (E)-3-subunit substituted phthalide compounds
ActiveCN107089960AReduce dosageEasy to operateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPentamethylcyclopentadieneSynthesis methods
The invention discloses a synthesis method of (E)-3-subunit substituted phthalide compounds. The method uses pentamethyl cyclopentadienyl rhodium dichloride as a catalyst, and a series of (E)-3-subunit substituted phthalide compounds is simply, conveniently and efficiently synthesized in a one-step mode through carboxyl ortho-position C-H key functionalization based cascade cyclization reaction between aromatic carboxylic acid and an acrylic compound. The synthesis method has the advantages that raw materials are simple and easy to obtain, the catalyst dosage is small, operation is simple, reaction conditions are mild and the like.
Owner:SHAANXI NORMAL UNIV
Organic metal rhodium compound and preparation method and application thereof
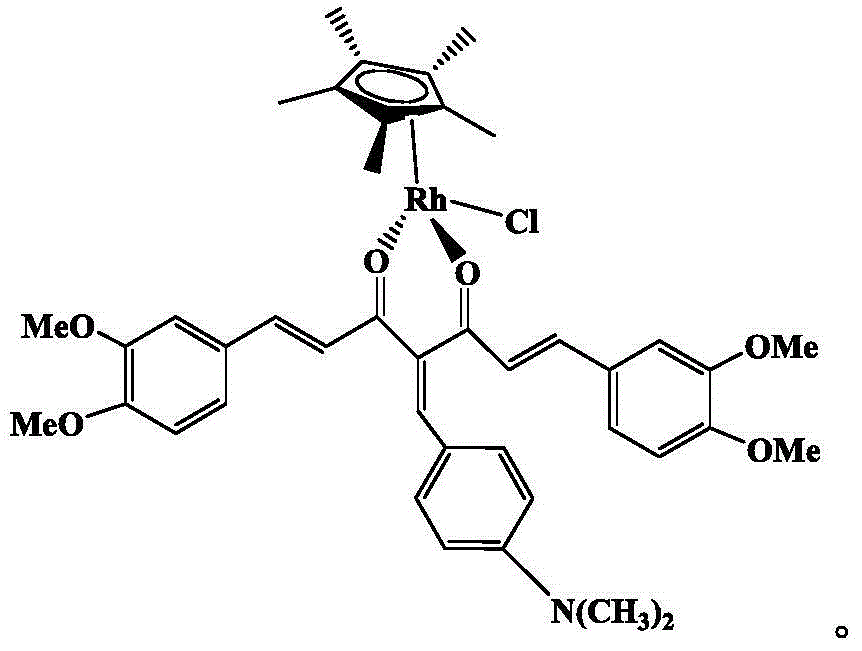
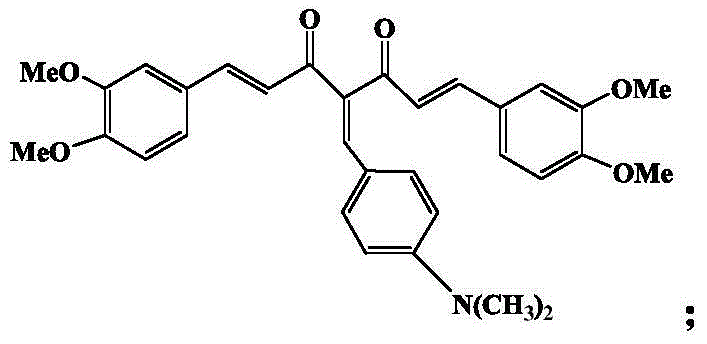
InactiveCN104478938AGood activityGood inhibitory effect on tumor cell activityAntimycoticsRhodium organic compoundsPentamethylcyclopentadieneDiketone
The invention discloses an organic metal rhodium compound and a preparation method and application thereof. The organic metal rhodium compound is chlorine-(1E,6E)-1,7-bis(3,4-dimethoxyphenyl)-4-(p-methylamino phenyl vinyl)-1,6-heptadiene-2,5-diketone-pentamethyl cyclopentadienyl rhodium (III). The organic metal rhodium compound can be applied to preparation of a drug for treating cancer and resisting bacteria. The structural formula of the organic metal rhodium compound is shown in the specification.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com