Catalyst for catalyzing formaldehyde or derivative thereof to prepare hydrogen, and synthesis method and application thereof
A synthesis method and technology of derivatives, which can be used in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. poisoning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The embodiment of the present invention also provides a synthesis method of the aforementioned catalyst that catalyzes the production of hydrogen from formaldehyde or its derivatives, which includes:
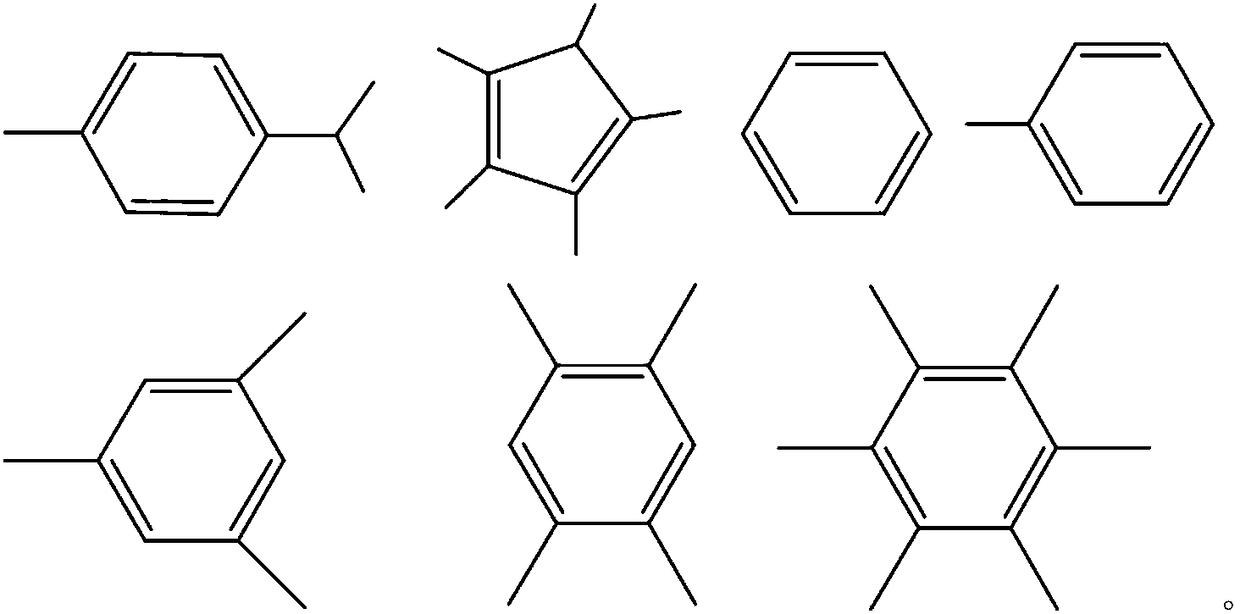
[0034] Provides [Ru(Y)] n Z m , wherein Y includes 4-methylisopropylphenyl, pentamethylcyclopentadienyl, phenyl, tolyl, trimethylphenyl, 1,2,4,5-tetramethylphenyl, hexamethylphenyl Any one, but not limited thereto, Z includes and is not limited to any one or a combination of two or more of I, Cl, Br, n and m are natural numbers, and the value range of n and m is 1-10;
[0035] The [Ru(Y)] n Z m Dispersed in water to form a suspension, the temperature of the suspension is controlled between 0 and 100°C, preferably 60°C, then silver salt is added, and the water is removed after the reaction to obtain Ru(Y)(H 2 O) n x m .
[0036]In some embodiments, the synthesis method further comprises: combining the Ru(Y)(H 2 O) n x m Add water, then add ammonia water, remove w...
Embodiment 1
[0057] 1. the method for preparing described catalyst in the present embodiment is:
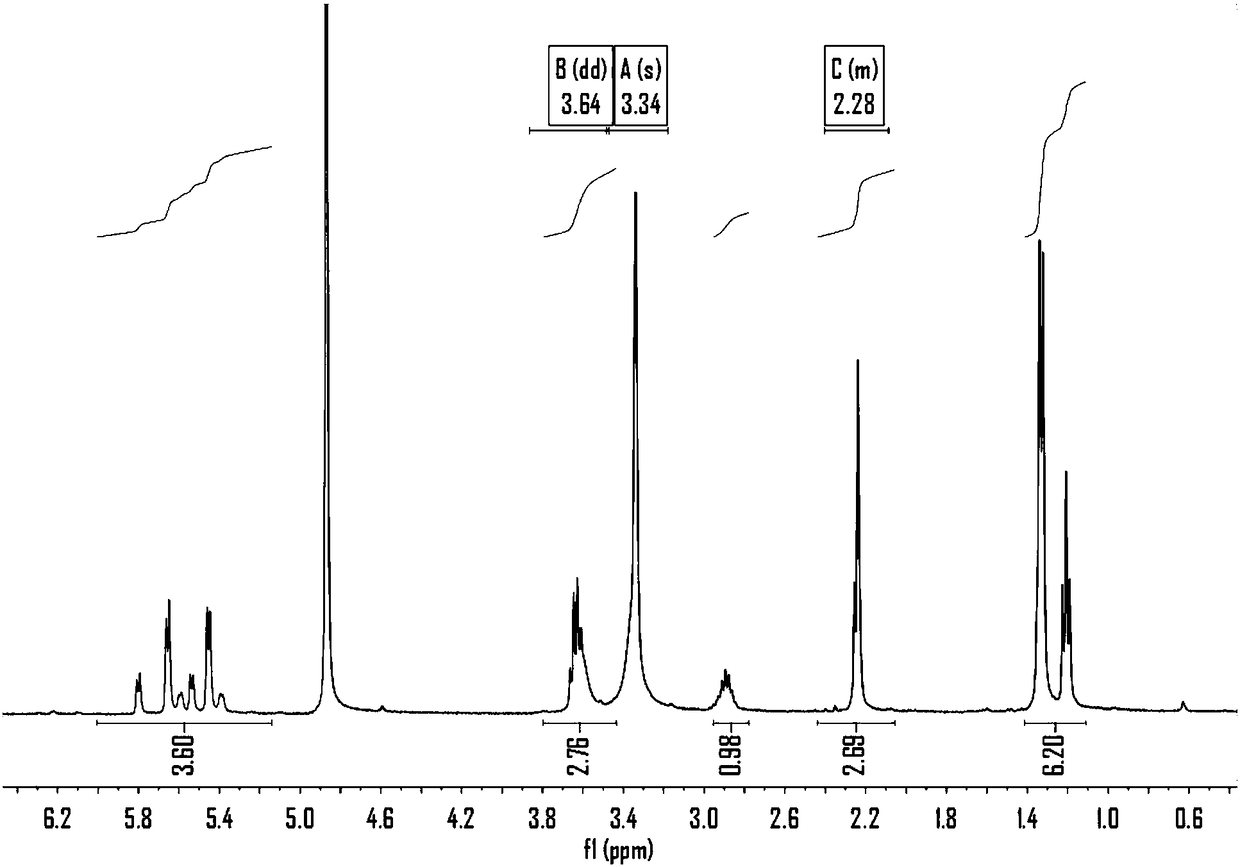
[0058] 1) Put [Ru(Y)] n Z m (wherein Y is 4-methylisopropylphenyl, Z is Cl, n is 2, and m is 4), add in water, make suspension after stirring, be heated to 60 ℃, then add 2 equivalents of silver salt ( It can be silver nitrate, silver sulfate, silver phosphate, silver tetrafluoroborate, silver hexafluorophosphate) reaction, during [Ru(Y)] n Z m Will gradually dissolve, while forming a white silver chloride precipitate. Then the solvent water was removed by a rotary evaporator, and a series of water-soluble catalysts Ru(Y)(H 2 O) n x m , where Y is 4-methylisopropylphenyl, X can be PO 4 , NO 3 , BF 4 , SO 4 、PF 6 Any one of them, n and m are natural numbers with a value range of 1-9 (the values of n and m vary according to X).
[0059] 2) The aforementioned series of Ru(Y)(H 2 O) n x m Add water, stir to make a solution, slowly add ammonia solution dropwise to the solution, an...
Embodiment 2- Embodiment 7
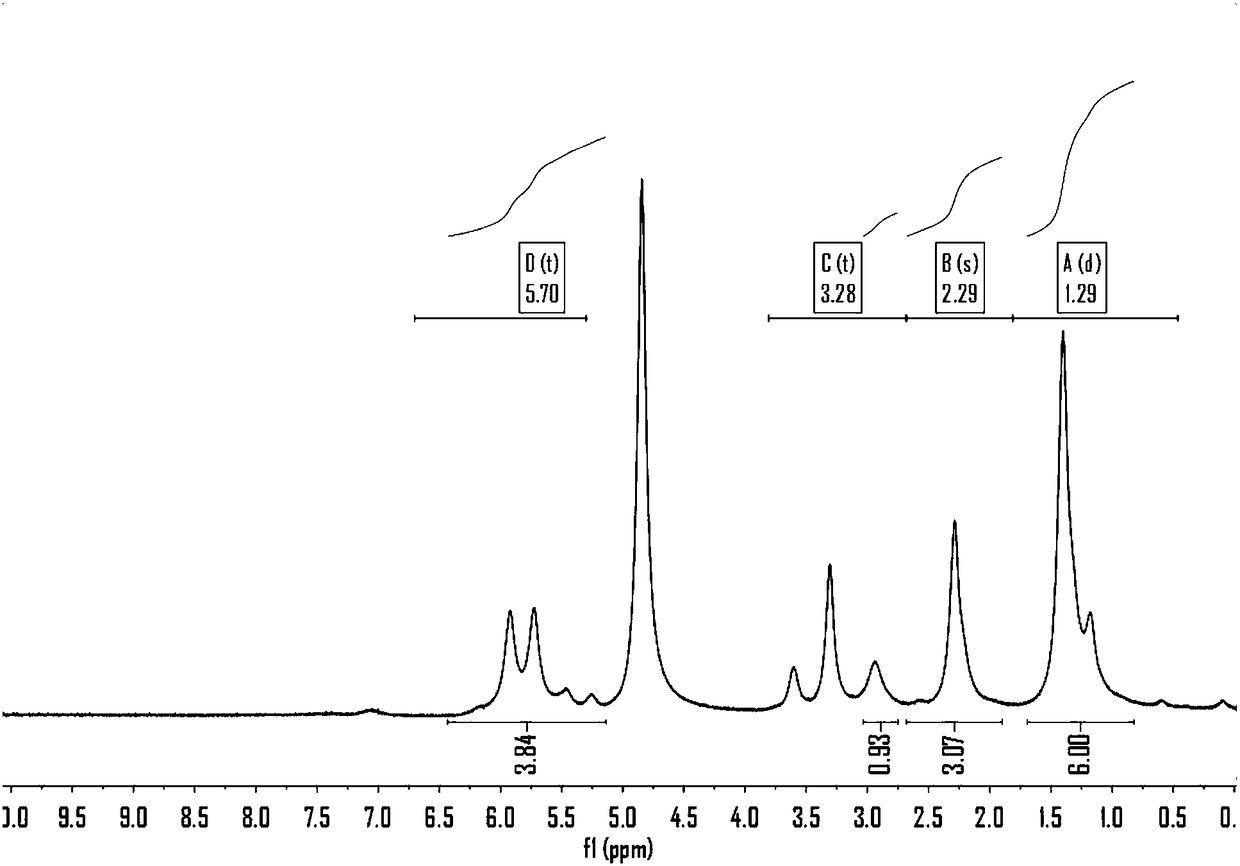
[0066] The preparation method of catalyst in this embodiment 2-7 is basically the same as embodiment 1, and difference is the raw material [Ru(Y)] that adopts n Z m Among them, Y is respectively pentamethylcyclopentadienyl, phenyl, tolyl, trimethylphenyl, 1,2,4,5-tetramethylphenyl, hexamethylphenyl, Z is any of I, Cl, Br one. This embodiment 2-7 also made a series of catalysts Ru(Y)(NH 3 ) n x m 、Ru(Y)(H 2 O) n x m , where n and m are natural numbers with a value range of 1-9, and the values of n and m vary depending on X. Similarly, when these catalysts are used to catalyze formaldehyde, paraformaldehyde and polyoxymethylene to react directly with water to produce hydrogen, it can be found that the molar ratios of hydrogen and carbon dioxide in the gas products produced are basically in the 2:1 or so, the carbon monoxide content is lower than 10ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com