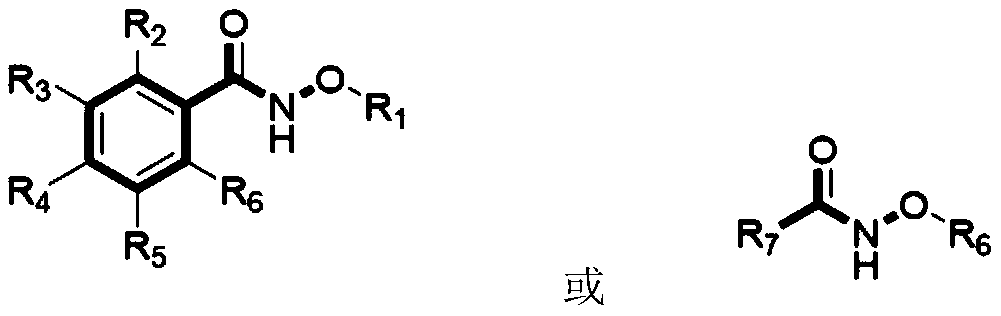
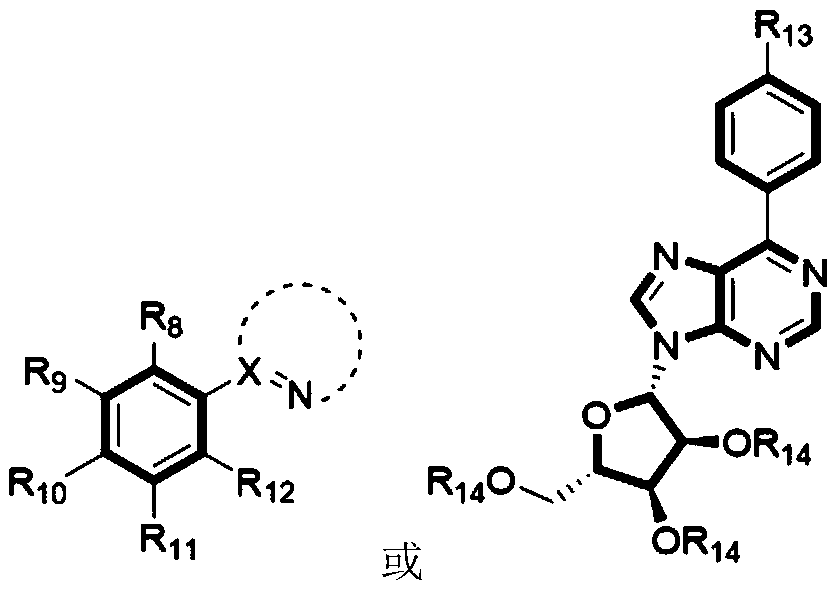
Practical method for synthesizing novel bioactive molecule with N-methoxy-amide as nitrogen source
A bioactive molecule, methoxyamide technology, applied in the field of synthesizing new bioactive molecules, can solve the problems of dangerous azide nitrogen sources, cumbersome synthesis routes, inconvenient storage, etc., and achieves reaction conditions that are simple, easy to operate, abundant The effect of reducing structure and by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
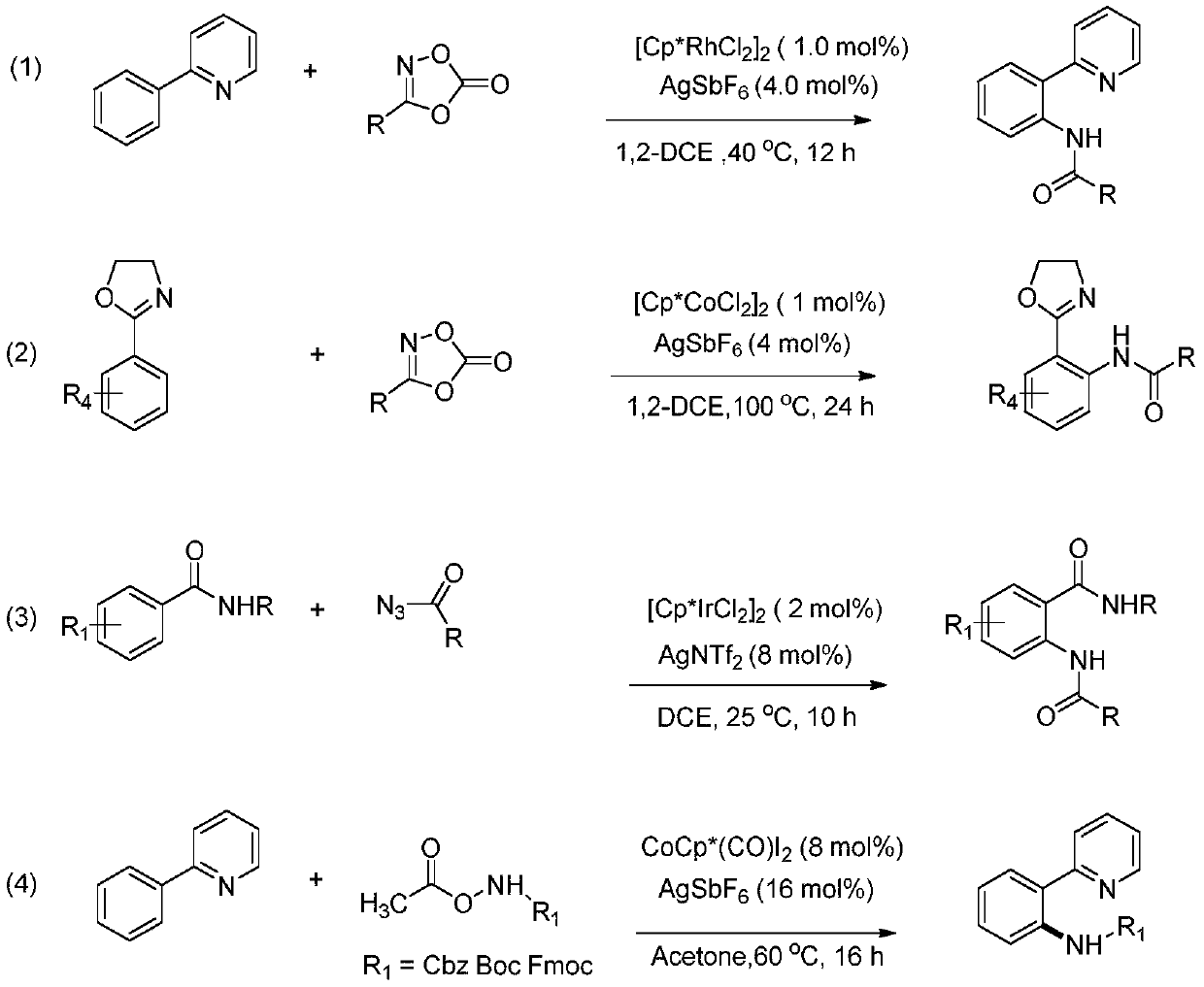
[0046] This implementation case shows a practical method for synthesizing new biologically active molecules using N-methoxyamide as a nitrogen source: using 3-methyl-2-phenylpyridine and N-methoxybenzamide as raw materials , The reaction formula is as follows:
[0047]
[0048] (1) Add 0.0338 g (0.2 mmol) of 3-methyl-2-phenylpyridine, 0.0040 g (0.005 mmol) of dichloro (pentamethylcyclopentadienyl) iridium (III) dimer, and six 0.0069 g (0.02 mmol) of silver fluoroantimonate, 0.045 g (0.3 mmol) of N-methoxybenzamide, and 1.00 mL of 1,2-dichloroethane, reacted at 120°C for 12 hours in an air atmosphere;
[0049] (2) TLC follows the reaction until it is completely over;
[0050] (3) The crude product obtained after the reaction is separated by column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain the target product (yield 96%).
[0051] 1 H NMR(400MHz, CDCl 3 )δ13.45(s,1H),8.94-8.63(m,1H),8.44(s,1H),8.05(dd,J=7.6,1.8Hz,2H),7.69-7.56(m,3H),7.55 –7.47(m,3H),7.47–7.40(m,1...
Embodiment 2
[0053] This example shows a practical method for synthesizing new biologically active molecules using N-methoxyamide as a nitrogen source according to the following steps: nitrogen heterocyclic compounds substituted with aryl groups and 2-chloro-4-methylsulfonyl-N- Methoxybenzamide is the raw material, and the reaction formula is as follows:
[0054]
[0055] (1) Add 0.0379 g (0.2 mmol) of 2-(2-chlorophenyl) pyridine, 0.0040 g (0.005 mmol) of dichloro (pentamethylcyclopentadienyl) iridium (III) dimer, and six 0.0069 g (0.02mmol) of silver fluoroantimonate, 0.0789 g (0.3mmol) of 2-chloro-4-methylsulfonyl-N-methoxybenzamide and 1.00mL of 1,2-dichloroethane, in an air atmosphere React at 120°C for 12 hours;
[0056] ⑵ TLC follows the reaction until it is completely over;
[0057] (3) The crude product obtained after the reaction is separated by column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain the target product (yield 70%).
[0058] 1 H NMR(400MHz, CDCl 3 )δ9.9...
Embodiment 3
[0060] This example shows a practical method for the synthesis of new bioactive molecules using N-methoxyamide as a nitrogen source according to the following steps: 2-phenylpyridine and N-(benzyloxy)undec-10-ene Amide is a raw material, and its reaction formula is as follows:
[0061]
[0062] (1) Add 0.0310 g (0.2 mmol) of 2-phenylpyridine, 0.0040 g (0.005 mmol) of dichloro (pentamethylcyclopentadienyl) iridium (III) dimer, and silver hexafluoroantimonate 0.0069 in the reaction tube G (0.02 mmol), 0.0867 g (0.3 mmol) of N-(benzyloxy)undec-10-enamide, and 1.00 mL of 1,2-dichloroethane, reacted at 120°C for 12 hours in an air atmosphere ;
[0063] ⑵ TLC follows the reaction until it is completely over;
[0064] (3) The crude product obtained after the reaction is separated by column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain the target product (yield 48%).
[0065] 1 H NMR(400MHz, CDCl 3 )δ12.09(s,1H),8.64-8.63(m,1H),8.56(d,J=8.2Hz,1H),7.84(td,J=7.9,1.8Hz,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com