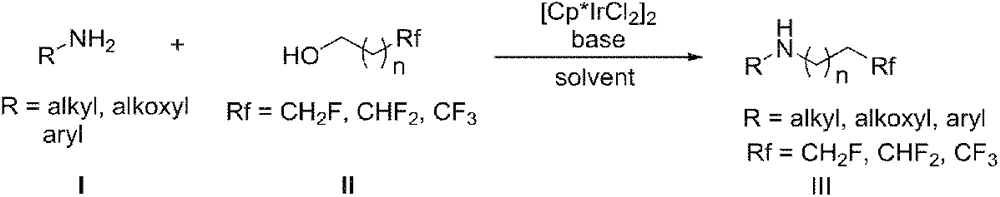
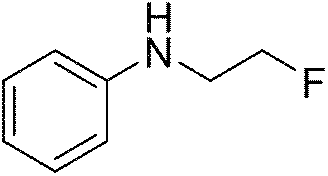
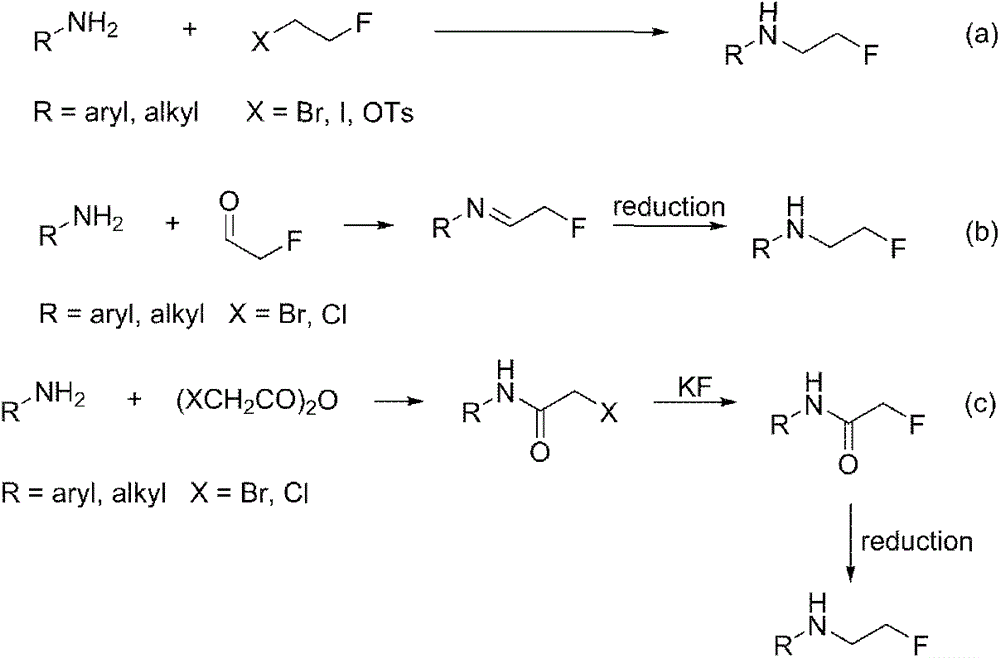Method for preparing fluorine-containing secondary amine
A technology for fluorine-containing secondary amines and primary amines, applied in the field of preparation of fluorine-containing secondary amines, to achieve the effects of easy access, low toxicity, high atom economy and step economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Under nitrogen protection, sequentially add [Cp*IrCl 2 ] 2 0.006mmol, aniline 0.3mmol, fluoroethanol 0.33mmol, sodium bicarbonate 0.006mmol and toluene 0.5mL, heated to 100°C and reacted for 24h. After the reaction, cool down to room temperature, add a small amount of water, extract three times with 15 mL of ethyl acetate, combine the organic phases, then wash with saturated brine, dry over anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and separate the secondary amine by column chromatography. 76%
Embodiment 2
[0023]
[0024] Under nitrogen protection, sequentially add [Cp*IrCl 2 ] 2 0.006mmol, 0.3mmol of aniline, 0.33mmol of fluoroethanol, 0.03mmol of triethylamine and 0.5mL of toluene, heated to 100°C and reacted for 24h. After the reaction, cool down to room temperature, add a small amount of water, extract three times with 15 mL of ethyl acetate, combine the organic phases, then wash with saturated brine, dry over anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and separate the secondary amine by column chromatography. 75%.
Embodiment 3
[0026]
[0027] Under nitrogen protection, sequentially add [Cp*IrCl 2 ] 2 0.006mmol, aniline 0.3mmol, fluoroethanol 0.33mmol, sodium bicarbonate 0.006mmol and dioxane 0.5mL, heated to 100°C and reacted for 24h. After the reaction, cool down to room temperature, add a small amount of water, extract three times with 15 mL of ethyl acetate, combine the organic phases, then wash with saturated brine, dry over anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and separate the secondary amine by column chromatography. 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com