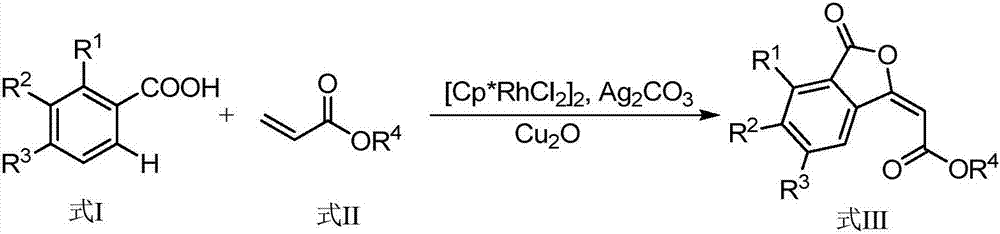
Synthesis method of (E)-3-subunit substituted phthalide compounds
A synthetic method and compound technology, applied in the -3- field, can solve the problems of toxicity, high reaction temperature, and unavailable raw materials, and achieve the effects of less catalyst consumption, simple reaction raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
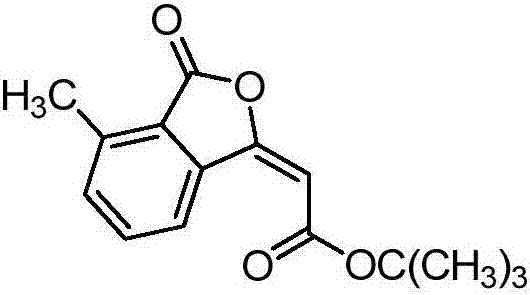
[0013] Preparation of (E)-tert-butyl-2-(4-methyl-3-oxoisobenzofuran-1(3H)-ylidene)acetate of the formula
[0014]
[0015] Add 13.6mg (0.1mmol) o-toluic acid, 14.5μL (0.1mmol) tert-butyl acrylate, 2.3mg (0.00375mmol) pentamethylcyclopentadienyl rhodium dichloride, 41mg (0.15mmol) of silver carbonate, 1.4mg (0.01mmol) of cuprous oxide, and 0.5mL of toluene were stirred and reacted at 105°C for 1.5 hours in a closed system, cooled to room temperature after the reaction, and filtered through a column chromatography silica gel column to remove Salt and catalyst, separated by TLC to give (E)-tert-butyl-2-(4-methyl-3-oxoisobenzofuran-1(3H)-ylidene)acetate, which The rate is 71%, and the structural characterization data are as follows:
[0016] 1 H NMR (400MHz, CDCl 3 ):δ(ppm)=8.86(d,J=7.9Hz,1H),7.65(t,J=7.8Hz,1H),7.44(d,J=7.5Hz,1H),6.07(s,1H), 3.81(s,3H),2.69(s,3H);
[0017] 13 C NMR (100MHz, CDCl 3 ): δ (ppm) = 166.0, 165.7, 157.8, 139.6, 136.3, 134.9, 134.4, 125.63, 123....
Embodiment 2
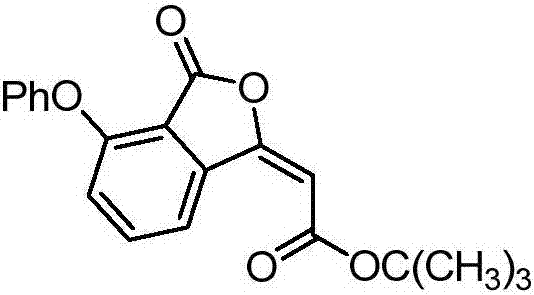
[0020] Preparation of (E)-tert-butyl-2-(4-phenoxy-3-oxoisobenzofuran-1(3H)-ylidene)acetate of the following structural formula
[0021]
[0022] In Example 1, the o-toluic acid used was replaced with equimolar 2-phenoxybenzoic acid, and the other steps were the same as in Example 1 to obtain (E)-tert-butyl-2-(4-phenoxy Base-3-oxoisobenzofuran-1(3H)-ylidene) acetate, its yield is 75%, and the structural characterization data are as follows:
[0023] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) = 8.64 (d, J = 7.8Hz, 1H), 7.56 (t, J = 8.1Hz, 1H), 7.35 (t, J = 7.6Hz, 2H), 7.19-7.16 (m, 1H ), 7.06(d, J=7.8Hz, 2H), 6.86(s, 1H), 6.02(s, 1H), 1.48(s, 9H);
[0024] 13 C NMR (100MHz, CDCl 3 ): δ (ppm) = 164.8, 163.2, 156.6, 156.4, 154.7, 138.3, 136.9, 130.1, 125.3, 121.9, 120.6, 119.5, 114.7, 104.6, 81.5, 28.2;
[0025] HRMS(ESI)m / z:C 20 h 18 o 5 ,[M+Na] + , The theoretical value is 361.1052; the measured value is 361.1054.
Embodiment 3
[0027] Preparation of (E)-tert-butyl-2-(4-ethyl-3-oxoisobenzofuran-1(3H)-ylidene)acetate of the following structural formula
[0028]
[0029] In Example 1, the o-toluic acid used is replaced with equimolar 2-ethylbenzoic acid, and other steps are the same as in Example 1 to obtain (E)-tert-butyl-2-(4-ethyl- 3-oxoisobenzofuran-1(3H)-ylidene) acetate, its yield is 74%, and the structural characterization data are as follows:
[0030] 1 H NMR (400MHz, CDCl 3 ):δ(ppm)=8.87(d,J=7.9Hz,1H),7.69(t,J=7.8Hz,1H),7.47(d,J=7.6Hz,1H),6.03(s,1H), 3.13(q, J=7.5Hz, 2H), 1.55(s, 9H), 1.29(t, J=7.6Hz, 3H);
[0031] 13 C NMR (100MHz, CDCl 3 ): δ (ppm) = 165.8, 165.0, 156.9, 145.8, 136.8, 135.1, 132.5, 125.7, 123.2, 103.7, 81.3, 28.2, 24.3, 14.8;
[0032] HRMS(ESI)m / z:C 16 h 18 o 4 ,[M+Na] + , The theoretical value is 297.1103; the measured value is 297.1104.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com