Method for converting alkyne into alcohol
An alkyne and acetylene technology, which is applied in the field of formic acid to promote the conversion of alkynes to alcohols, can solve the problems of flammable and explosive handling of hydrogen, harsh reaction conditions, long reaction time, etc., and achieves low production cost, mild reaction conditions and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
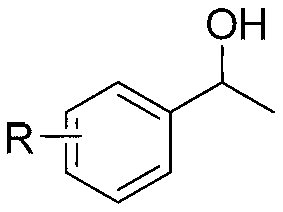
[0020] Taking the preparation of phenylethyl alcohol as an example, its structural formula is shown in formula I, where R is H, and the raw materials used and the preparation method are as follows:
[0021]
[0022] Under the protection of argon, add 306mg (3.0mmol) phenylacetylene and 3.5mL formic acid into the thick-walled pressure-resistant tube, the molar ratio of phenylacetylene and formic acid is 1:30, stir, react at 100°C for 0.5 hours, cool to room temperature, Use saturated sodium formate aqueous solution to adjust the pH value to 3~4, add 1.85 mg of (pentamethylcyclopentadiene) chloride-(5-methoxy-2-{1-[(4-methoxyphenyl) Amine iridium, (pentamethylcyclopentadiene)-(5-methoxy-2-{1-[(4-methoxyphenyl)imide) molar ratio of iridium to phenylacetylene is 1:1000 , react at 80°C for 6 hours, add methanol 8 times its volume to the reaction solution, adjust to alkalinity with potassium hydroxide, hydrolyze for 30 minutes, extract with ethyl acetate, dry the organic phase wi...
Embodiment 2
[0025] Taking the preparation of 1-(4-methylphenyl)ethanol as an example, its structural formula is shown in formula I, where R is a methyl group, and the raw materials used and the preparation method are as follows:
[0026] Under argon protection, add 348mg (3.0mmol) p-methylphenylacetylene and 3.5mL formic acid into a thick-walled pressure-resistant tube, the molar ratio of p-methylphenylacetylene and formic acid is 1:30, stir, and react at 100°C for 3 hours, cooled to room temperature, adjusted the pH value to 3-4 with saturated aqueous sodium formate solution, added 3.7 mg of (pentamethylcyclopentadiene) chloride-(5-methoxy-2-{1-[(4-methylcyclopentadiene) Oxyphenyl)imide iridium, (pentamethylcyclopentadiene)-(5-methoxy-2-{1-[(4-methoxyphenyl)imide iridium chloride and p-methyl The mol ratio of phenylacetylene is 1:500, 80 ℃ of reactions 6 hours, other steps are identical with embodiment 1, are prepared into 1-(4-methylphenyl) ethanol, and its productive rate is 85%, and t...
Embodiment 3
[0028] Taking the preparation of 1-(4-bromophenyl)ethanol as an example, its structural formula is shown in formula I, where R is Br, and the raw materials used and the preparation method are as follows:
[0029] Under the protection of argon, add 543mg (3.0mmol) p-bromophenylacetylene and 3.5mL formic acid into the thick-walled pressure-resistant tube, the molar ratio of p-bromophenylacetylene and formic acid is 1:30, stir, and react at 100°C for 5 hours. Cool to room temperature, adjust the pH value to 3-4 with saturated aqueous sodium formate solution, add 3.7 mg of (pentamethylcyclopentadiene)-(5-methoxy-2-{1-[(4-methoxy Phenyl)imide iridium, (pentamethylcyclopentadiene)-(5-methoxy-2-{1-[(4-methoxyphenyl)imide iridium chloride and p-bromophenylacetylene Molar ratio is 1:500, 80 ℃ of reaction 6 hours, other steps are identical with embodiment 1, are prepared into 1-(4-bromophenyl) ethanol, and its productive rate is 86%, and the spectral data of product is: 1 H NMR (CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com