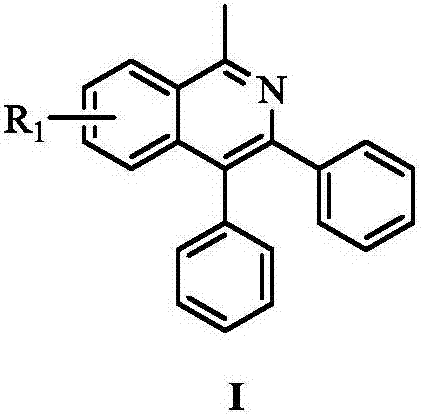
Novel polysubstitution isoquinoline derivative and synthesis method thereof
A synthetic method and isoquinoline technology, applied in the direction of drug combination, antipyretics, antineoplastic drugs, etc., can solve the problems of harsh reaction conditions, large amount of catalysts, expensive raw materials, etc., and achieve less side reactions and high reactivity , The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
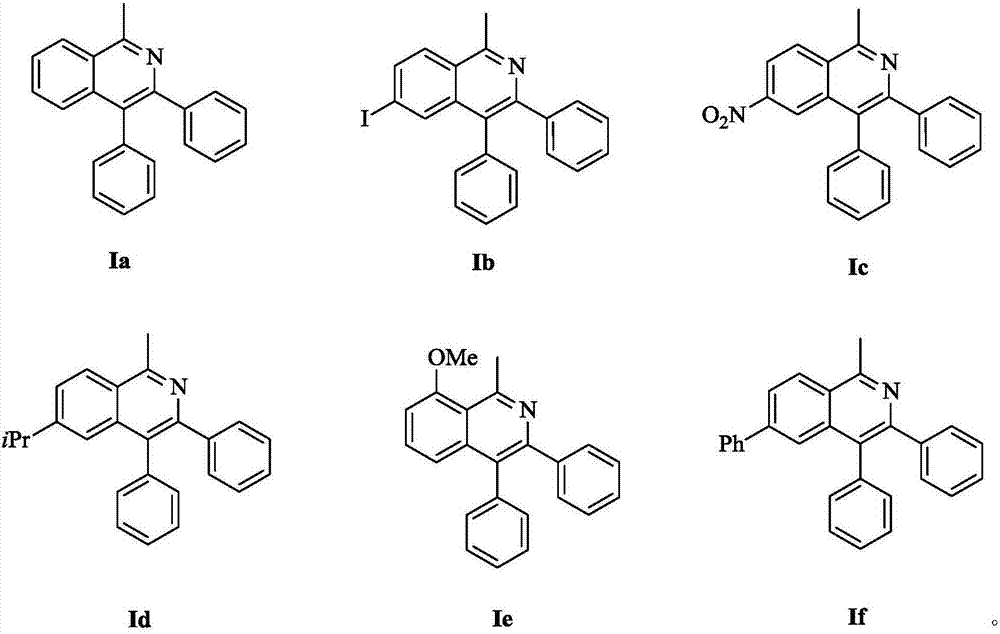
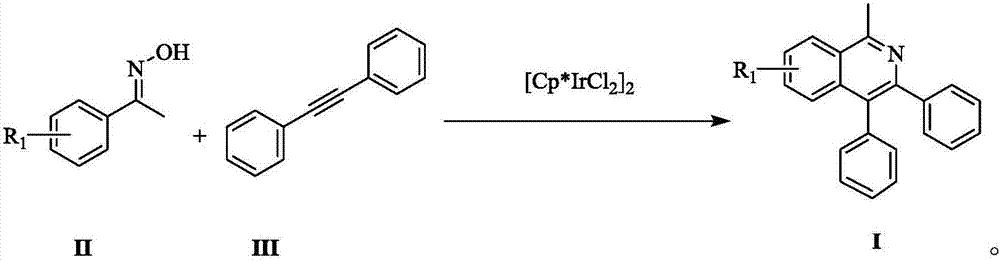
[0021] Add acetophenone oxime (0.1mmol), tolan (0.12mmol) in the reaction tube, then add pivalic acid (0.3 equivalents) and pentamethylcyclopentadiene iridium dichloride catalyst (0.025 equivalents), and then Add 1 mL of methanol, and stir the mixture at 60°C for 24 hours. After the reaction, cool the reaction system to room temperature. Suction filtration is performed after the solid precipitates, and the filtrate is rotary evaporated and dried to obtain a crude product. The crude product is separated and purified by column chromatography to obtain the target product Product 1-methyl-3,4-diphenylisoquinoline (Ia): The yield is 88%.
[0022] 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.28-8.25(m,1H,ArH),7.74-7.72(m,1H,ArH),7.66-7.64(m,2H,ArH),7.45-7.39(m,5H,ArH), 7.31-7.24(m,5H,ArH),3.14(s,3H,CH 3 );
[0023] 13 C NMR (100MHz, CDCl 3 )δ (ppm): 157.9, 149.6, 147.6, 141.1, 137.7, 136.1, 131.5, 130.4, 130.0, 129.3, 128.3, 127.7, 127.2, 127.1, 126.7, 126.3, 125.7, 122.9;
[0024] HRMS...
Embodiment 2
[0026] According to the method of Example 1, 4-iodo-acetophenone oxime, with pivalic acid (0.3 equivalents) and pentamethylcyclopentadiene iridium dichloride (0.025 equivalents) as catalyst, add methanol 1mL, at 60 ℃ and stirred for 24 hours. After the reaction, the reaction system was cooled to room temperature. Suction filtration was performed after the solid was precipitated. The filtrate was rotary evaporated and dried to obtain a crude product. The crude product was separated and purified by column chromatography to obtain the target product 6-iodo-1- Methyl-3,4-diphenylisoquinoline (Ib): Yield 78%.
[0027]1 H NMR (400MHz, CDCl 3 )δ(ppm):8.04-8.03(m,1H,ArH),7.92-7.84(m,2H,ArH),7.40-7.32(m,5H,ArH),7.21-7.18(m,5H,ArH), 3.04(s,1H,CH 3 );
[0028] 13 C NMR (100MHz, CDCl 3 )δ (ppm): 158.0, 150.6, 140.8, 137.7, 136.9, 135.5, 135.2, 131.5, 130.3, 128.6, 128.1, 127.8, 127.6, 127.3, 127.1, 125.0, 197.8, 122.8;
[0029] HRMS: m / z, [M+H] + :422.04.
Embodiment 3
[0031] According to the method of Example 1, 4-nitroso-acetophenone oxime, with pivalic acid (0.3 equivalents) and pentamethylcyclopentadiene iridium dichloride (0.025 equivalents) as a catalyst, was added methanol 1mL, Stir the reaction at 60°C for 24 hours. After the reaction, cool the reaction system to room temperature. After the solid precipitates, perform suction filtration. The filtrate is rotary evaporated and dried to obtain a crude product. The crude product is separated and purified by column chromatography to obtain the target product 1-methyl -6-nitro-3,4-diphenylisoquinoline (Ic): Yield 92%.
[0032] 1 H NMR (400MHz, CDCl 3 )δ(ppm):8.60-8.59(m,1H,ArH),8.37-8.33(m,2H,ArH),7.42-7.37(m,5H,ArH),7.24-7.21(m,5H,ArH), 3.14(s,3H,CH 3 );
[0033] 13 C NMR (100MHz, CDCl 3 )δ (ppm): 158.2, 152.1, 151.8, 148.4, 140.1, 136.1, 136.0, 131.3, 130.6, 130.3, 128.9, 128.2, 128.0, 127.9, 127.7, 122.9, 120.0, 123.1;
[0034] HRMS: m / z, [M+H] + :341.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com