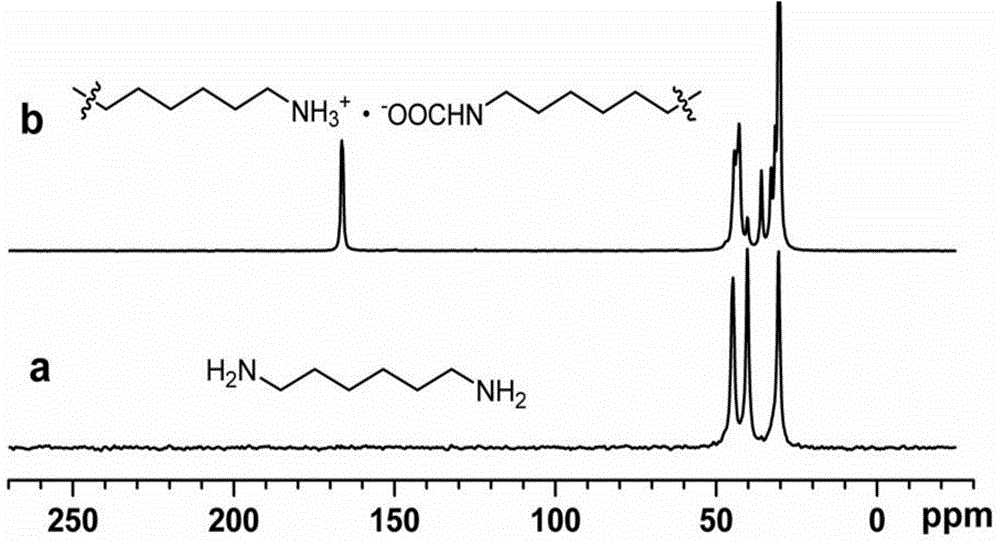
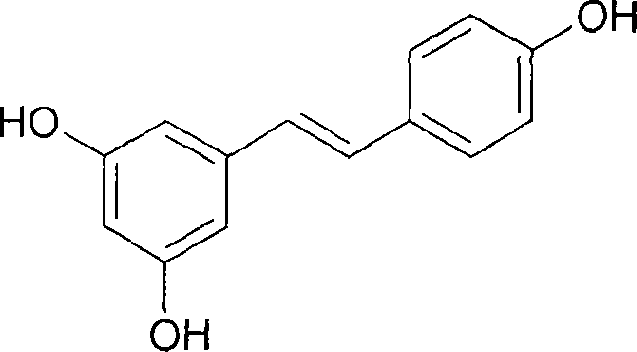
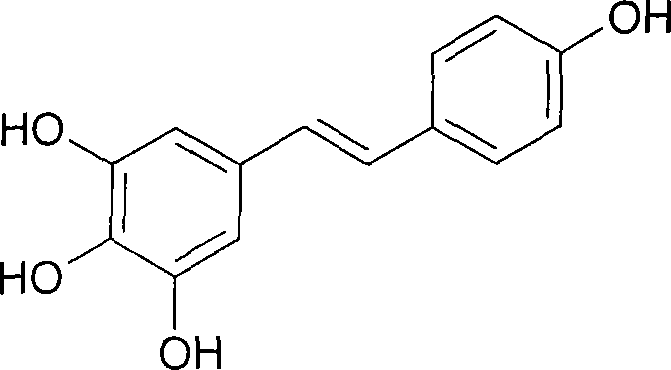
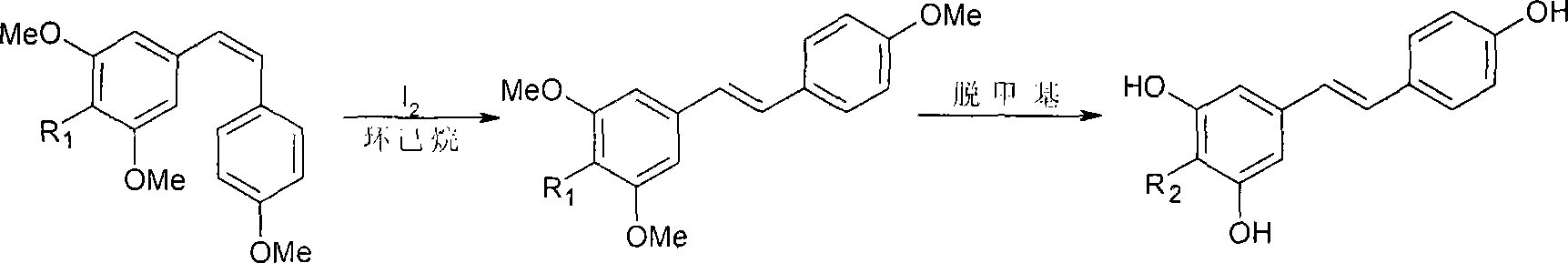
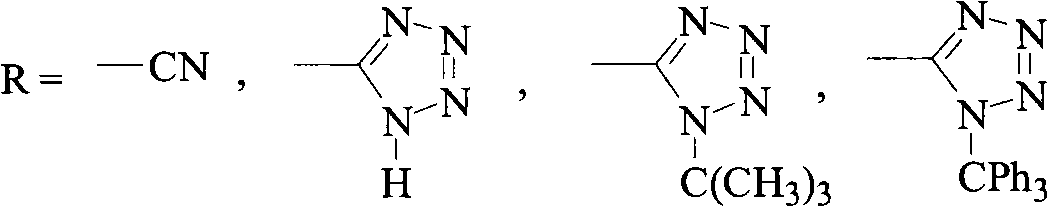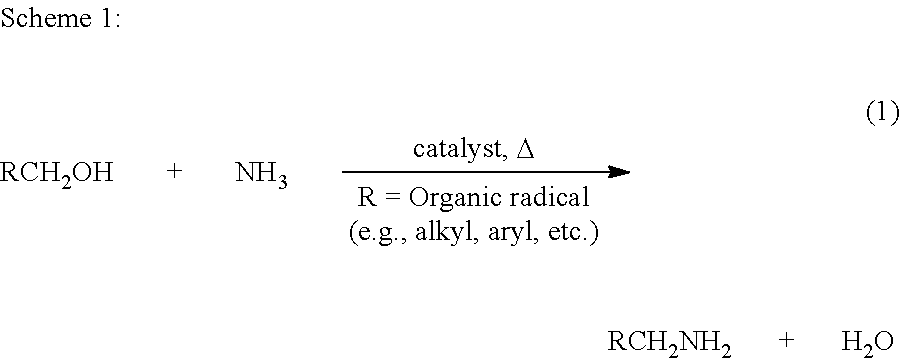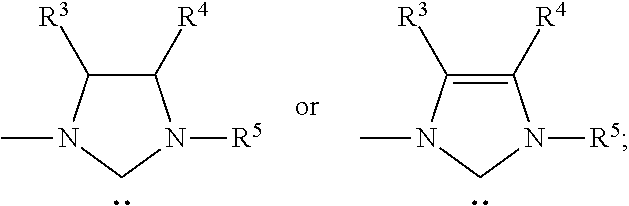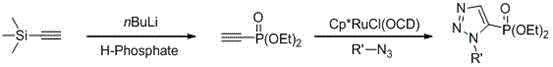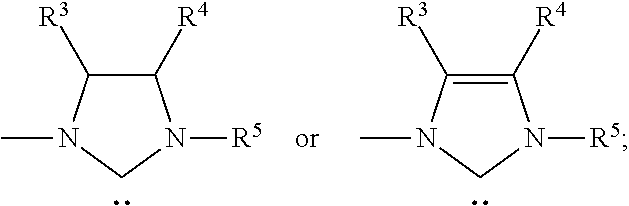Patents
Literature
444results about How to "Good atom economy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
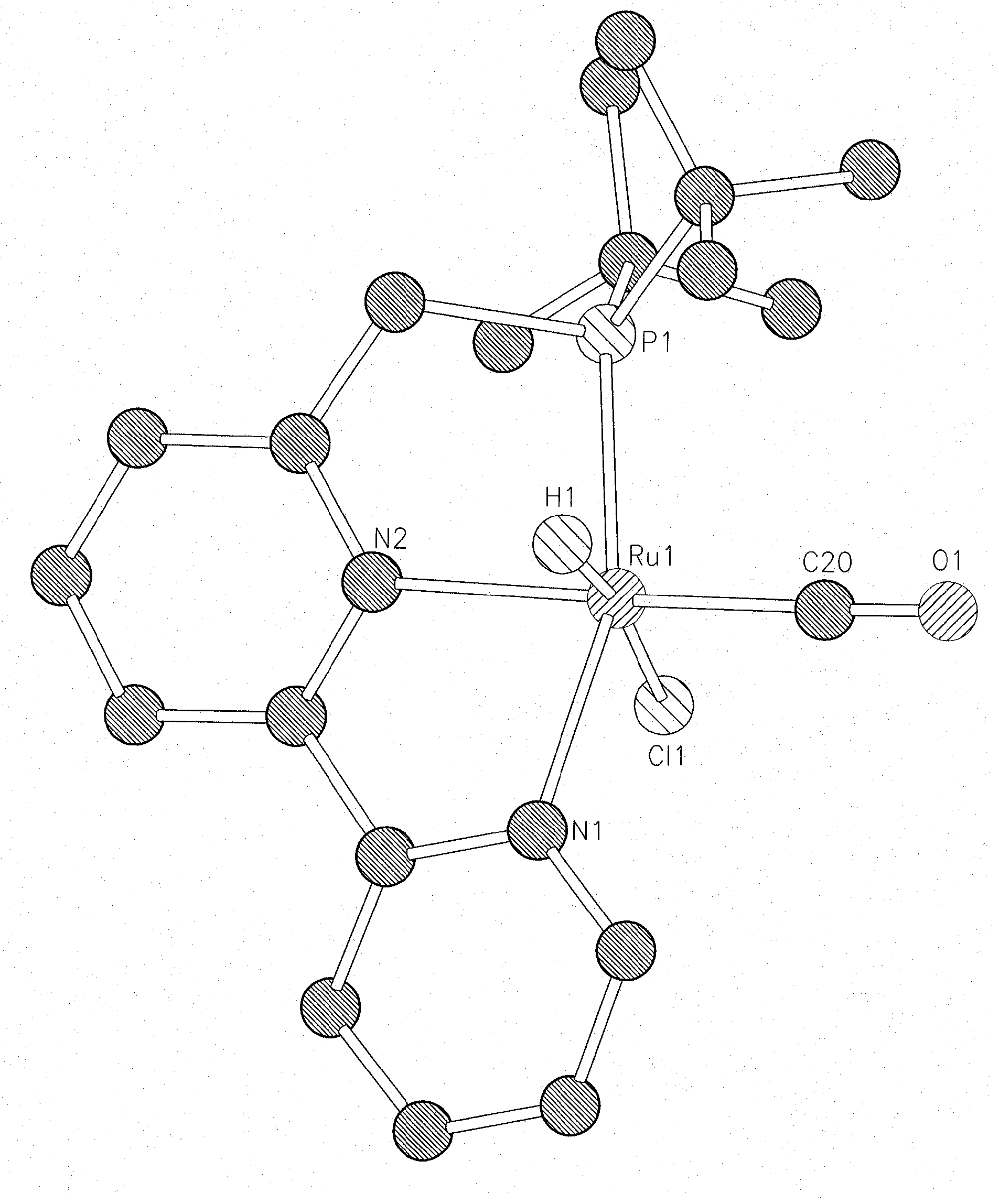
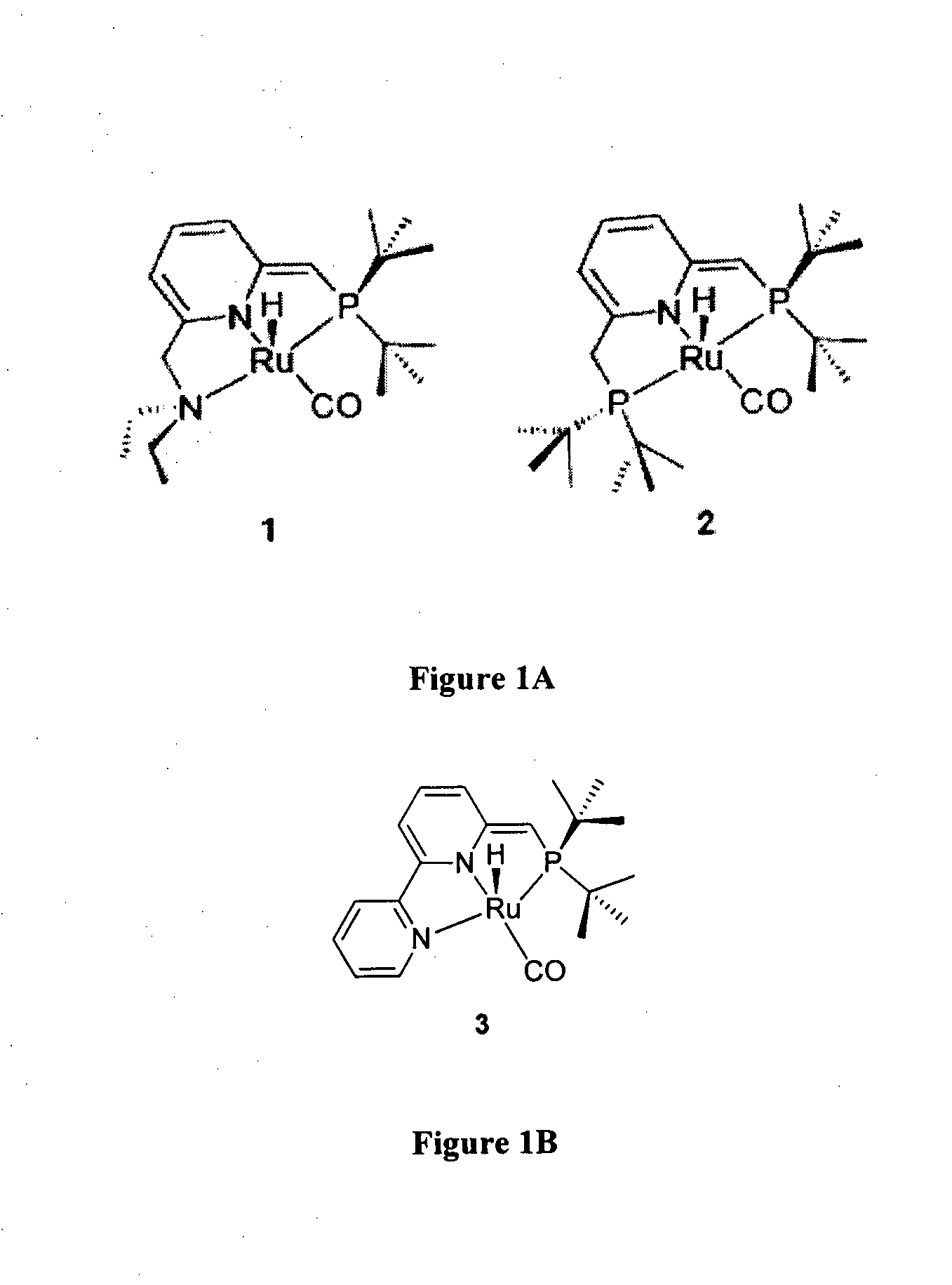
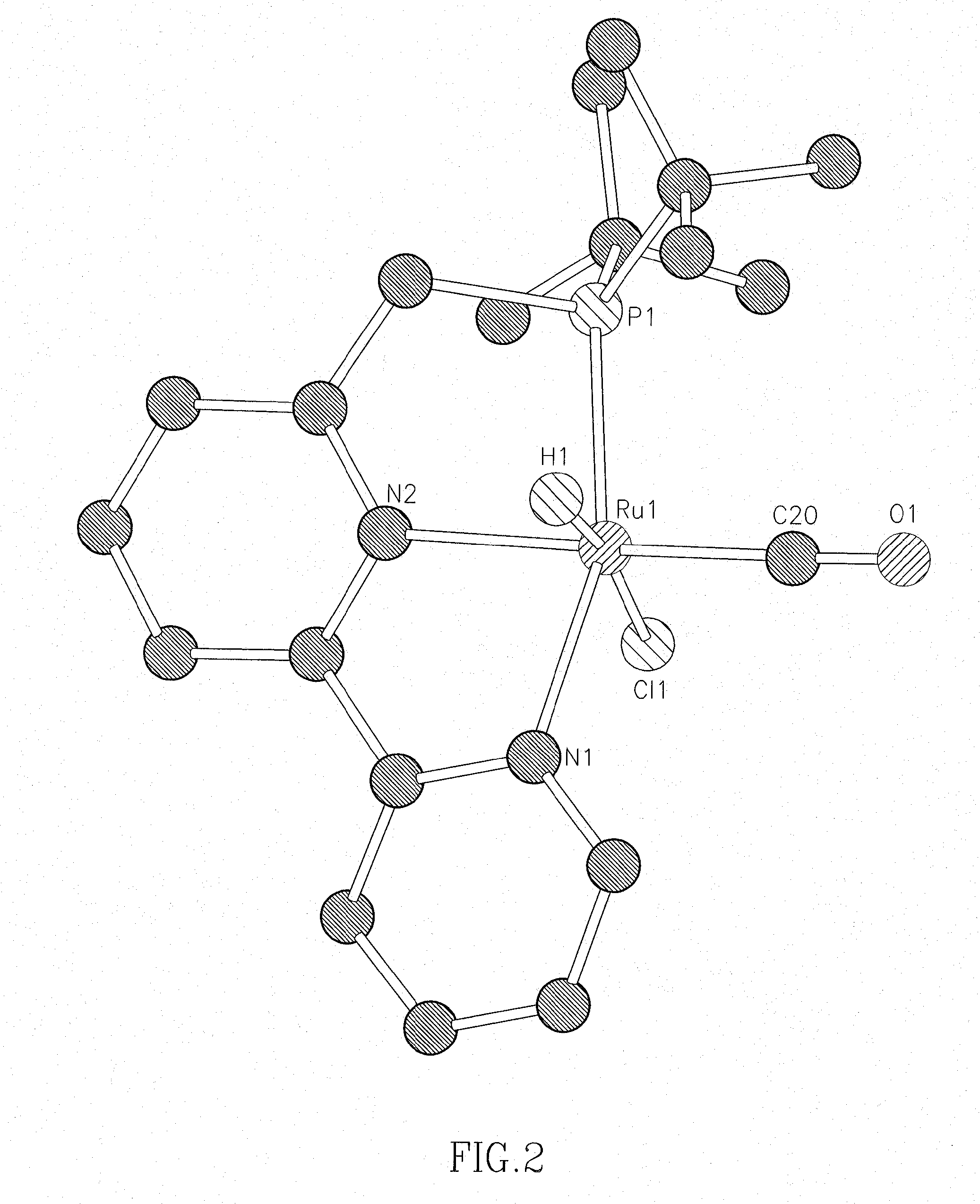
Novel ruthenium complexes and their uses in processes for formation and/or hydrogenation of esters, amides and derivatives thereof
ActiveUS20130281664A1High yieldImprove turnover ratePreparation from carboxylic acid saltsPlatinum group organic compoundsUrea derivativesPolyester
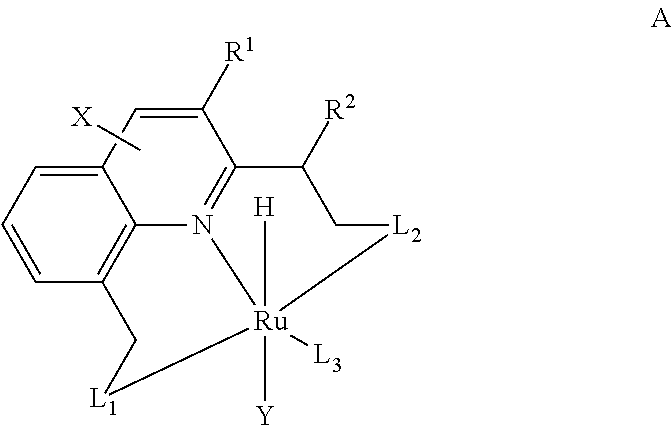
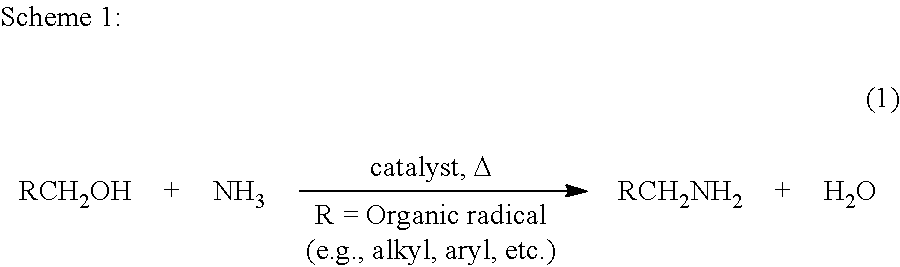
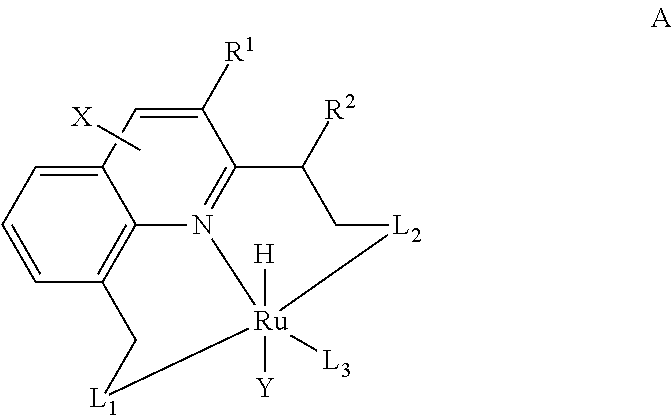
The present invention relates to novel Ruthenium catalysts and related borohydride complexes, and the use of such catalysts, inter alia, for (1) hydrogenation of amides (including polyamides) to alcohols and amines; (2) preparing amides from alcohols with amines (including the preparation of polyamides (e.g., polypeptides) by reacting dialcohols and diamines and / or by polymerization of amino alcohols); (3) hydrogenation of esters to alcohols (including hydrogenation of cyclic esters (lactones) or cyclic di-esters (di-lactones) or polyesters); (4) hydrogenation of organic carbonates (including polycarbonates) to alcohols and hydrogenation of carbamates (including polycarbamates) or urea derivatives to alcohols and amines; (5) dehydrogenative coupling of alcohols to esters; (6) hydrogenation of secondary alcohols to ketones; (7) amidation of esters (i.e., synthesis of amides from esters and amines); (8) acylation of alcohols using esters; (9) coupling of alcohols with water to form carboxylic acids; and (10) dehydrogenation of beta-amino alcohols to form pyrazines. The present invention further relates to the novel uses of certain pyridine Ruthenium catalysts.
Owner:YEDA RES & DEV CO LTD
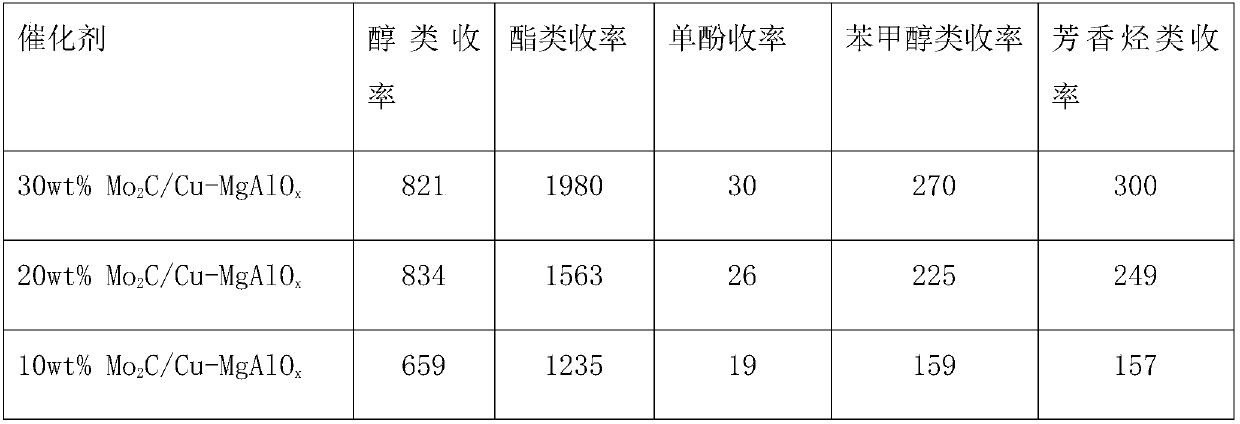
Application of molybdenum-based catalyst to prepare organic chemicals from lignin
The invention discloses a method for preparing organic chemicals from lignin. The method comprises: taking a catalyst employing a transition metal molybdenum as an active site to perform catalysis on reactions, heating to 230-350 DEG C, stirring to react for 0.5 h-12 h, after the reaction is finished, filtering out the solid catalyst, and performing rotary evaporation to obtain liquid products. The catalytic process of the technical scheme has extremely high product efficiency, the product total yield is up to 90%, the products comprise monophenols and other aromatic compounds used in large scale in industry, the added value is high, and the application has extremely good industrial application prospect.
Owner:TIANJIN UNIV
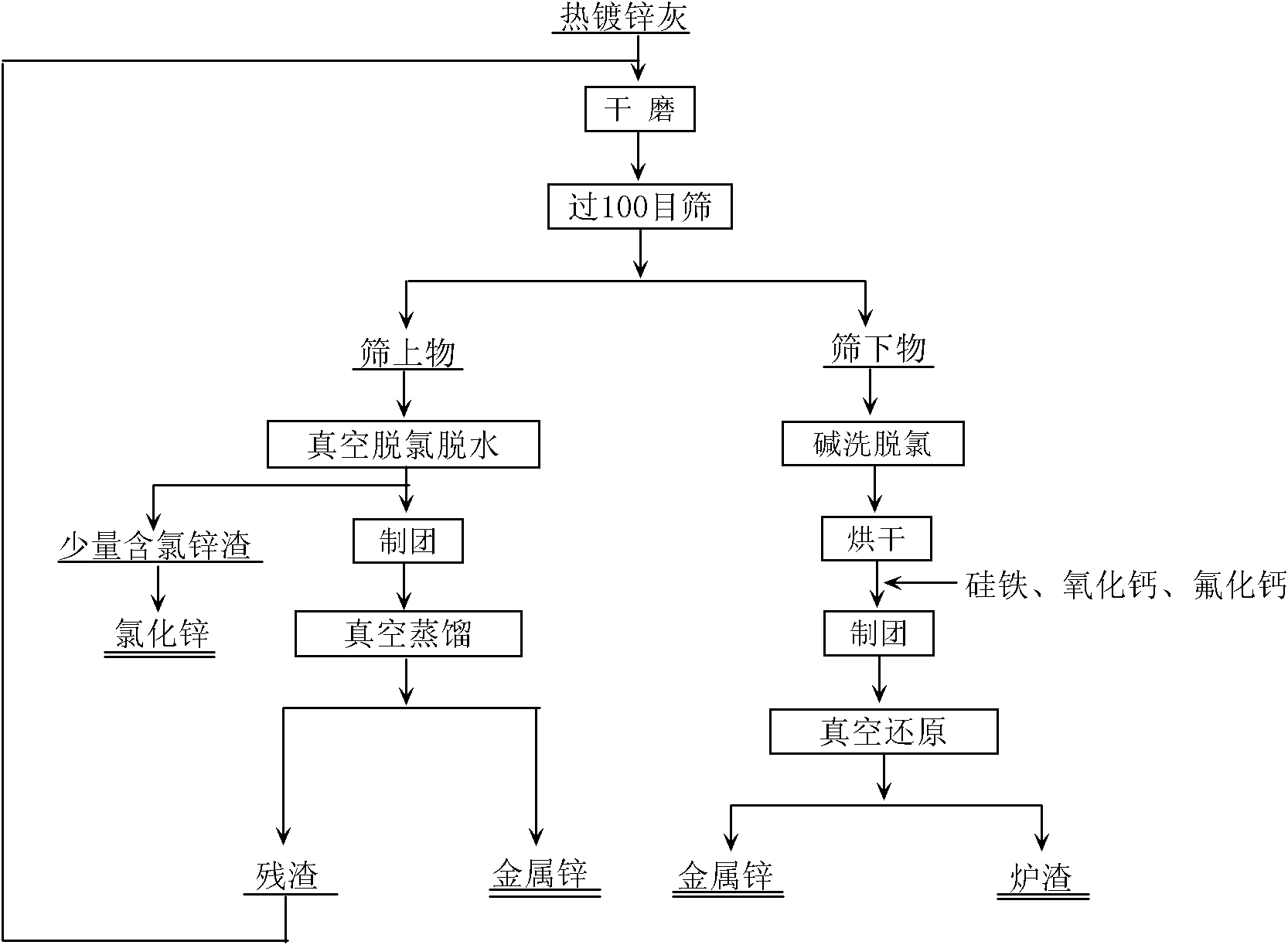
Process for recovering zinc from hot-dip coating zinc ash
InactiveCN101979684AReduce energy consumptionHigh purityProcess efficiency improvementBound waterFerrosilicon
The invention relates to a process for recovering zinc from hot-dip coating zinc ash. The process comprises the following steps of: separating the hot-dip coating zinc ash after dry grinding and screening to obtain an oversize product and an undersize product; removing bound water and a small amount of zinc chloride from the oversize product under the condition that the temperature is between 400 and 500 DEG C and the vacuum degree is 10 to 50 Pa; performing vacuum distillation on the oversize product under the condition that the temperature is between 650 and 800 DEG C and the vacuum degree is 10 to 30 Pa to obtain zinc; and performing alkali cleaning on the undersize product to remove chlorine, and then performing vacuum thermal reduction on the undersize product to obtain the zinc from zinc oxide by using ferrosilicon as a reducing agent and calcium oxide as a slagging agent under the condition that the vacuum degree is 10 to 30 Pa and the temperature is between 1,050 and 1,200 DEG C. The process has a high recovery rate for recovering the zinc from the hot-dip coating zinc ash, and the obtained zinc has a good crystallized shape.
Owner:CENT SOUTH UNIV
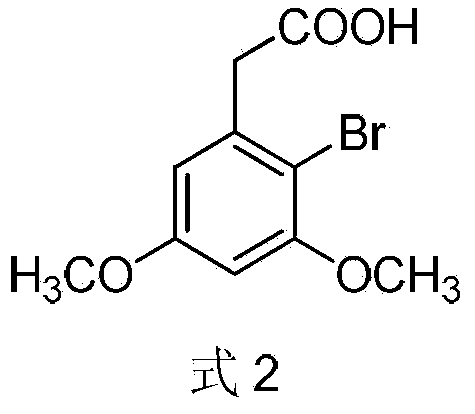
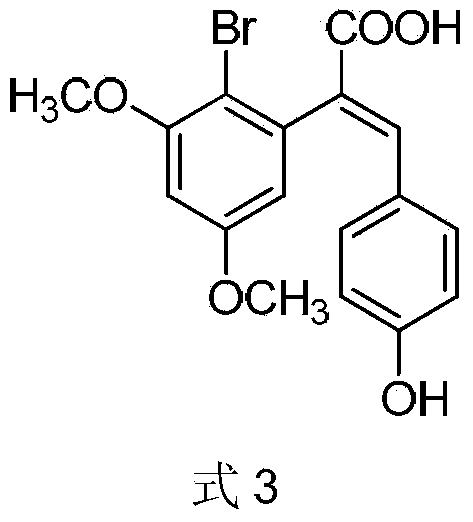
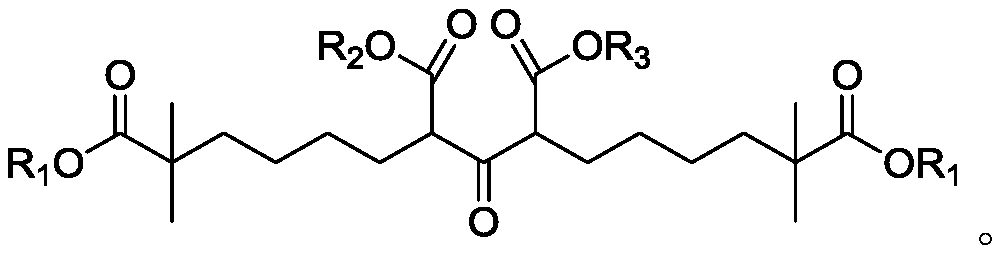
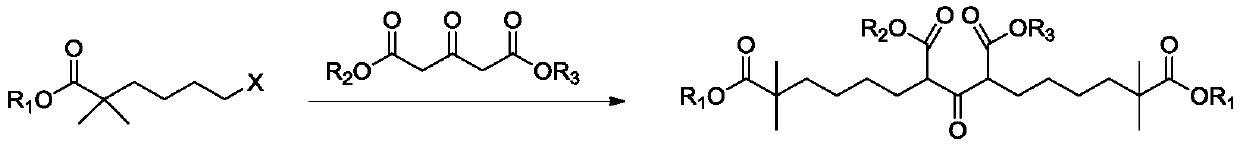
Compound and method for synthesizing 8-hydroxy-2,2,14,14-tetramethyl pentadecanedioic acid by adopting compound
ActiveCN111170855AReduce usageShort reaction stepsOrganic compound preparationCarboxylic acid esters preparationChemical synthesisBiochemical engineering
The invention belongs to the field of chemical synthesis, and relates to a compound with a structural formula as described in the specification. In the structural formula, R1, R2 and R3 are independently selected from C1-C6 alkyl groups, alkenyl groups and cycloalkyl groups respectively. The invention also provides a method for synthesizing 8-hydroxy-2,2,14,14-tetramethyl pentadecanedioic acid byusing the compound. The method has the advantages of short reaction steps, simplified operation and greatly reduced production cost; and the use of raw materials with high toxicity and danger is avoided, so the method is safer.
Owner:AURISCO PHARM(TIANJIN) INC +2
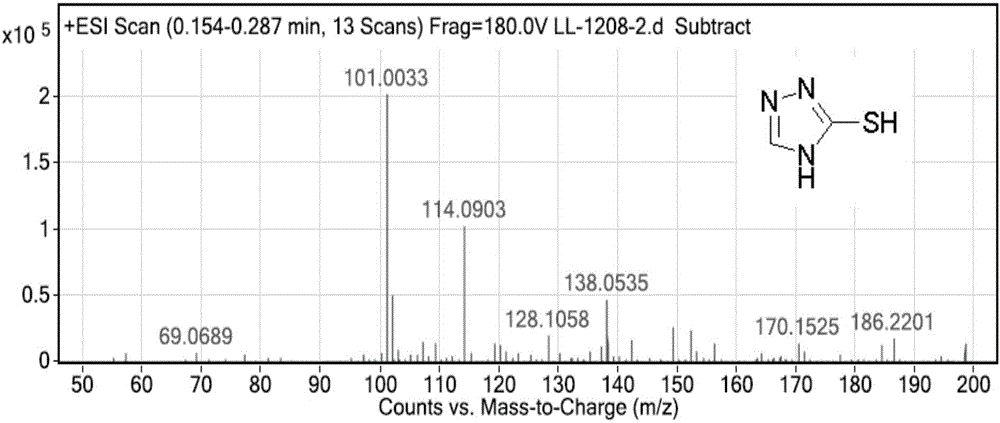
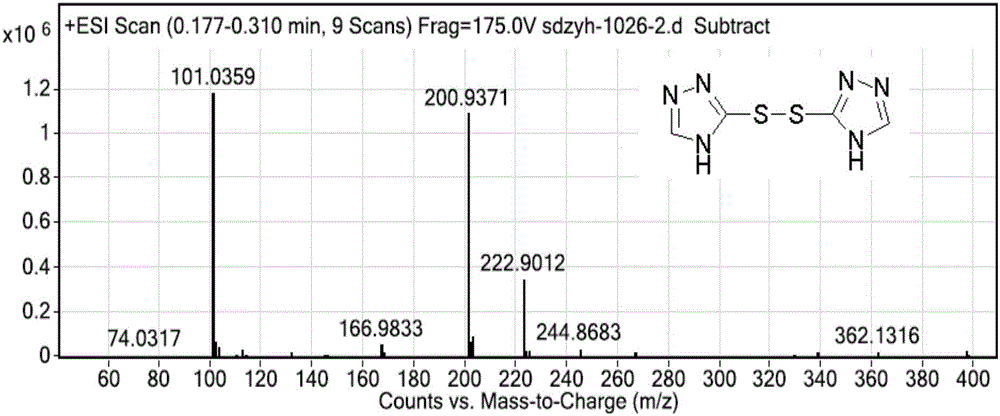
Intermediate compound, and synthetic method of prothioconazole
ActiveCN106749057ASynthetic raw materials are cheap and easy to obtainReduce manufacturing costOrganic chemistryPropanolGreen chemistry
The invention discloses an intermediate compound, and a synthetic method of prothioconazole. The method comprises the following steps: carrying out a substation reaction on 5,5'-dithio-bis(1,2,4-triazole) and 2-(1-chlorocyclopropyl)-3-chloro-1-(2-chlorophenyl)-2-propanol to obtain the key intermediate compound; and reducing the key intermediate compound to obtain the target product prothioconazole. The synthetic method has the advantages of high conversion rate, high selectivity, cheap and easily available synthesis raw materials, reduction of the production cost, mild and easily controlled technologic reaction conditions, simplicity in operation, easiness in product purification, obtaining of the product through direct re-crystallization, simple and accurate control method of intermediates in all steps, high product yield, good atom economy, avoiding of tedious post-treatment, large competition advantages and industrial production utilization values, avoiding of strong alkalis and other raw materials, extremely low three wastes, and according with the green chemistry idea.
Owner:NANJING TECH UNIV
Process for recovering triphenyl phosphine oxide and 2-mercaptobenzothiazole from production waste liquid of cephalothin active ester
The invention discloses a method for recycling triphenyl phosphine oxide and 2-mercaptobenzothiazole from the production waste liquid of cephalosporin active ester, which includes the following steps: 1 to 10 percent sodium hydroxide solution is added drop by drop into the production waste liquid of the cephalosporin active ester under 0 to 80 DEG C; the pH value of the system is adjusted to be 10 to 12, and then the waste liquid is mixed fully for 0.5 to 6 hours, standing still and layering; an organic layer is decompressed to recycle an organic solvent; the solid waste slag is recrystallized directly to obtain the triphenyl phosphine oxide; the 2-mercaptobenzothiazole is extracted and obtained from water layer by a using acid neutralization method. The recycling method of the invention has the advantages of the simple operation, the high recycling rate, the good product purity, the good atom economy, etc., which effectively solves the problems existing in the prior art, such as the complicated operation, the high energy consumption, the low yield rate, the serious environment pollution, etc., thus having a wide implementary value and potential social economic benefits.
Owner:ZHEJIANG UNIV OF TECH +2
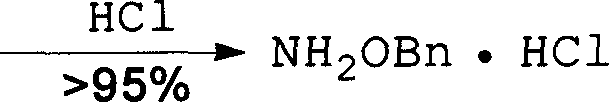
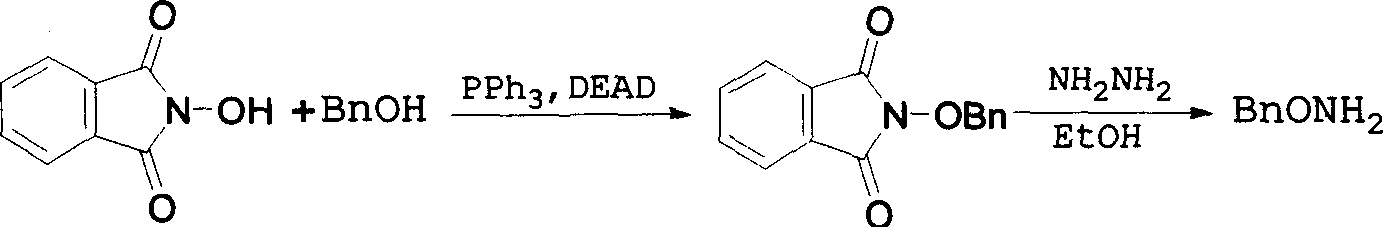
Method for synthesizing benzyloxy amine hydrochloride
The invention refers to a manufacturing method of an important medicine and the organic synthesis midbody-benzyl oxide amine hydrochlorate. The invention starts from hydroxylamine hydrochlorate and gets acetoxime through batching reaction. There obtains the product by the liquor hydrochloric acid under the controlled temperature. The increasing of midbody molecular is little, and the reaction efficiency is high, the whole line atom economic is good. The cost is low.
Owner:XIAMEN UNIV
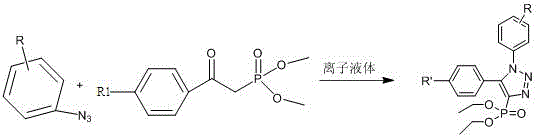
Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid
The invention discloses a method for catalytic synthesis of a 4H-benzo[b]pyran derivative with a basic ionic liquid and belongs to the technical field of organic synthesis. The molar ratio of aromatic aldehyde to malononitrile to a 1,3-cyclohexanedione compound in a synthetic reaction is 1:1:1, the molar weight of a basic ionic liquid catalyst is 4%-7% of that of the used aromatic aldehyde, the volume dose of a reaction solvent ethanol aqueous solution in terms of milliliters is 4-6 times the molar weight of the aromatic aldehyde in terms of millimoles, the reaction pressure is one atmosphere, the reflux reaction is performed for 5-30 min, the mixture is cooled to the room temperature after the reaction is finished, a large quantity of solids are separated out, suction filtration is performed, filter residues are subjected to vacuum drying, and the pure 4H-benzo[b]pyran derivative is obtained. Compared with synthetic methods in which other basic ionic liquids are adopted as catalysts, the method has the characteristics that the catalyst is easy to biodegrade, simple to prepare, low in cost and easy to obtain, the utilization rate of raw materials is high in the whole synthetic process, the operation is simple and convenient and the like, and industrial mass production is facilitated.
Owner:山东润耀环保科技有限公司
Synthesis process of nicosulfuron original medicine
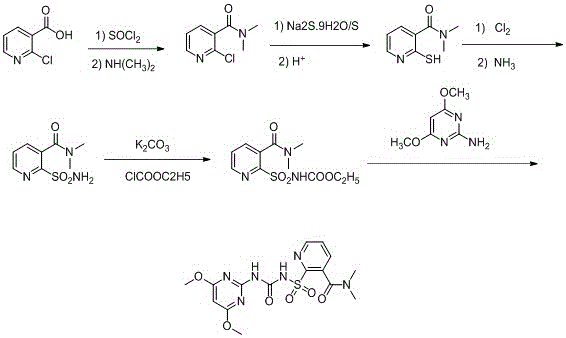
The invention relates to a synthesis process of a nicosulfuron original medicine. In the method, 2-chloronicotinic acid is used as a raw material to synthesize an intermediate 2-sulfunylchloro-N,N-dimethyl nicotinamide, which is then reacted with amino pyrimidine to produce the nicosulfuron. The method has good atom economy and meanwhile avoids use of highly-toxic raw materials, such as phosgene, and expensive catalysts. The method has simple operations, is low in pollution and is low in cost; by means of continuous material feeding and continuous distillation, continuous synthesis of the nicosulfuron original medicine is achieved, and synthesis quality and yield of the nicosulfuron are greatly improved.
Owner:江苏长青生物科技有限公司
Preparation method for organic amine carbaminate
InactiveCN104592058AHas a fixed melting pointIncrease reaction rateCarbamic acid derivatives preparationOrganic compound preparationOrganic solventReaction rate
The invention discloses a preparation method for organic amine carbaminate, belongs to the technical field of preparation of organic amine carbaminate, and solves the problems that a solvent is difficult to separate out and the energy consumption is high of the preparation method for the organic amine carbaminate in the prior art. The preparation method comprises the steps of adding organic amine and an organic solvent into a reaction device according to the mass ratio of 100:(2-500), continuously stirring under the temperature of 10 DEG C below zero to 100 DEGC, filling the reaction device with CO2 gas to enable the gas pressure in the reaction device to reach 0.1-15 MPa, reacting for 2 minutes to 10 hours, and removing the organic solvent to obtain the organic amine carbaminate, wherein the organic solvent can be used for dissolving organic amine and the CO2 gas or is swelled by supercritical CO2. According to the method, the reaction rate and the salting rate are greatly increased; the organic amine carbaminate generated by reaction is easy to separate.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Process of preparing trans-polyhydroxy diphenyl ethylene
InactiveCN101066912AEasy to operateLower reaction costOrganic chemistryOrganic compound preparationIsomerizationSolvent
The present invention discloses process of preparing trans-polyhydroxy diphenyl ethylene. In solvent and at 50-100 deg.c, cis-polymethoxyl substituted diphenyl ethylene under the action of demethylating reagent produces demethylating and isomerizing reaction, and through subsequent cooling, suction filtering, concentrating, re-crystallization, trans-polyhydroxy diphenyl ethylene is prepared. The preparation process of the present invention has simple operation, low reaction cost, high yield and excellent industrial application foreground.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
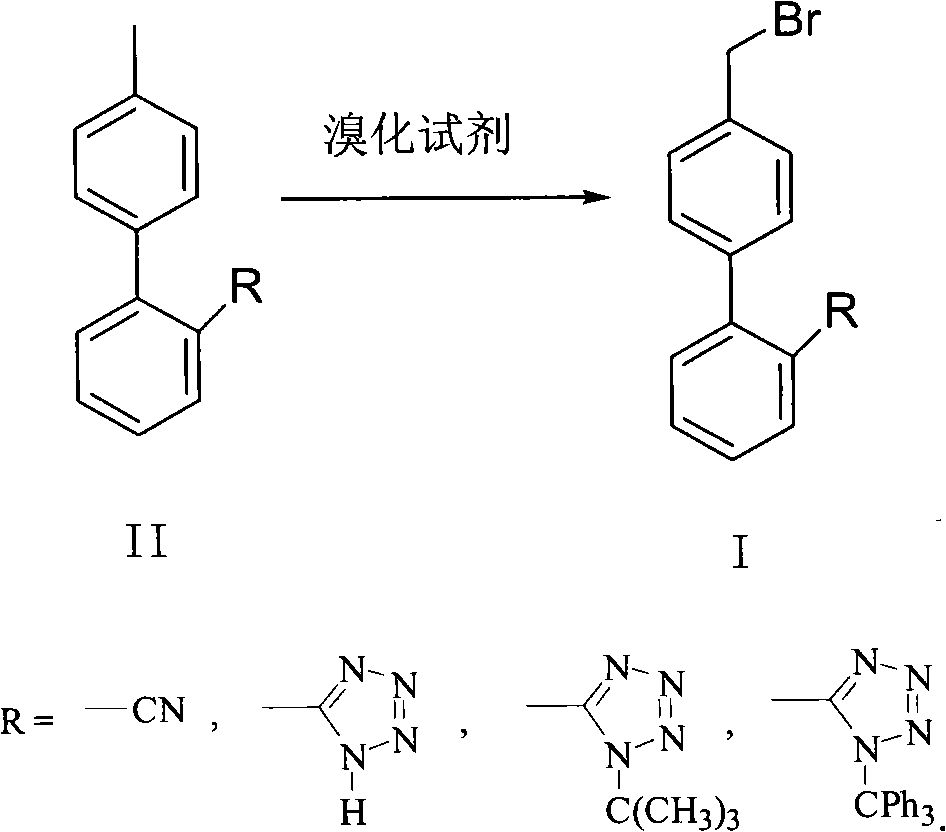
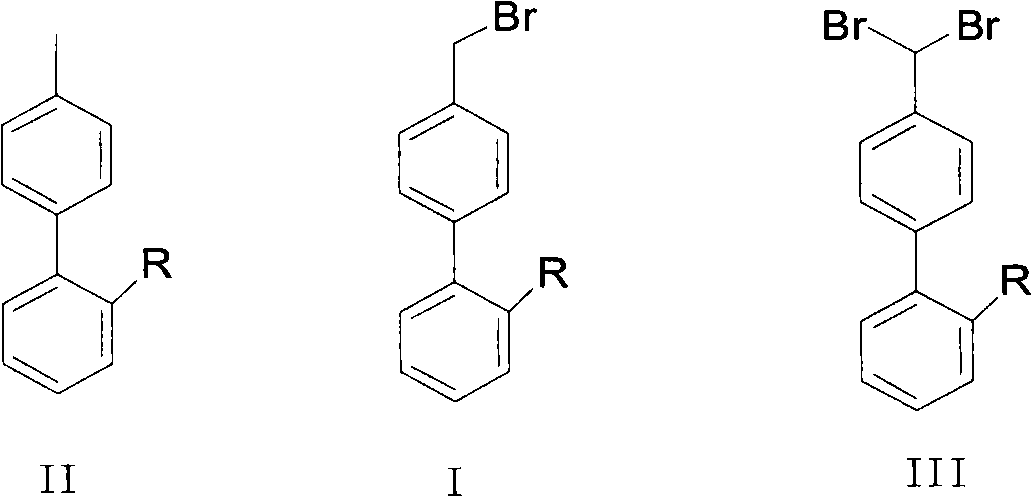
Green synthesis method of bromomethylbiphenyl compound
ActiveCN101648839AHigh selectivityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationBenzeneOrganic solvent
The invention relates to a green synthesis method of a bromomethylbiphenyl compound, comprising the following steps: dissolving a 4'-methyl-2-substituted biphenyl compound in an organic solvent, adding a bromizating agent to carry out bromination reaction and controlling the reaction conditions to obtain the bromomethylbiphenyl compound. The invention is characterized in that the reaction is carried out in light.
Owner:CHINA RESOURCES SAIKE PHARMA
Porous organic polymer framework material and preparation method and application thereof
ActiveCN106496530AEasy to prepareMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkDimethyl acetal
The invention discloses a porous organic polymer framework material and a preparation method and application thereof, and belongs to the technical field of chemical and new materials. The porous organic polymer framework material connected by using methylene is obtained under the catalysis of a lewis acid catalyst by using 2,7-di(nitrogen-carbazolyl)-9 fluorenone as a structural unit and formaldehyde dimethyl acetal as a cross-linking agent, and the porous organic polymer framework material can be applied to selective imine synthesis from photocatalysis of organic amine or selective sulfoxide synthesis from photocatalysis of thioether. The porous organic polymer framework material has the characteristics of simple operation, mild reaction conditions, wide applicability and the like.
Owner:JILIN UNIV
Process for preparing amines from alcohols and ammonia
ActiveUS20110152525A1Good atom economyHighly economicalRuthenium organic compoundsOrganic compound preparationHigh turnoverAlcohol
The present invention provides novel ruthenium based catalysts, and a process for preparing amines, by reacting a primary alcohol and ammonia in the presence of such catalysts, to generate the amine and water. According to the process of the invention, primary alcohols react directly with ammonia to produce primary amines and water in high yields and high turnover numbers. This reaction is catalyzed by novel ruthenium complexes, which are preferably composed of quinolinyl or acridinyl based pincer ligands.
Owner:YEDA RES & DEV CO LTD
Trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof
ActiveCN104649857AMild reaction conditionsHigh selectivityCarbamic acid derivatives preparationSugar derivativesTrifluoromethylationAzidotrimethylsilane
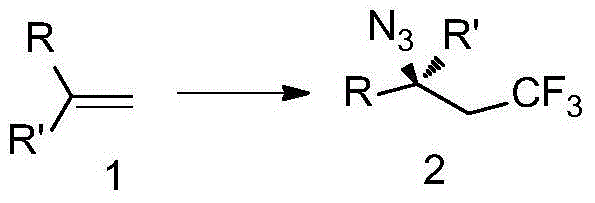
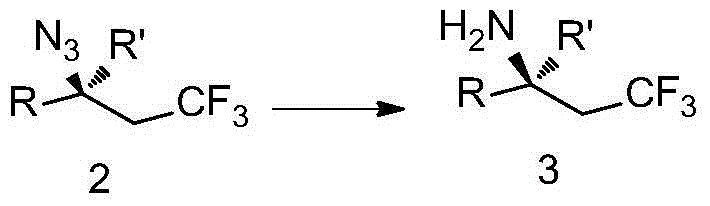
The invention discloses trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof. The preparing method of the trifluoromethyl-substituted azide compounds includes following steps of: subjecting a trifluoromethylation agent, azidotrimethylsilane and a carbon-carbon double bond of an olefin to addition in an organic solvent under the existence of a catalyst to obtain a compound in which one carbon in the carbon-carbon double bond of the olefin has trifluoromethyl and the other carbon has an azide group. The preparing methods utilize the trifluoromethylation agent which is mild relatively, directly form a carbon-nitrogen bond and a carbon-carbon bond by double-functionalization of olefins, and efficiently synthesize the trifluoromethyl-substituted azide, amine and heterocycle compounds with high selectivity. The preparing methods are easily available in raw materials, mild in reaction conditions, good in atom economy, high in selectivity, simple in after-treatment, environmental friendly, high in yields and suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method of carbon-dioxide-base polyurea high polymer material
ActiveCN104650322APerformance variesThe preparation method is simple and environmentally friendlyEpoxy resin adhesivesEpoxyOligomer
The invention discloses a preparation method of a carbon-dioxide-base polyurea high polymer material, belonging to the technical field of preparation of high polymer materials. The method solves the problem that the carbon-dioxide-base polyurea in the prior art can not satisfy the requirements, and widens the application range of the carbon-dioxide-base polyurea. The preparation method comprises the following step: polymerizing amino-terminated polyurea oligomer with a chain extender, an epoxy resin, an alkyd resin, a phenol formaldehyde resin, a urea-formaldehyde resin or other active-terminated oligomers to obtain the carbon-dioxide-base polyurea high polymer material. The structural formula of the amino-terminated polyurea oligomer is disclosed as Formula I. The preparation method is green and environment-friendly, has the advantages of no pollution, simple technique, favorable atomic economical efficiency and wide application range, can prepare high polymer materials with various properties through further reaction between amino-terminated polyurea oligomers with different structures and different substrates, and is suitable for multiple purposes.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
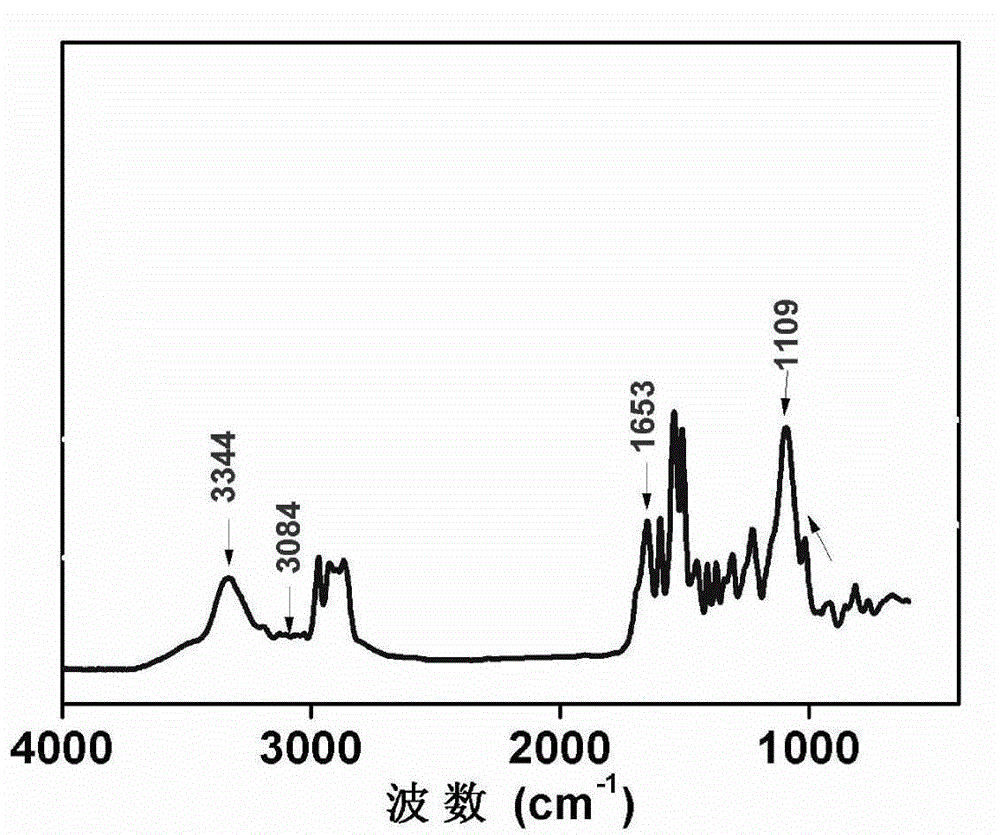
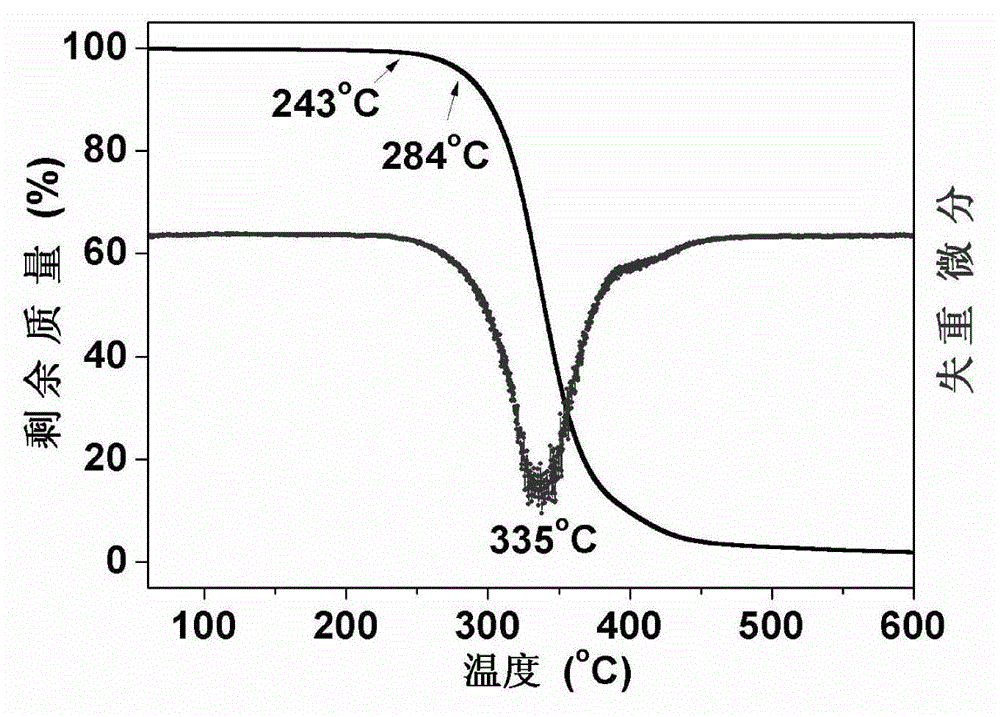
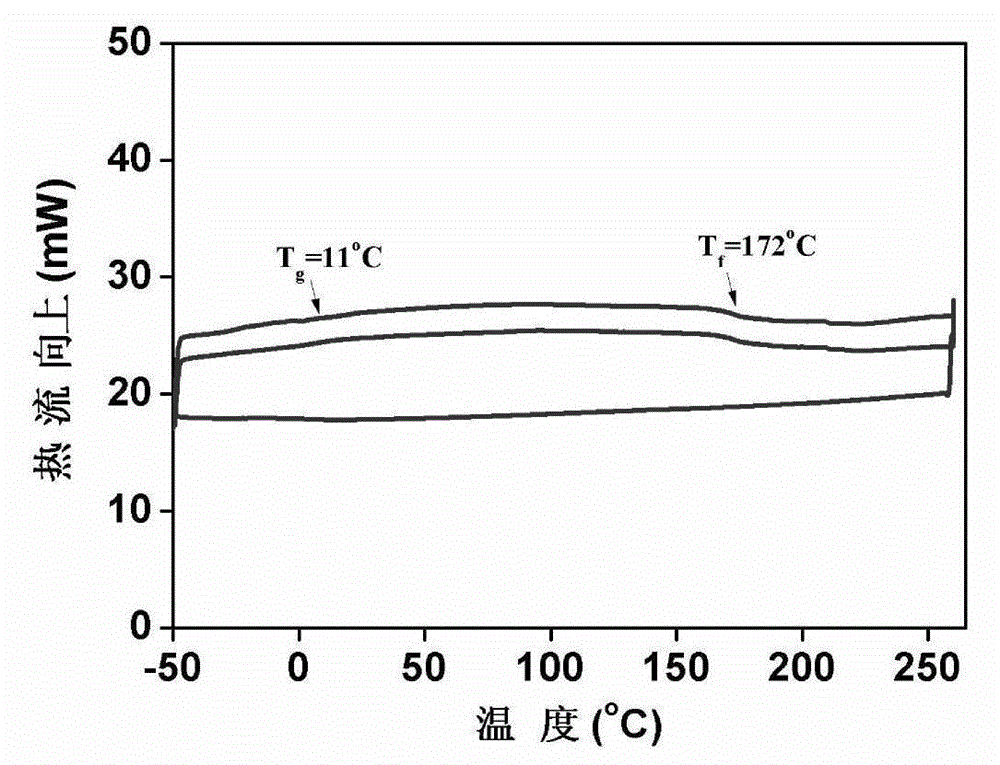
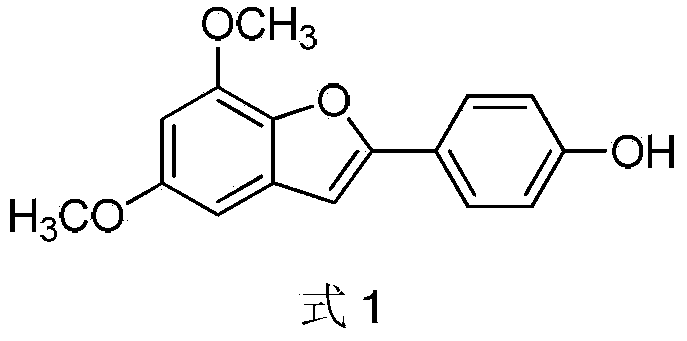
Preparation method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran
The invention discloses a synthetic method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. The synthetic method comprises the following steps of: firstly, performing bromination reaction and Perkin reaction on 3,5-dimethoxy phenylacetic acid and parahydroxyben-zaldehyde which are used as raw materials to obtain 2-(2-bromo-3,5-dimethoxy phenyl)-3-(4-hydroxyphenyl) acroleic acid; then performing series-wound hydroxylation / intramolecular cyclization / dehydrogenation reaction to obtain 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran-3-carboxylic acid; and finally, performing decarboxylic reaction to prepare the 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. According to the method, raw materials are low in price and easy to obtain, the synthetic route is simple, quick and efficient, a noble metal and a ligand are not needed for catalysis, and the method is simple and convenient to operate and good in atom economy.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing lactic acid through selective catalytic conversion of ethylene glycol
ActiveCN110357770AHigh selectivityHigh purityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaline earth metalCatalytic oxidation
The invention specifically relates to a method for preparing lactic acid through selective catalytic conversion of ethylene glycol, belonging to the technical field of fine chemical engineering. The invention provides an azacyclocarbene metal complex catalyst for the selective catalytic conversion of ethylene glycol to produce lactic acid at first. According to the method of the invention, ethylene glycol and methanol are used as raw materials, an alkali metal or alkaline earth metal hydroxide is used as a base, an azacyclocarbene metal complex is used as a catalyst, and a closed reaction is carried out at 100-200 DEG C for 0.5-12 h to prepare lactic acid. Compared with the prior art, the method of the invention has the following advantages: the cheap and easily-available ethylene glycol and methanol are used as substrates; a catalytic oxidation reaction is performed under mild conditions; quantitative lactic acid yield is obtained; reaction conversion efficiency is high; operation iseasy; high-purity lactic acid can be obtained without complicated post-treatment; and the method is suitable for large-scale industrial application.
Owner:FUDAN UNIV
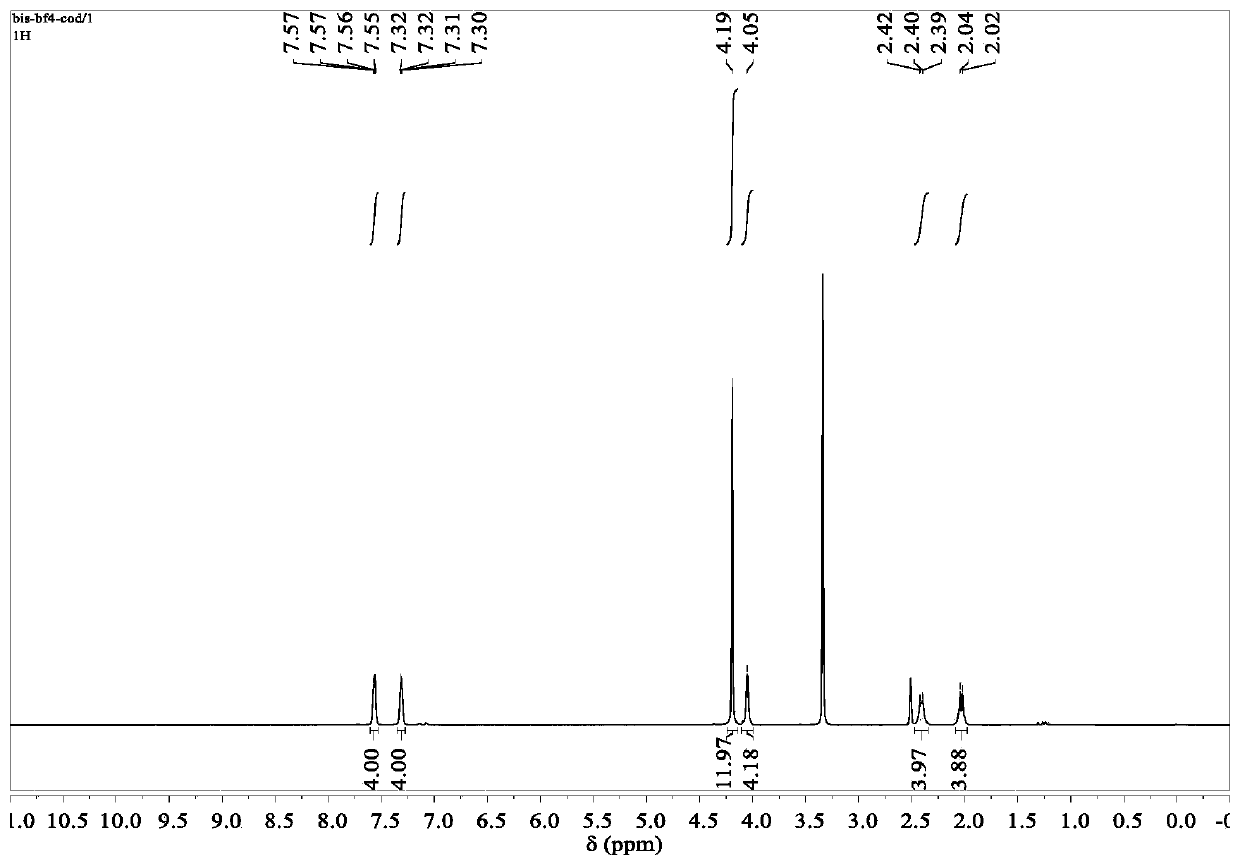
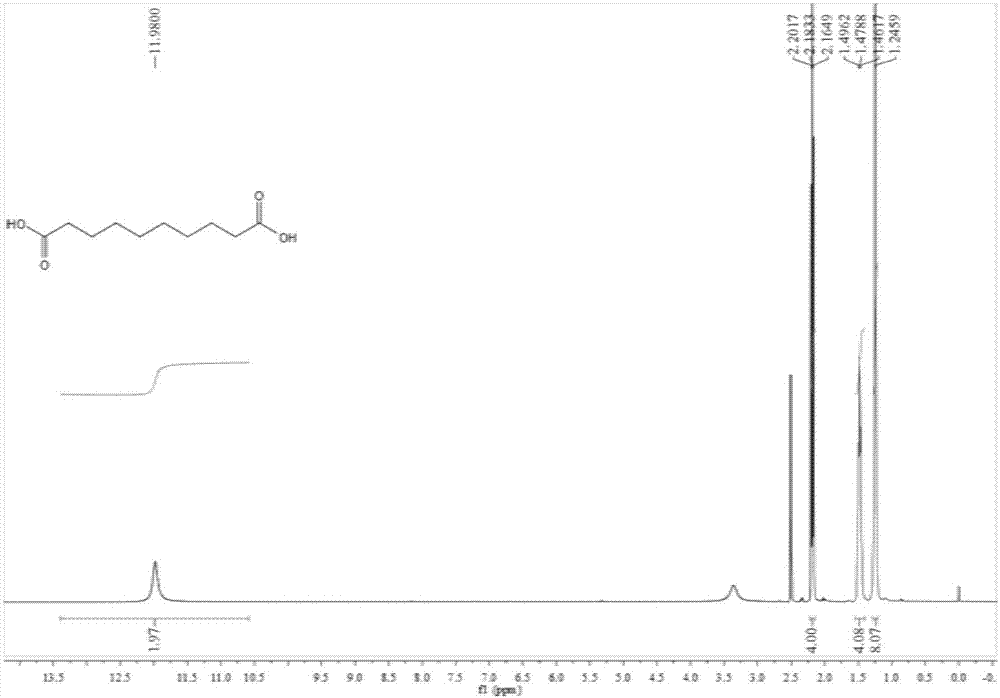
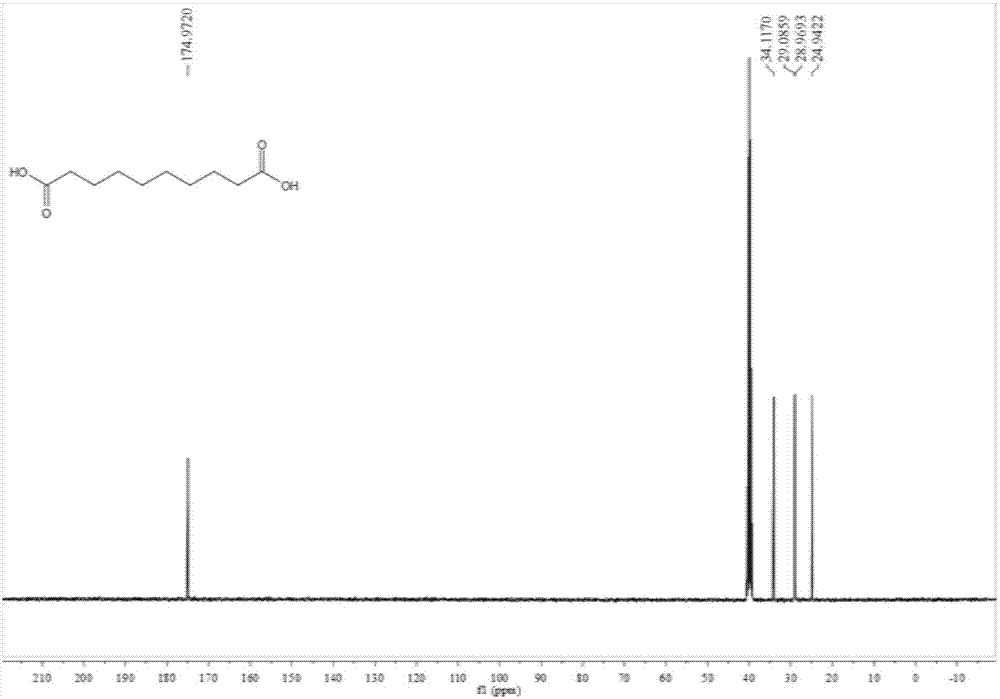
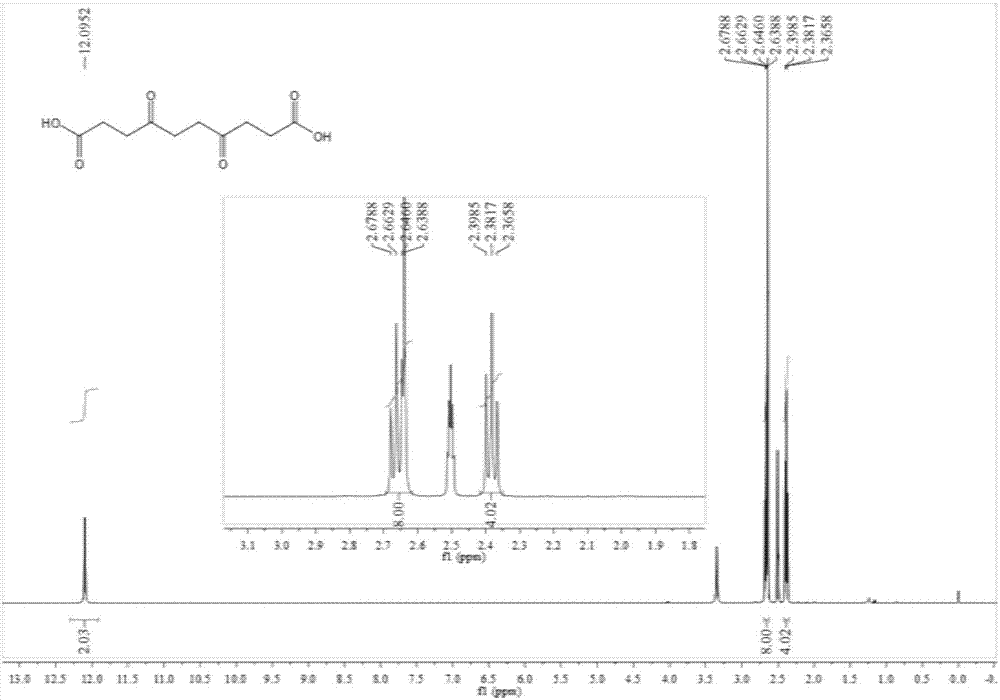
Preparation method of 1,10-sebacic acid
ActiveCN107382712AReduce pollutionHigh yieldOrganic compound preparationCarboxylic compound preparationDiketoneHydrogen
The invention discloses a preparation method of 1,10-sebacic acid. The preparation method includes: subjecting substance A to hydrolyzing and ring opening in an acid environment to obtain 4,7-diketone-1, and 10-sebacic acid; subjecting 4,7-diketone-1, 10-sebacic acid, hydrogen, trifluoromethanesulfonic salt and a hydrogenation catalyst to hydrodeoxygenation to obtain the 1,10-sebacic acid. The preparation method is good in atom economy, high in product yield, high in product purity, less in environment pollution, simple in process route, convenient in operation, cheap in raw materials which are easy to obtain and suitable for industrialized mass production.
Owner:合肥利夫生物科技有限公司
Method for synthesizing 4-phosphonic acid-1,5-substituted-1,2,3 triazole compounds through catalysis of ionic liquid
ActiveCN105949240AShort reaction timeMild reaction conditionsGroup 5/15 element organic compoundsSolubilityFluorescence
The invention discloses a method for synthesizing 4-phosphonic acid-1,5-substituted-1,2,3 triazole compounds through catalysis of an ionic liquid and belongs to the technical field of synthesis of the triazole compounds. The technical scheme is characterized in that azide compounds and phosphorus ester compounds are taken as substrates, the ionic liquid is taken as a catalyst, methyl alcohol is taken as a solvent, the materials are subjected to stirring reaction at the temperature of 25-50 DEG C, and the 4-phosphonic acid-1,5-substituted-1,2,3 triazole compounds are prepared. The reaction time for the synthesis method is shorter, the reaction conditions are mild, the reaction temperature is 25-50 DEG C, the method requires no metal catalyst while a traditional click reaction requires transition metal as a catalyst, and accordingly, toxicity of metal ions is greatly reduced; phosphorylation is a method capable of improving properties of an index compound, with the introduction of a phosphoric acid group in drug design, the water solubility of the compound can be improved, so that bioavailability is improved, and part of prepared compounds have the fluorescence characteristic.
Owner:HENAN NORMAL UNIV
Application of mo-based catalyst loaded by unreduced or partially reduced polymetallic oxide in preparation of organic chemical product with lignin
InactiveCN107759444AHigh catalytic activityWide variety of sourcesOxygen-containing compound preparationOrganic compound preparationOrganic chemicalsEthyl caproate
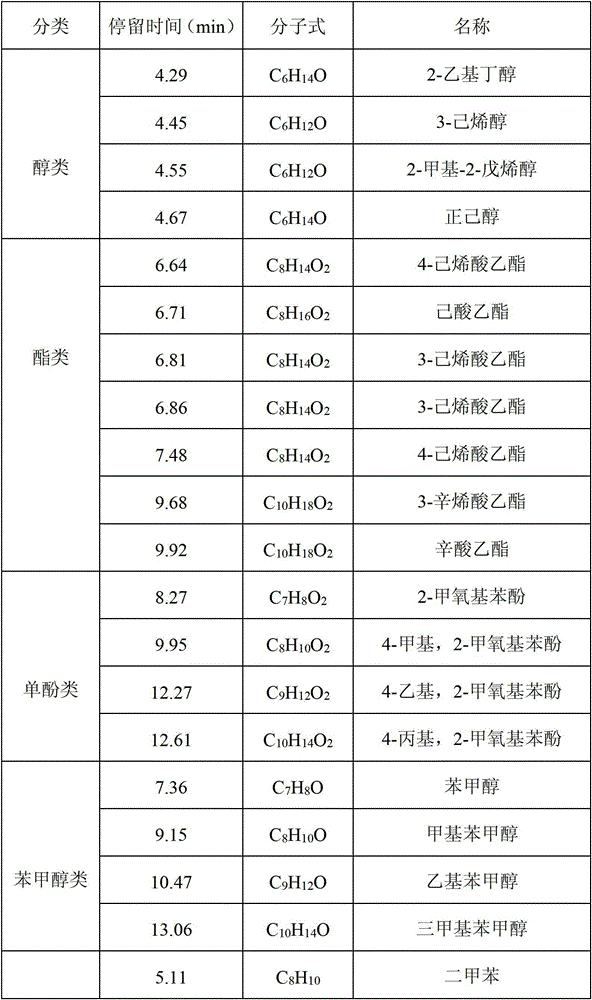
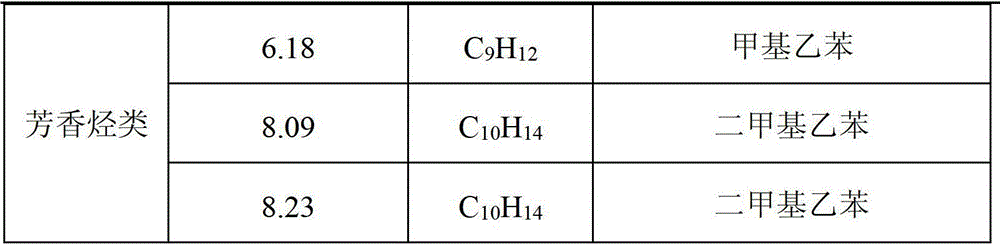
The invention provides application of a mo-based catalyst loaded by an unreduced or partially reduced polymetallic oxide in preparation of an organic chemical product with lignin. The application comprises the steps of after the lignin, the catalyst and a reaction solvent are mixed, adding a mixture into a sealed reaction container, introducing gas into the container, increasing the temperature at230-350 DEG C, stirring the mixture and making the mixture react for 0.5-12 h, after reaction, filtering out the catalyst, conducting rotary evaporation, and obtaining the liquid product. The application of the mo-based catalyst loaded by the unreduced or partially reduced polymetallic oxide in preparation of the organic chemical product with the lignin has the advantages that the catalyzing process has a high yield of product, on the optimized reaction condition, the total mass yield of a micromolecule organic product reaches 349%, which is because solvent molecules and lignin depolymerizedsmall molecules are combined further to generate useful molecules such as ethyl caproate, ethyl cis-3-hexenoate, 4-ethyl cis-3-hexenoate, ethyl 3-methylvalerate, 3-ethyl trans-2-octenoate and ethyl caprylate, the product contains aromatic compounds such as monophenol applied to industry in large amounts, the additional value of the catalyst is high, and the catalyst has a good industrial application prospect.
Owner:TIANJIN UNIV +1
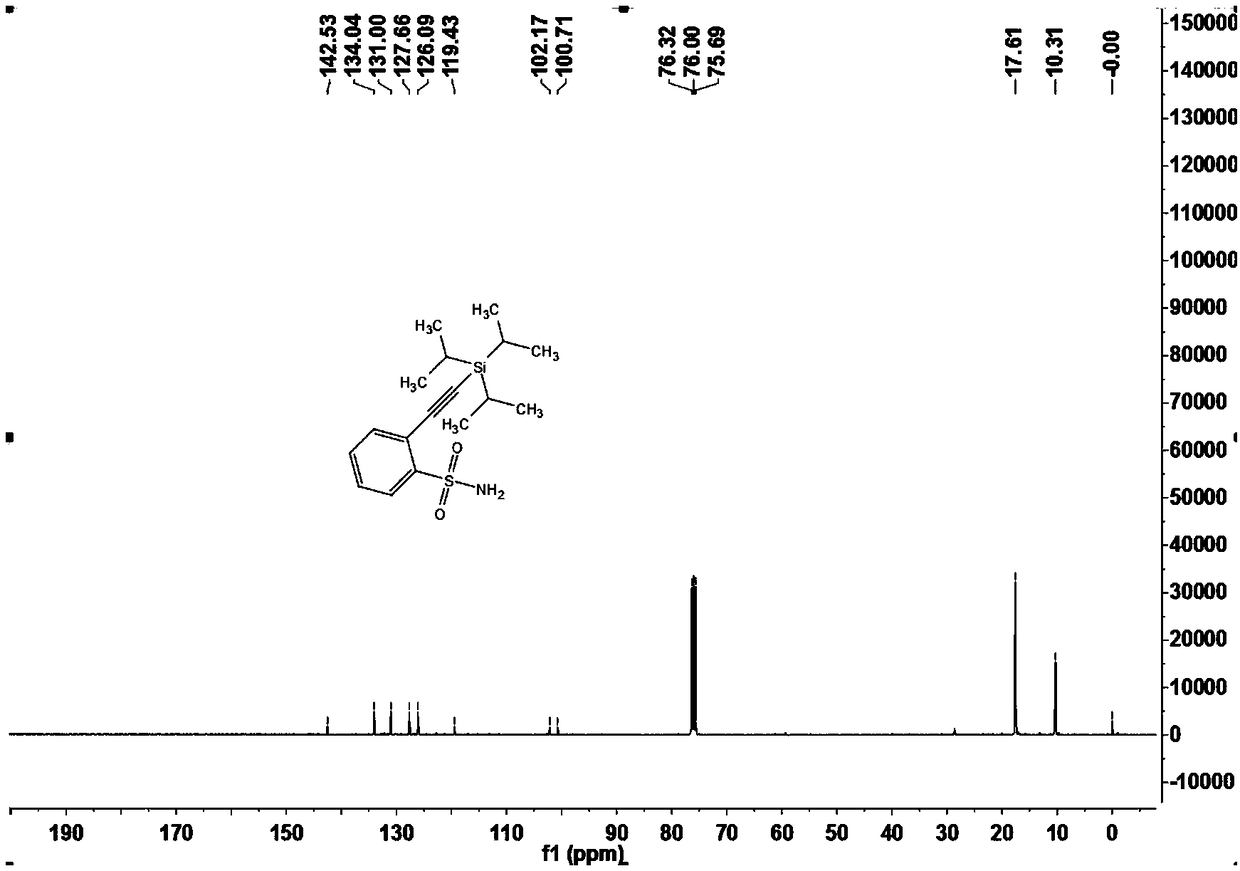
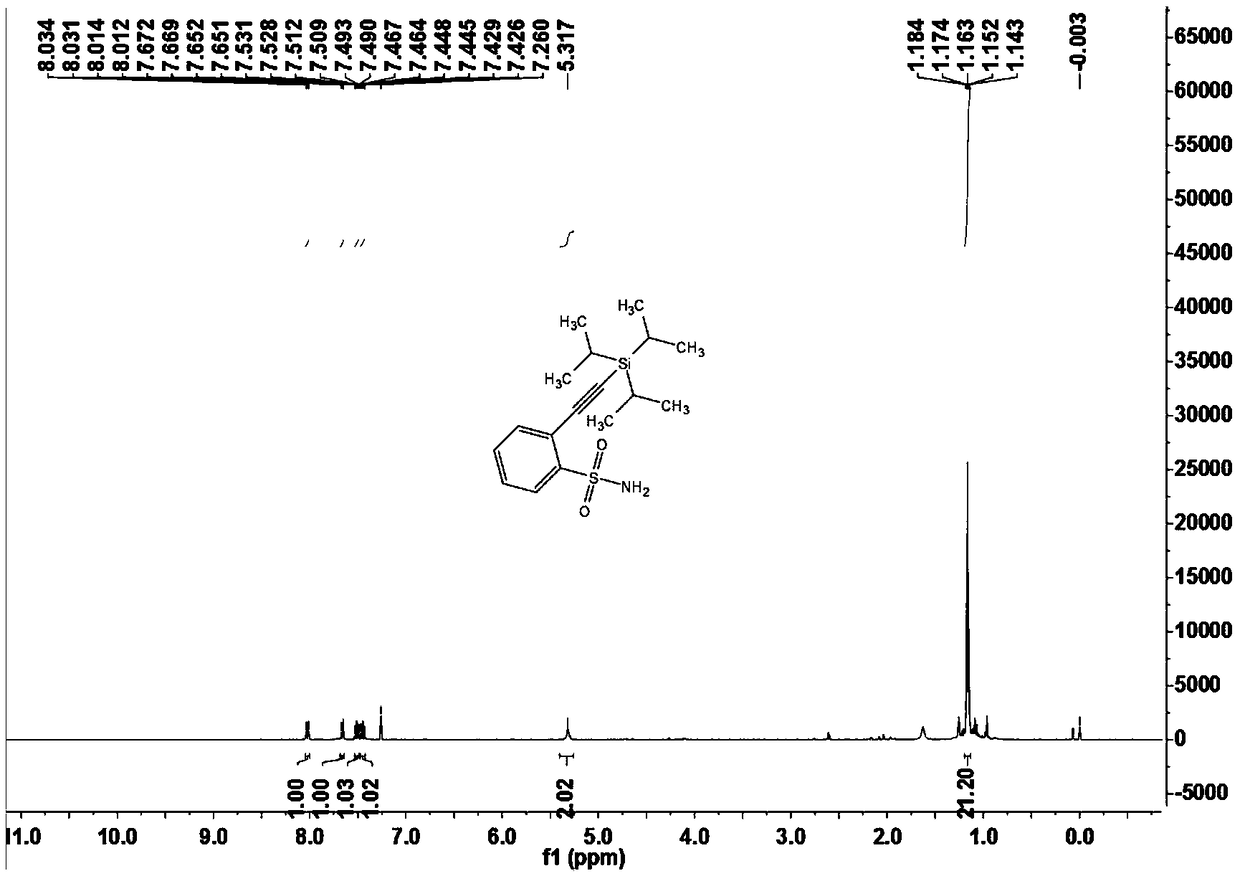
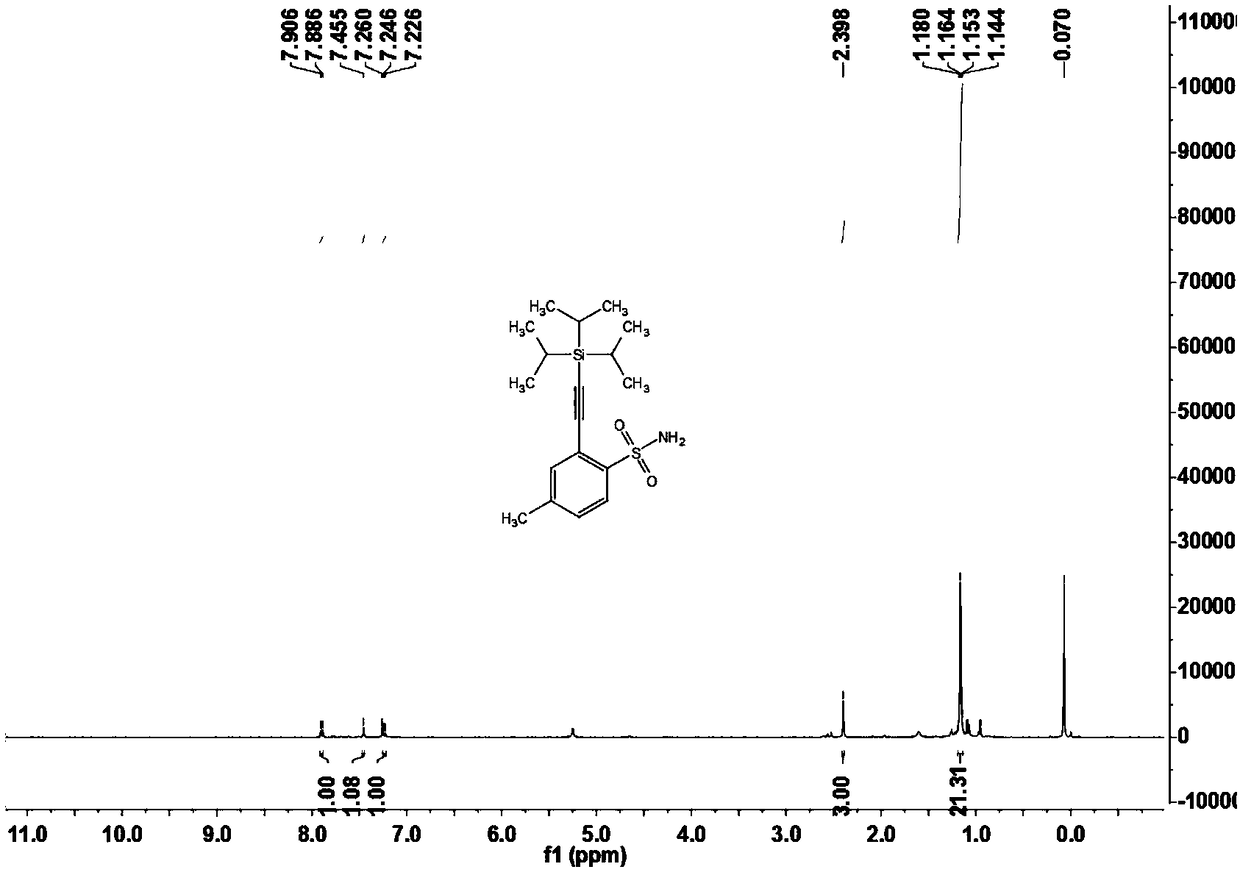
Sulfonamide compounds as well as preparation method and application thereof
ActiveCN108822145ARaw materials are easy to obtainFew stepsSilicon organic compoundsHydrogenOrganic synthesis
The invention relates to the technical field of organic synthesis, in particular to sulfonamide compounds as well as a preparation method and an application thereof. The preparation method of the sulfonamide compounds comprises the following step: compounds shown as formula (I) are prepared from compounds shown as formula (II) and compounds shown as formula (III) under the action of a catalyst inpresence of an insert solvent, the compounds shown as the formula (I) are brand-new sulfonamide compounds and can be applied to the field of biomedicine. Substrates of the compounds are simple and easily available, for alkynylation reactions of direct carbon-hydrogen bonds of primary sulfonamides or secondary sulfonamides without additional orienting groups, few steps are exerted, operation is simple, the compounds have good atom economy and industrial application value, and the technical problems that the conventional sulfonamide compounds are synthesized through complicated steps and application to industrial production is difficult are solved.
Owner:GUANGDONG UNIV OF TECH
Method for synthesizing cyanomethyl carboxylate
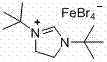
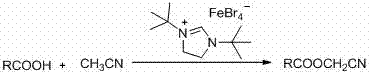
ActiveCN107311890ARealize the esterification reactionReduce chemical pollutionCarboxylic acid nitrile preparationOrganic compound preparationDi-tert-butyl peroxideAcetonitrile
The invention discloses a method for synthesizing cyanomethyl carboxylate. According to the invention, the method employs an ionic iron (III) complex containing 1,3-di-tert-butylimidazoline cations and having a molecular formula of [(tBuNCH2CH2NtBu)CH][FeBr4] as a catalyst and di-tert-butyl peroxide as an oxidant, and synthesizes cyanomethyl carboxylate through an oxidative coupling reaction of carboxylic acid with acetonitrile. Applicative carboxylic acid substrates in the invention include aliphatic carboxylic acids, aromatic carboxylic acids and heterocyclic carboxylic acids. The method realizes synthesis of cyanomethyl carboxylate through the oxidative coupling reaction of carboxylic acid with acetonitrile under the action of an iron-based catalyst for the first time.
Owner:SUZHOU UNIV
3-amino indanone compound synthesis method
ActiveCN107033016AImprove conversion efficiencyGood atom economyOrganic compound preparationGroup 5/15 element organic compoundsMANGANESE ACETATEPentamethylcyclopentadiene
The invention discloses a 3-amino indanone compound synthesis method which includes the steps: adding an imine derivative, an olefin compound, pentamethyl dichloride rhodium cyclopentadiene and manganese acetate into an organic solvent; performing heating reaction under air conditions; performing post-treatment after complete reaction to obtain a 3-amino indanone compound. According to the method, simple and easily obtained raw materials are synthesized at one step to obtain the 3-amino indanone compound, conversion efficiency is high, and atom economy is good. Besides, the synthesis method is simple in operation and high in reaction yield, and a substrate is wide in adaptability.
Owner:ZHEJIANG UNIV
Bisbenzothiazole disulfide and triphenylphosphine preparation by means of one pot
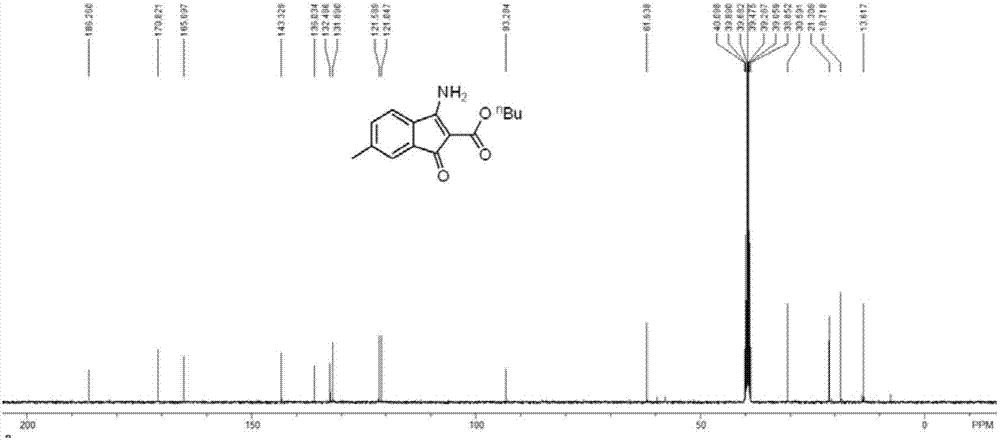
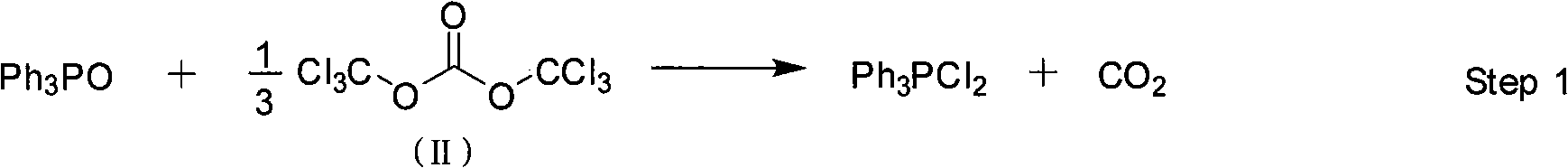
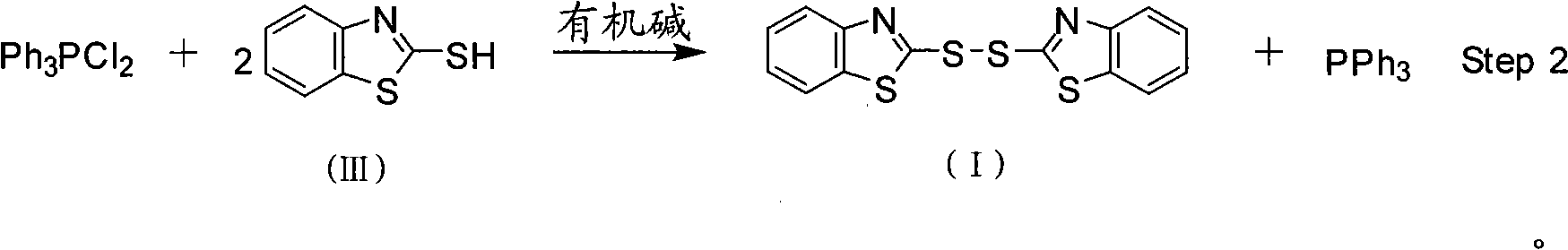
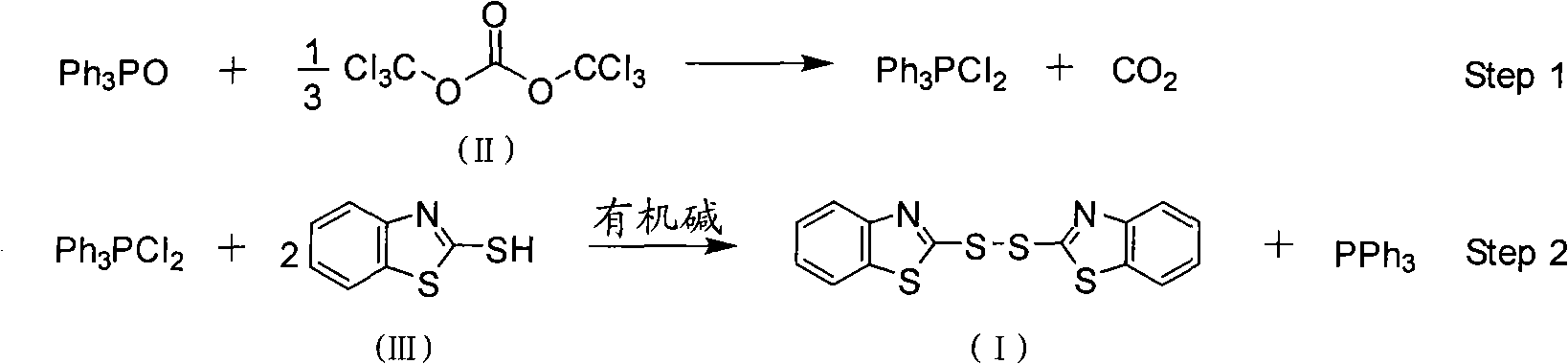
InactiveCN101357908AEasy to separate and purifyHigh yieldGroup 5/15 element organic compoundsOrganic baseBottle
The invention discloses a method for preparing bisbenzothiazole disulfide (DM) and triphenylphosphine (TPP) by a pot method, comprising the following processes: an organic solvent A, bis (trichloromethyl) carbonate shown in formula (II) and triphenyl phosphine oxide are sequentially added to a reaction bottle, and the insulating reaction is carried out for 0.5 to 10 hours at the temperature of minus 30 DEG C to 90 DEG C; the reaction solution is added with mixed solution of 2-sulfhydryl benzothiazole and organic base which are dissolved by an organic solvent B shown in formula (III), and the mixture reaction is carried out for 0.5 to 10 hours at the temperature of -30 DEG C to 90 DEG C; after the reaction is completed, drawing and filtering are carried out, the filter cake is dried and then the bisbenzothiazole disulfide is obtained, the organic solvent in the filter solution is recycled to obtain crude products, the crude products are re-crystallized to obtain the triphenylphosphine. The method has the advantages of simple operation, high reaction yield, high product purity and good atom economy, thus solving the problem that by-products can cause environment pollution, and the like, owning to difficult recovery and utilization in the process of producing cephalothin active ester, and the method has great implement value and potential social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH +2
Preparation method of o-hydroxybenzonitrile
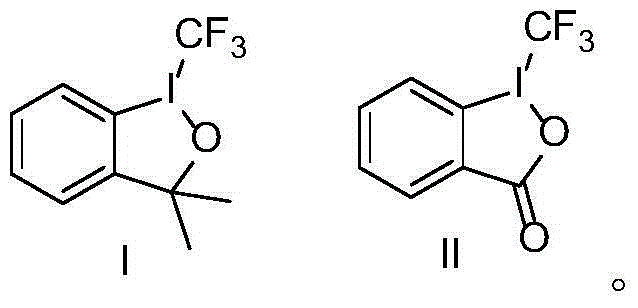
InactiveCN104610095AHigh catalytic activityAtom economy is highPreparation by carboxylic acid amide dehydrationBoiling pointReaction temperature
The invention discloses a preparation method of o-hydroxybenzonitrile. The preparation method comprises the following step: taking o-hydroxybenzoyl amide as a raw material, an amino acidic compound as a catalyst and dichloroethane as a solvent, and dehydrating to prepare o-hydroxybenzonitrile under the action of phosgene. According to the preparation method, the amino acidic compound is used as the catalyst, the catalytic activity is high, the product yield reaches 98%, and the atom economy is high; at the reaction temperature of 75-80 DEG C, a reaction system is in a micro reflowing state and sufficient reaction of the phosgene can be ensured, so that use of a high-boiling point solvent and loss of the phosgene in reflowing reaction are avoided and the energy consumption and the cost are reduced.
Owner:尹山红
Process for preparing amines from alcohols and ammonia
ActiveUS8586742B2Good atom economyHighly economicalRuthenium organic compoundsOrganic compound preparationHigh turnoverAlcohol
The present invention provides novel ruthenium based catalysts, and a process for preparing amines, by reacting a primary alcohol and ammonia in the presence of such catalysts, to generate the amine and water. According to the process of the invention, primary alcohols react directly with ammonia to produce primary amines and water in high yields and high turnover numbers. This reaction is catalyzed by novel ruthenium complexes, which are preferably composed of quinolinyl or acridinyl based pincer ligands.
Owner:YEDA RES & DEV CO LTD
Isoindoline derivative and preparation method thereof
ActiveCN110156660AIncreased complexityEfficient synthesisOrganic chemistryMetal catalystOrganic synthesis
The invention belongs to the technical field of organic synthesis, and particularly relates to an isoindoline derivative and a preparation method thereof. The invention provides the isoindoline derivative, and the structural formula of the isoindoline derivative is shown in a formula I. The invention further provides the preparation method of the isoindoline derivative. The preparation method comprises the following steps: placing a compound represented by a formula II, a compound represented by a formula III and a compound represented by a formula IV in an inert solvent, and under the actionof an oxidizing agent and a metal catalyst, adding an alkali to carry out a reaction under an alkaline condition to obtain the isoindoline derivative. The invention provides the isoindoline derivativeand the preparation method thereof, and can effectively solve the technical problems that an existing preparation method of isoindoline derivatives is poor in compatibility and the amount of byproducts is large.
Owner:GUANGDONG UNIV OF TECH
Preparation method of capric acid
ActiveCN107445819AHigh yieldHigh purityOrganic compound preparationPreparation from carboxylic acid esters/lactonesHydrogenCapric Acid
The invention discloses a preparation method of capric acid. The preparation method comprises the following steps: carrying out hydrodeoxygenation on a substance A and hydrogen, trifluoromethanesulfonic acid and a hydrogenation catalyst so as to obtain capric acid. According to the preparation method, the atom economy is good, the product yield is high, the product purity is high, the pollution to the environment is slight, the process route is simple, the operation is convenient, and the preparation method is suitable for industrial large-scale production.
Owner:合肥利夫生物科技有限公司
Method for preparing aryl acetonitrile compound
ActiveCN102452867AReduce usageLow toxicityCarboxylic acid nitrile preparationOrganic compound preparationCarboxylateCatalytic efficiency
The invention provides a method for preparing an aryl acetonitrile compound, which obtaines an aryl acetonitrile compound by performing a decarboxylation coupling reaction of heating a decarboxylation coupling reagent and an electrophilic substrate in the presence of a palladium catalyst, a phosphine ligand, and an organic solvent. The method provided by the invention prevents the application of toxic reagents, and the reaction substrate is carboxylic acid or carboxylates, which is low in price, safe and stable, low in toxicity, and convenient for operation; the yield is high; the operation is easy; and the economy is good; compared with traditional synthetic methods reported before, the method has good compatibility for groups such as ester groups and carbonyls which are sensitive to alkali; the by-product is few; the atom economy is better than that of previous methods; the requirements for green chemistry are met; the catalytic amount is few; the catalytic efficiency is high; the separation is easy; the conversion rate is high; the yield is high; and the method has industrial and synthetic value.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
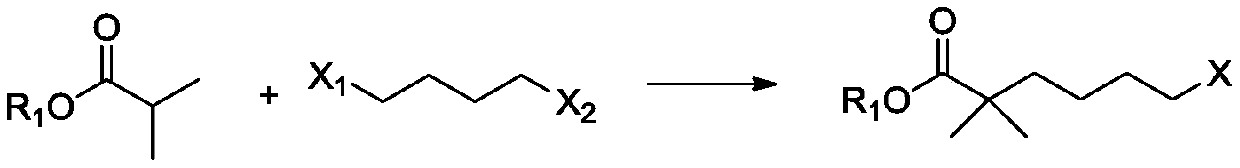
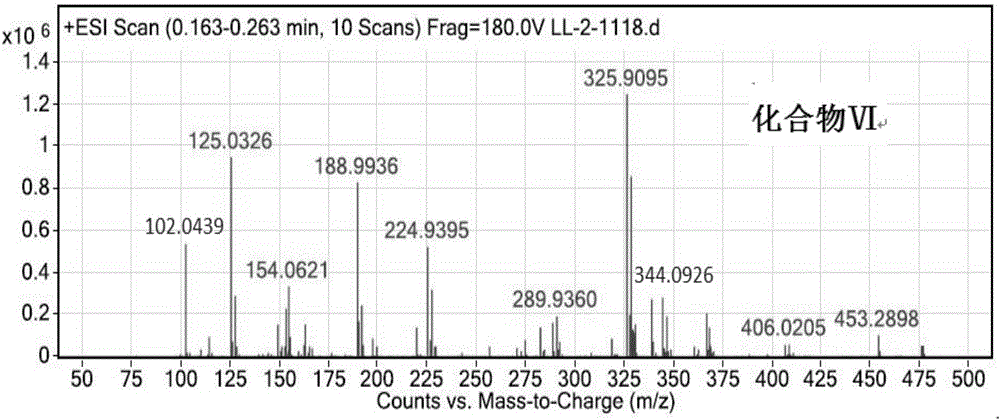
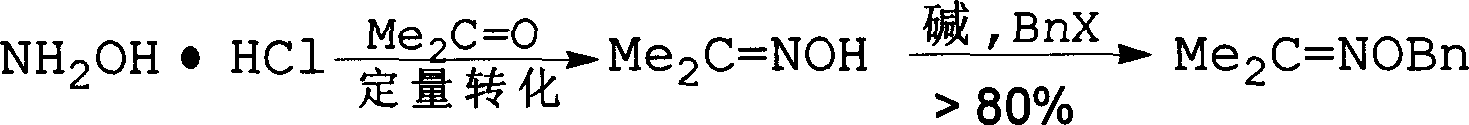
![Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid](https://images-eureka.patsnap.com/patent_img/57767ea6-20b4-474c-8501-820b82377553/HDA0000778861820000011.PNG)
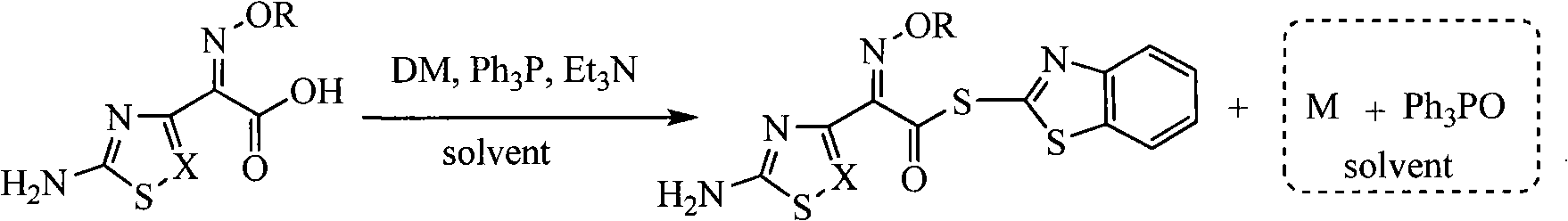
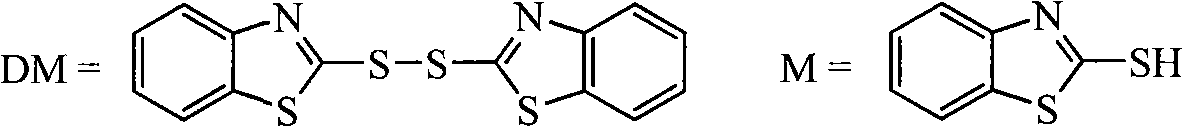
![Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid](https://images-eureka.patsnap.com/patent_img/57767ea6-20b4-474c-8501-820b82377553/HDA0000778861820000012.PNG)
![Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid Method for catalytic synthesis of 4H-benzo[b]pyran derivative with basic ionic liquid](https://images-eureka.patsnap.com/patent_img/57767ea6-20b4-474c-8501-820b82377553/HDA0000778861820000021.PNG)