Trifluoromethyl-substituted azide, amine and heterocycle compounds and preparing methods thereof
A technology for azide compounds and amine compounds, which is applied in the field of amines and heterocyclic compounds, trifluoromethyl-substituted azides, and can solve problems such as difficult synthesis, high reaction risk, and long reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
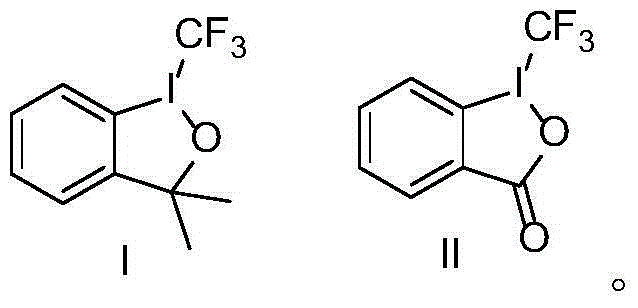
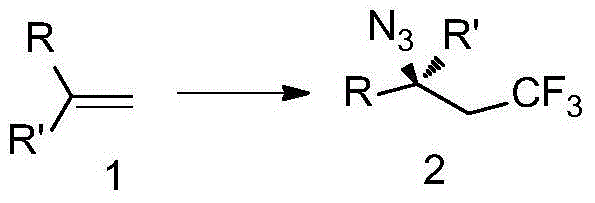
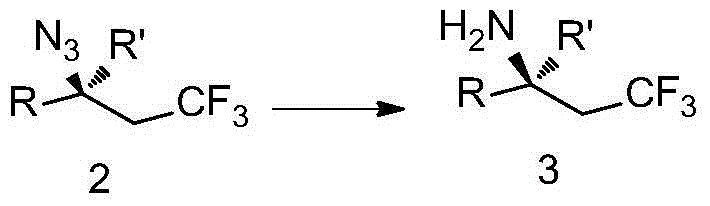
[0098] Add Togni reagent (99.2mg, 0.3mmol) into a 10mL sealed tube, weigh the catalyst Cu(CH 3 EN) 4 PF 6 (3.7mg, 0.01mmol) and transferred out of the glove box, under the protection of nitrogen, add magneton and 1mL solvent, and then add TMSN 3 (54μL, 0.4mmol) and styrene (0.2mmol) were added to the sealed tube, and after the reaction was stirred at room temperature for 6h, 5mL of ethyl acetate was added. The reaction solution was washed twice with water (20mL×2), and once with saturated brine (20mL). The organic layer was spin-dried and directly subjected to column chromatography to obtain 37.4 mg of product The total yield is 87%, 1 H NMR, 13 C NMR and 19 The purity of F NMR is greater than 95%.
[0099] 1 H NMR (400MHz, CDCl 3 )δ7.44-7.28(m,5H),4.77(dd,J=8.4,5.2Hz,1H),2.65(dqd,J=15.6,10.4,8.4Hz,1H),2.48(dqd,J=15.6, 10.4,5.2Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ137.6, 129.2, 129.0, 126.6, 125.2(d, J=276.4Hz), 59.9(q, J=3.2Hz), 40.3(q, J=28.3Hz). 19 F NMR (376MHz,...
example 2
[0102] Add Togni reagent (99.2mg, 0.3mmol) into a 10mL sealed tube, weigh the catalyst Cu(CH 3 EN) 4 PF 6 (3.7mg, 0.01mmol) and transferred out of the glove box, under the protection of nitrogen, add magneton and 1mL solvent, and then add TMSN 3 (54 μL, 0.4 mmol) and (0.2mmol) was added to the sealed tube, and after the reaction was stirred at room temperature for 6h, 5mL of ethyl acetate was added. The reaction solution was washed twice with water (20mL×2), and once with saturated brine (20mL). The organic layer was spin-dried and directly subjected to column chromatography to obtain 48.6 mg of product The total yield is 89%, 1 H NMR, 13 C NMR and 19 The purity of F NMR is greater than 95%.
[0103] 1 H NMR (400MHz, CDCl 3 )δ7.34(d,J=8.1Hz,2H),7.29(d,J=8.1Hz,2H),6.21(b,1H),4.77(dd,J=8.6,5.0Hz,1H),3.66( s,2H),2.61(dqd,J=15.2,10.4,8.6Hz,1H),2.47(dqd,J=15.2,10.4,5.0Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ172.9,136.8,134.2,130.2,126.9,125.1(q,J=277.4Hz),59.5(q,J=3.1Hz),...
example 3
[0106] Add Togni reagent (99.2mg, 0.3mmol) into a 10mL sealed tube, weigh the catalyst Cu(CH 3 EN) 4 PF 6 (3.7mg, 0.01mmol) and transferred out of the glove box, under the protection of nitrogen, add magneton and 1mL solvent, and then add TMSN 3 (54 μL, 0.4 mmol) and (0.2mmol) was added to the sealed tube, and after the reaction was stirred at room temperature for 6h, 5mL of ethyl acetate was added. The reaction solution was washed twice with water (20mL×2), and once with saturated brine (20mL). The organic layer was spin-dried and directly subjected to column chromatography to obtain 42.8 mg of product The total yield is 93%, 1 H NMR, 13 C NMR and 19 The purity of F NMR is greater than 95%.
[0107] 1 H NMR (400MHz, CDCl 3 )δ7.49-7.39(m,4H),7.37-7.32(m,1H),2.66(dq,J=15.6,10.4Hz,1H),2.60(dq,J=15.6,10.4Hz,1H),1.88 (q,J=0.9Hz,3H). 13 C NMR (100MHz, CDCl 3)δ141.9,128.7,128.1,125.3,125.0(d,J=278.3Hz),62.8(q,J=2.1Hz),45.2(q,J=27.3Hz),24.2(q,J=1.7Hz). 19 F NMR (376MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com